Advances in Enzyme Research
Vol.2 No.2(2014), Article ID:46657,8 pages
DOI:10.4236/aer.2014.22010
Aliskiren Augments the Activities of Anti-Oxidant Enzymes in Liver Homogenates of DOCA Salt-Induced Hypertensive Rats
Sahar Kamal
Pharmacology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Email: saharkamal2003@hotmail.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 2 May 2014; revised 30 May 2014; accepted 7 June 2014
Hypertension is a serious problem that is recently thought to be associated with damaging effects on target organs partially via oxidative stress. On the other hand, there is accumulating literature describing some sort of therapeutic interaction between antioxidant enzymes in vital organs and hypertension. Therefore, the aim of this study is to investigate the possible effect of a direct renin inhibitor, aliskiren, used in treatment of hypertension via renin-angiotensin-aldosterone system (RAAS), on selected anti-oxidant enzymes in hepatic homogenates in DOCA salt-induced hypertesnive albino rats. Thirty male wister albino rats were assigned randomly into 3 groups (n = 10/ group). Group 1 received no treatement and serves as control. Group 2 received 0.5% carboxymethylcellulose sodium ip as a solvent of aliskiren, as a direct renin inhibitor (DRI). Group 3 received aliskiren 100 mg/kg/day ip for 4 weeks through gastric tube. Systolic blood pressure (SBP) was measured every week and its mean was recorded at the end of the study. Superoxide dismutase (SOD) enzyme in RBCs lysates, activities of catalase (CAT) and glutathione peroxidase enzymes and thiobarbituric acid reactive substance (TBARS), as a marker of lipid peroxidation, in hepatic homogenates were measured at the end of the study. DRI produced a marked reduction in mean SBP of hypertensive rats. It also significantly (p < 0.05) increased the activities of measured anti-oxidant enzymes while it significantly (p < 0.05) reduced TBARS in liver homogenates. These results indicated that renin possesses an oxidative effect in the liver in hypertensive rats. Aliskiren, in addition to its powerful anti-hypertensive effect, it could induce a great anti-oxidant effect in liver homogenates of DOCA salt-hypertensive rats.
Keywords:Aliskiren, DOCA Hypertensive Rats, Anti-Oxidant Enzymes, TBARS, Liver

The renin angiotensin aldosterone system (RAAS) plays an important role in the regulation of blood pressure and body fluid volume. It stimulates the synthesis of angiotensin II (Ang II) via two main steps: production of angiotensin I from angiotensinogen via cleavage by renin enzyme to convert it to Ang II by angiotensin-converting enzyme (ACE). RAAS has an important role in the development of many pathological states. This role could be related to the increase in blood pressure and aldosterone levels by Ang II and also to its remodeling effects on cardiac, vascular and renal tissue that finally lead to end-organ damage [1] .
It was suggested that there is a strong relationship between reactive oxygen species (ROS) and oxidant stress in the pathogenesis of hypertension. High salt diet and angiotensin II, as hypertensive stimuli, enhance the synthesis of ROS in vital organs as brain, kidney, heart and endothelium of blood vessels. Angiotensin II increases sympathetic outflow while sympathetic nerve terminals in lymph nodes activate T cells and attract them to these organs. T cells stimulate the release of cytokines that lead to vasoconstriction and sodium retention contributing to the development of hypertension [2] .
Many experimental studies were designed to demonstrate the role of ROS, mainly of superoxide anion, in the pathogenesis of hypertension. They found a marked alteration in the cellular signal transduction systems accompanied by an increase in the production of inositol triphosphate and a reduction in the production of cyclic GMP in cultured vascular smooth muscle cells (SMC), thus enhancing the occurrence of vasoconstriction and hypertension. Those effects were very apparent in spontaneously hypertensive rats (SHR) that possess a greater sensitivity of the vascular tissue to the oxidative stress. They also reported an increase in the synthesis of superoxide anion in the aorta of hypertensive rats either SHR or DOCA-salt models during the induction of hypertension. The studies concluded a great chronic treatment with drugs that are characterized by a strong antioxidative therapeutic effect such as alpha lipoic acid or aspirin. These findings also support the major effect of vascular superoxide anion on the pathogenesis of hypertension in such models. It seems that oxidative stress may have a major pathogenic role in the development of hypertension and that the treatment with appropriate antioxidative drugs could provide a protection against the development of hypertension and its complications in experimental models of hypertension [3] .
Aliskiren, as a DRI, is the first orally active renin inhibitor approved for treatment of essential hypertension [4] . There are as yet no enough experimental or clinical data regarding the ability of aliskiren to prevent or decrease the damaging effect of oxidative stress in case of hypertension on vital organs like liver, kidney and heart [5] .
The aim of this study was to determine if aliskiren possesses a possible protective anti-oxidant effect on hepatic homogenates, in addition to its anti-hypertensive effect, in a DOCA-salt induced hypertensive model in rats.
2.1. Materials
Aliskiren (Novartis Pharma. Co.), superoxide dismutase enzyme [RANSOD, by Randox Laboratories]. Deoxycorticosterone acetate (DOCA) (Sigma Chemicals Co. available as powder suspended in corn oil). All other chemicals were obtained from Sigma Chemical Co. Aliskiren’s powder was dissolved in 0.5% carboxymethylcellulose sodium. The solution of the tested drug was freshly prepared throughout the experimental study.
N.B. a pilot study was done to test the solvent (0.5% carboxymethylcellulose sodium) on tested rats to exclude its possible effects on the tested parameters of the present study.
2.1.1. Animal Protocols
Thirty wister albino rats weighing 200 - 250 gm, each were randomized into 3 groups [N = 10, in each group].
DOCA-Salt induced Hypertension [6] Twenty wister albino rats [group 2 & 3] were treated twice weekly with DOCA and administered subcutaneously (15 mg/kg), and 1% NaCl was added to their tap water for drinking. Two weeks after the start of DOCA-salt treatment, these rats were randomly divided into 2 groups (each group, n = 10).
Animal grouping: [duration of the study = 4 weeks].
Group 1 (control non-treated) received no treatment with ip injection of 0.5% carboxymethylcellulose sodium as a solvent of aliskiren.
Group 2 were hypertensive with ip injection of 0.5% carboxymethylcellulose sodium as a solvent of aliskiren. [Rats were already rendered hypertensive as mentioned above].
Group 3 were hypertensive + aliskiren administration ip 30 mg/day for 4 weeks [Rats were already rendered hypertensive as mentioned above].
Dose of aliskiren was used according to Rashikh et al. [7] .
At the end of the study, each rat was anesthetized with urethane [1 g/kg], dissected and the livers were removed for anti-oxidant enzymes and TBARS measurement of their homogenates. Additionally, blood samples were collected from tails of all tested rats of all groups for measurement of SOD levels in erythrocyte lysates using commercially available colorimetric assay kit as described above in material section. We used kits not Western blot or qPCR due to economic reasons for our laboratory. The kits are lesser in cost than these procedures. N.B. We match the results of some samples with that of qPCR, they showed the same levels but this step was done for reassurance and we can not continue as kits are available with lesser cost as I mentioned before.
2.1.2. Ethics
All procedures were in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, as well as the guidelines of the Animal Welfare Act.
2.2. Measurements
2.2.1. Assessment of Systolic Blood Pressure (SBP) Changes: [8]
SBP was measured by a tail-cuff sphygmomanometer (UR-5000, Ueda Co., Ltd., Japan). SBP measurements were conducted before starting treatment with aliskiren then at the end of the study. Measurements were made at 2:00 to 5:00 p.m. (5 to 6 h after treatment administration) to minimize circadian influences. For each animal an average of at least three consecutive measurements was taken to reduce variability.
2.2.2. Determination of SOD Enzyme Level in Erythrocyte Lysates [9]
At the end of the 4th week of the study, blood samples were collected from tested rats of all groups for measurement of SOD levels in erythrocyte lysates, using commercially-available colorimetric assay kits, based on an indirect xanthine-xanthine oxidase method as described by and results were expressed in IU/mL.
2.2.3. Determination of Catalase Enzyme Activity
Catalase (CAT) activity in the liver homogenates was assayed colorimetrically as described by Sinha [10] using dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The intensity was measured at 620 nm and the amount of hydrogen peroxide hydrolyzed was calculated for the catalase activity.
2.2.4. Determination of Glutathione Peroxidase (GPx) Enzyme Activity
Glutathione peroxidase (GPx) activity in the liver homogenates was measured by the method described by Rotruck et al. [11] . Glutathione peroxidase (GPx) was assayed in the liver sample homogenized in 8 volumes of cold buffer (50 mM Tris-HCl, pH 7.5, containing 5 mM EDTA and 1 mM 2-mercaptoethanol), next centrifuged 8500 × g for 10 minutes at 40C. GPx activity was determined in supernatant using BIOXYTECH GPx-340TM Assay kit produced by OXIS International, Inc., USA. The GPx assay was based on the oxidation of NADPH to NADP+, which is accompanied by a decrease in absorbance at 340 nm. The rate of this decrease is directly proportional to the GPx activity in the sample.
2.2.5. Hepatic Lipid Peroxidation Assay as Thiobarbituric Acid-Reactive Substance (TBARS)
Hepatic lipid peroxidation was quantified by measuring thiobarbituric acid-reactive substance (TBARS) according to Fraga et al. [12] . Liver tissue of each rabbit was homogenized in 9 volumes of 50 mmol/L Tris-HCl buffer (pH 7.4) containing 180 mmol/L KC1, 10 mmol/L EDTA, and 0.02% butylated hydroxytoluene. To 0.2 ml of the tissue homogenate, 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid, 1.5 mL of 0.9% thiobarbituric acid, and 0.6 ml of distilled water were added and vortexed. The reaction mixture was placed in a water bath at 95˚C for 1 hour. After cooling on ice, 1.0 ml of distilled water and 5.0 mL of butanol/pyridine mixture (15:1, v/v) were added and vortexed. After centrifugation at 10,000 × g for 10 minutes, absorbance of the resulting precipitate was determined at 532 nm. The TBARS concentration was calculated using 1,1,5,5-tetraethoxypropane as standard.
2.2.6. Protein Determination
The protein content of liver homogenates was determined by spectrophotometer according to the method of Bradford [13] . The aim is to relate TBARS concentrations as nmol/mg tissue protein, CAT and GPx enzyme activities as unit/mg tissue protein.
2.3. Statistical Analysis
Results are expressed as mean ± SD [Standard Deviation]. Statistical analysis was performed by analysis of variance followed by Tukey’s post hoc using GraphPad Prism version 3.00 for Windows 97 (Graph Pad Software, San Diego, CA, USA). Differences with p < 0.05 were considered to be statistically significant.
3.1. Effect of 4 Weeks Administration of Aliskiren on SBP of Tested Rats
Figure 1: SBP was significantly (P < 0.05) lowered by aliskiren (group 3) compared to non-treated DOCA-salt hypertensive rats (group 2). Results were comparable to that reported with normal control rats (group 1) (Figure 1).
The mean ± SD of SBP for each group remained constant all over the 8 hours period of measurement of SBP.
Figure 2: Measurement of SOD enzyme levels (IU/mL) in erythrocyte lysates of all tested rats.
Table 1: Changes in the activity of liver catalase (CAT) and glutathione peroxidase (GPx) anti-oxidant enzymes.
Figure 3: Effect of 4 weeks administration of aliskiren on thiobarbituric acid-reactive substance (TBARS) in nmol/mg tissue protein of the liver tissue homogenates of the tested rats.
3.2. Recaptulation of the Results of the Present Study
Intraperitoneal [ip] daily dose administration of aliskiren for 4 weeks, as a DRI acting on RAAS which is an
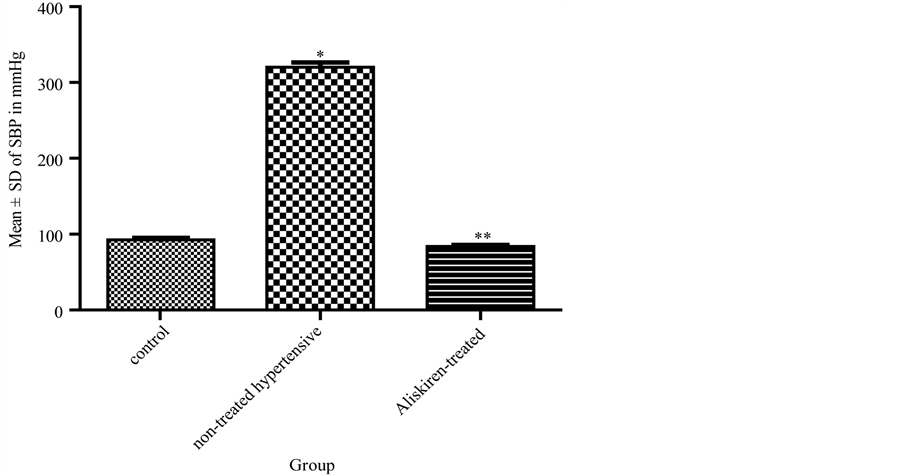
Figure 1. Effect of aliskiren on systolic blood pressure (SBP) in DOCAsalt hypertensive rats. *significant (p < 0.05) increase in SBP in tested rats without aliskiren [group 2] compared to group 1. **significant (p < 0.05) decrease in SBP in aliskiren-treated group (3) compared to group 2.
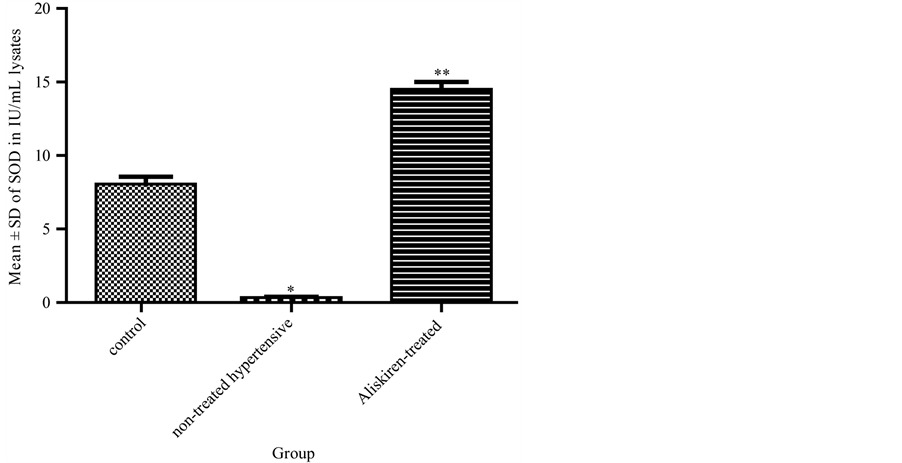
Figure 2. Effect of 4 weeks administration of aliskiren on levels of superoxide dismutase [SOD] enzyme in IU/mL in erythrocyte lysates of the tested rats. Results are expressed as mean ± SD (n = 10 rats/group). *p < 0.05 = significant decrease in SOD enzyme levels in group 2 compared to the control group 1. **p < 0.05 = significant increase in SOD enzyme levels in aliskiren-treated group 3 compared to the non-treated hypertensive rats group 2.
Table 1. Shows changes in the activities of anti-oxidant enzymes CAT, GPx enzymes in liver of tested rats. There is a significant (p < 0.05) reduction in their activities in non-treated hypertensive group 2 compared to control group 1. However, aliskiren-treated group shows significant (p < 0.05) increase in their activities.
Notes: a = Moles of hydrogen peroxide consumed per minute; b = μg of glutathione; consumed per minute. *p < 0.05, significant reduction in activity of both enzymes versus group 1. **p < 0.05, significant increase in activity of both enzymes versus group 2.
important system in regulating blood pressure results in: a significant (p < 0.05) reduction of SBP, an increase in levels of SOD enzyme in erythrocyte lysates, augmentation in the activity of CAT and GPx anti-oxidant enzymes and a reduction in TBARS, as a marker of lipid peroxidation, in liver homogenates of hypertensive rats.
The present study was conducted to determine a possible antioxidant effect of aliskiren, as a DRI, on liver homogenates in DOCA-salt induced hypertensive rats. The results revealed that ip daily dose for 4 weeks of the tested drug significantly reduced SBP, increased levels of SOD enzyme in erythrocyte lysates, augmented the activity of CAT and GPx anti-oxidant enzymes and reduced TBARS, as a marker of lipid peroxidation, in liver homogenates of hypertensive rats.
These results are in agree with that found by Rashikh [7] whose study was planned to test the possible protective effects of aliskiren in 100 mg/kg/day for 42 days in a model of cardiomyopathy in rats. Their results revealed that the drug significantly preserved the antioxidant defense and increased some anti-oxidant markers like glutathione (GSH) and superoxide dismutase (SOD). Furthermore, they performed electron microscopic studies that showed prevention of apoptosis in myocardium in cardiomyopathic rats by this drug treatment. They concluded that aliskiren protected rats from cardiomyopathy that was induced by oxidative damaging effect of
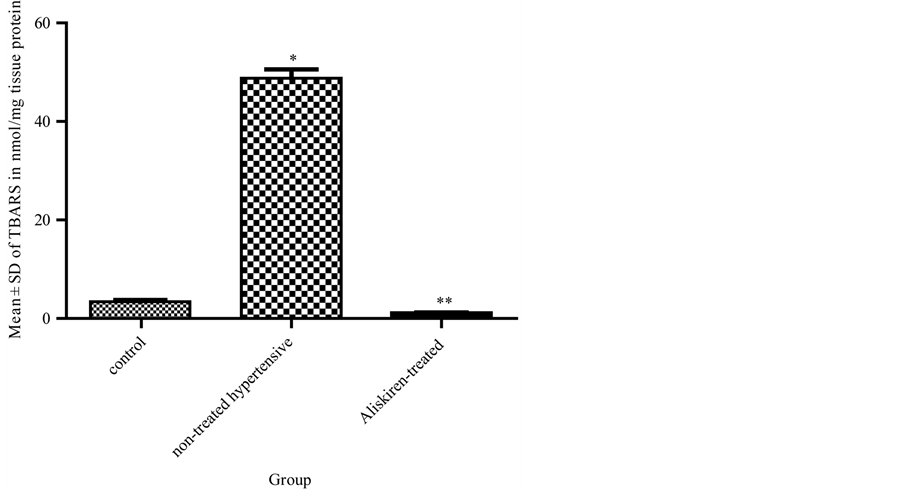
Figure 3. Effect of 4 weeks administration of aliskiren on thiobarbituric acid-reactive substance (TBARS) in nmol/mg tissue protein of the liver tissue homogenates of the Tested rats. Results are expressed as mean ± SD (n = 10 rats/group). A significant (*p < 0.05) increase in TBARS levels in comparison to the control group 1. A significant (**p < 0.05) decrease in TBARS levels in aliskirentreated group 3 compared to the non-treated hypertensive rats group 2.
doxorubicin (DXR).
Aliskiren also possesses a strong organoprotective ability beyond its antihypertensive properties. This fact was proved by a study that investigated its strong nephroprotective effects in a model of progressive renal fibrosis [14] .
In a rat’s model of non-alcoholic steatohepatitis (NASH), aliskiren induced a marked reduction of hepatic fibrosis by inhibition of oxidative damaging effects of many mediators e.g. ANG II. The authors concluded that DRI could be used in clinical practice safely in the future against the progression of NASH [15] .
Hypertension and oxidative stress:
It is well known that any enhancement of oxidative stress would result in a marked increase in the development of cardiovascular diseases and their complications like hypertension and hypertensive emergencies [16] [17] . Lipid peroxidation was measured in a clinical study [18] as an important marker to evaluate lipid status. This study reported a significant increase in malondialdhyde (MDA), as an end product of polyunsaturated fatty acid oxygenation. This result provides an indirect evidence of an increase in the level of oxygen free radicals. A similar finding was reported by other study [19] . Hypertension is always associated with hypercholesterolemia and an apparent increase in the levels of reactive oxygen species (ROS) [20] . Plasma levels of tumor necrosis factor-α (TNF-α) and ROS is elevated during synthesis of inflammatory mediators that mediate all damaging effects of oxidative stress [21] . However, it was found that aliskiren has an increasing effect on GSH in hypercholesterolemic rabbit with a significant improvement in oxidation status in hypercholesterolimic rabbit [22] .
In conclusion, the result of this study revealed that aliskiren significantly increased activities of some anti-oxidant enzymes with a marked reduction of TBARS, as a marker of lipid peroxidation. These findings would point to the hepatoprotective effect of aliskiren by its anti-oxidant effect, in addition to its powerful anti-hypertensive effect, in DOCA salt-induced hypertensive rats.
The author reports no conflicts of interest in this work.
This research was officially supported by the Medical Research Service of the Ain Shams University. It was financially supported by the laboratory of the Pharmacology Department, Faculty of Medicine, Ain Shams University.
- Fisher, N.D. and Hollenberg, N.K. (2001) Is There a Future for Renin Inhibitors? Expert Opinion on Investigational Drugs, 10, 417-426. http://dx.doi.org/10.1517/13543784.10.3.417
- Harrison D.G. and Gongora, M.C. (2009) Oxidative Stress and Hypertension. Medical Clinics of North America, 93, 621-635. http://dx.doi.org/10.1016/j.mcna.2009.02.015
- De Champlain J., Wu, R., Girouard, H., Karas, M., EL Midaoui, A., Laplante, M.A. and Wu, L. (2004) Oxidative Stress in Hypertension. Clinical and Experimental Hypertension, 26, 593-601. http://dx.doi.org/10.1081/CEH-200031904
- Staessen J.A., Li, Y. and Richart, T. (2006) Oral Renin Inhibitors. Lancet, 368, 1449-1456. http://dx.doi.org/10.1016/S0140-6736(06)69442-7
- Li Y.C. (2007) Inhibition of Renin: An Updated Review of the Development of Renin Inhibitors. Current Opinion in Investigational Drugs, 8, 750-757.
- Matsumura Y., Hashimoto, N., Taira, S., Kuro, T., Kitano, R., Ohkita, M., Opgenorth, J. and Takaoka, M. (1999) Different Contributions of Endothelin-A and Endothelin-B Receptors in the Pathogenesis of Deoxycorticosterone AcetateSalt-Induced Hypertension in Rats. Hypertension, 33, 759-765. http://dx.doi.org/10.1161/01.HYP.33.2.759
- Rashikh A., Ahmad, S.J., Pillai, K.K., Kohli, K. and Najmi, A.K. (2012) Aliskiren Attenuates Myocardial Apoptosis and Oxidative Stress in Chronic Murine Model of Cardiomyopathy. Biomedicine & Pharmacotherapy, 66, 138-143.
- Bunag R. (1973) Validation in Awake Rats of a Tail-Cuff Method for Measuring Systolic Pressure. Journal of Applied Physiology, 34, 279-282.
- Sato R., Goldstein, J. and Brown, M. (1993) Replacement of Serine-871 of Hamster 3-Hydroxy-3-Methylglutaryl-CoA Reductase Prevents Phosphorylation by AMP-Activated Kinase and Blocks Inhibition of Sterol Synthesis Induced by ATP Depletion. Proceedings of the National Academy of Sciences, 90, 9261-9265. http://dx.doi.org/10.1073/pnas.90.20.9261
- Sinha K.A. (1972) Colorimetric Assay of Catalase. Analytical Biochemistry, 47, 389-394. http://dx.doi.org/10.1016/0003-2697(72)90132-7
- Rotruck J., Pope, A., Ganther, H., Swanson, A., Hafeman, D. and Hoekstra, W. (1973) Biochemical Role as a Component of Glutathione Peroxidase. Science, 79, 588-590. http://dx.doi.org/10.1126/science.179.4073.588
- Fraga C., Leibovitz, B. and Tappel, A. (1988) Lipid Peroxidation Measured as Thiobarbituric Acid Reactive Substances in Tissue Slices: Characterization and Comparison with Homogenates and Microsomes. Free Radical Biology & Medicine, 4, 155-161. http://dx.doi.org/10.1016/0891-5849(88)90023-8
- Bradford M. (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry, 72, 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Oliver G., Rainer, G., Diana, R., Johanna, T., Stephanie, T. and Gerhard-Anton, M. (2011) Renal Protective Effects of Aliskiren Beyond Its Antihypertensive Property in a Mouse Model of Progressive Fibrosis. American Journal of Hypertension, 24, 355-361. http://dx.doi.org/10.1038/ajh.2010.231
- Aihara Y., Yoshiji, H., Noguchi, R., Kaji, K., Namisaki, T., Shirai, Y., Douhara, A., Moriya, K., Kawaratani, H. and Fukui, H. (2013) Direct Renin Inhibitor, Aliskiren, Attenuates the Progression of Non-Alcoholic Steatohepatitis in the Rat Model. Hepatology Research, 43, 1241-1250. http://dx.doi.org/10.1111/hepr.12081
- Aso Y. (2008) Cardiovascular Disease in Patients with Diabetic Nephropathy. Current Molecular Medicine, 8, 533- 543. http://dx.doi.org/10.2174/156652408785747960
- Rojas A., Mercadal, E., Figueroa, H. and Morales, M.A. (2008) Advanced Glycation and ROS: A Link between Diabetes and Heart Failure. Current Vascular Pharmacology, 6, 44-51. http://dx.doi.org/10.2174/157016108783331312
- Al-Aubaidy H., Sahib, H., Mohammad, B., Hadi, N. and Abas, S. (2013) Antiatherosclerotic Potential of Aliskiren: Its Antioxidant and Anti-Inflammatory Effects in Rabbits: A Randomized Controlled Trial. Journal of Pharmaceutical Technology and Drug Research.
- Prasanna G. and Purnima, A. (2011) Protective Effect of Leaf Extract of Trichilia Connaroides on Hypercholesterolemia Induced Oxidative Stress. International Journal of Pharmacology, 7, 106-112. http://dx.doi.org/10.3923/ijp.2011.106.112
- Prasad K. and Kalra, J. (1993) Oxygen Free Radicals and Hypercholesterolemic Atherosclerosis: Effect of Vitamin E. American Heart Journal, 125, 958-973. http://dx.doi.org/10.1016/0002-8703(93)90102-F
- Panganamala R.V., Sharma, H.M., Heikkila, R.E., Geer, J.C. and Cornwell, D.G. (1976) Role of Hydroxyl Radical Scavengers Dimethyl Sulfoxide, Alcohols and Methional in the Inhibition of Prostaglandin Biosynthesis. Prostaglandins, 11, 599-607. http://dx.doi.org/10.1016/0090-6980(76)90063-0
- Del Fiorentino A., Cianchetti, S., Celi, A. and Pedrinelli, R. (2010) Aliskiren, a Renin Inhibitor, Downregulates TNFAlpha-Induced Tissue Factor Expression in HUVECS. Journal of the Renin-Angiotensin-Aldosterone System, 11, 243- 247. http://dx.doi.org/10.1177/1470320310379449


