Journal of Minerals and Materials Characterization and Engineering
Vol. 1 No. 6 (2013) , Article ID: 40049 , 6 pages DOI:10.4236/jmmce.2013.16053
Crystallographic Study of Uranium-Thorium Bearing Minerals in Tranomaro, South-East Madagascar
1Institut National des Sciences et Techniques Nucléaires (Madagascar-INSTN), Antananarivo, Madagascar
2Institute of Nuclear Waste and Disposal (INE), Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany
Email: *instn@moov.mg
Copyright © 2013 Frank Elliot Sahoa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 20, 2013; revised October 21, 2013; accepted November 5, 2013
Keywords: Tranomaro; Madagascar; Uranothorianite; Crystallography; XRD
ABSTRACT
Studies are undertaken to characterize the uranium and thorium minerals of south-east Madagascar. Seven selected uranothorianite bearing pyroxenites samples from old abandoned uranium quarries in Tranomaro, south Amboasary, Madagascar (46˚28'00"E, 24˚36'00"S) have been collected. To determine the mineral micro-structure, they were investigated for qualitative identification of crystalline compounds by using X-ray powder diffraction analytical method (XRD). Results showed that the uranium and thorium compounds, as minor elements, were present in various crystalline structures. Thorium, as thorianite, is present in a simple ThO2 cubic crystalline system, whereas the uranium component of the Tranomaro uranothorianite samples is oxide-based and is a mixture of complex oxidation states and crystalline systems. Generally called uraninite, its oxide compounds are present in more than eight phases.
1. Introduction
In 1907, a Malagasy mineralogist, J. B. Rasamoel, discovered euxenite-bearing tertiary lake sediments near Vinaninkarena. French interest in the source of radium led to intensive search for primary uranium minerals in the adjacent upland plateau [1]. The central island is one of the regions of interest of a Manhattan District Project. In 1943-1944, the Kitsamby district, located in the region, was then explored for potential uranium ore deposit by the project specialists [2]. But Moreau [3] has observed uranium-thorium mineralization in Tranomaro, southeast of Madagascar, where major extracting activities were undertaken by the French Commissariat à l’Energie Atomique (CEA) during the fifties and sixties, to support their effort to develop nuclear project.
In 1979, using new analytical technology such as gamma spectrometry system, Raoelina Andriambololona et al. [4] have further investigated the radioactivity characteristics of uranium and thorium minerals from Madagascar, in particular from the South-East region [5-7]. Rabesiranana et al. [8] reported the natural radionuclide content in soil, focusing mainly on the comparison between on-site vs. off-site data, to detect significant differences, taking into account spatial variability. Using electronic microprobe analysis and X-ray fluorescence technique, Rakotondratsima and Moine et al. [9,10] studied the origin and concentration of uranothorianite bearing pyroxenite content in the region, and have demonstrated the formation of uranothorianite bearing pyroxenites with marble at Belafa and Marosohihy and mineralization without marble at Ambidandrakemba, two billion years ago. Rakotondrazafy used XRD technique and microprobe analysis for dating and determining the fomation conditions of hibonite in Madagascar. He has indicated that hibonite mineral has been plainly confined in uranothorianite bearing skarns formed 545 million years ago [11].
Recently, in order to supply worldwide increasing need for alternative energy, and in view of future shortage of conventional fossil fuel, there is a renew interest in nuclear energy. Consequently, mining companies are exploring old and newly discovered uranium-thorium provinces of the country. This is the case for the South East region of Madagascar, where the uranothorianite deposits of the Tranomaro province are re-investigated (Figure 1).
2. Rationale
The uranium and thorium compounds may be mobilized
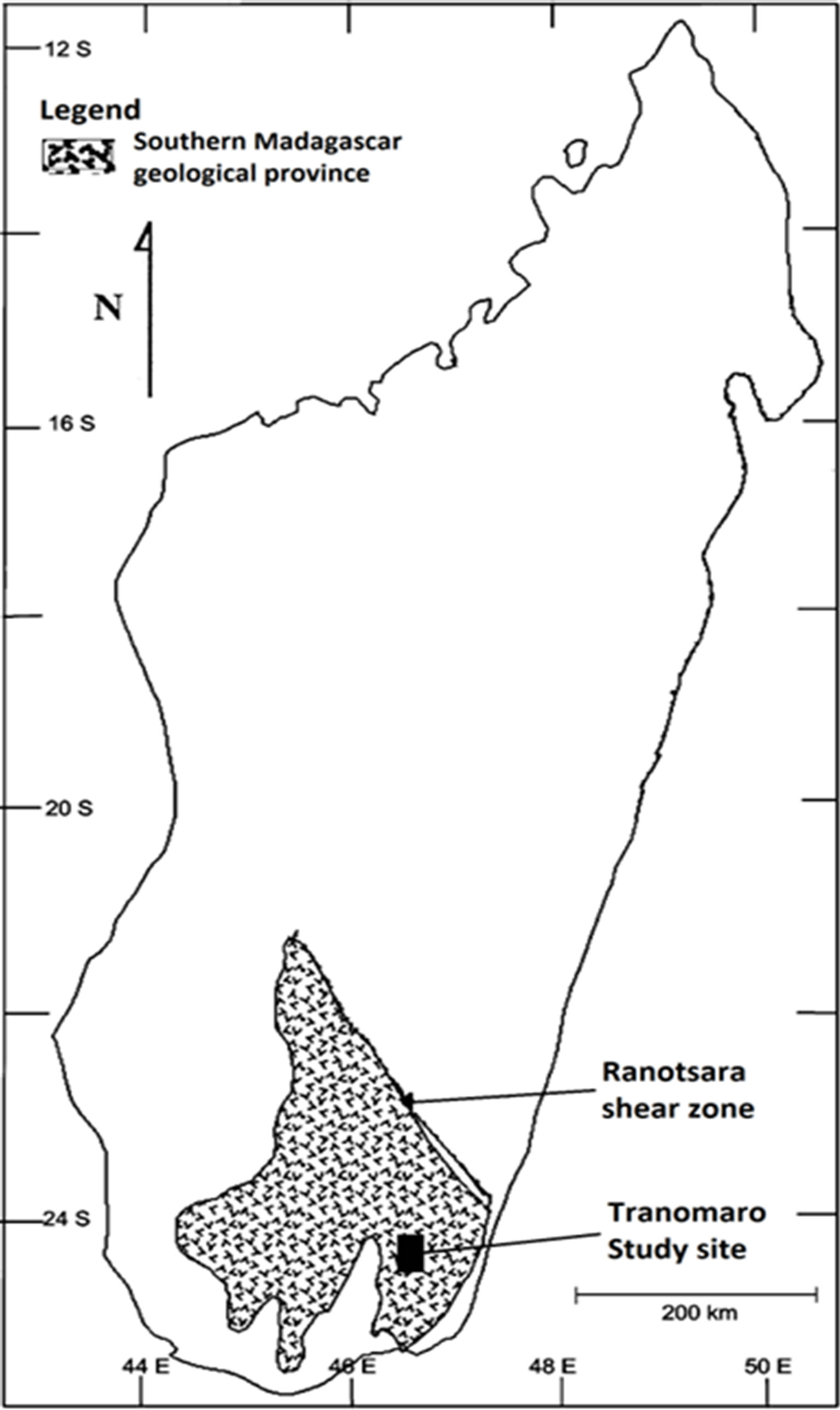
Figure 1. Geographical location of the tranomaro study site, south-east madagascar.
and transported into the environmental compartments, increasing the risk of human contamination and exposure from radioactive elements. If the mobilization is limited for an undisturbed geological formation, human activities such as mining may accelerate the process. The dispersion depends on 1) the weathering/leaching efficiency, 2) the uranium-thorium structure resistance and 3) the disturbance magnitude. Rock weathering is normally instigated by air and water from the earth’s surface gaining access to minerals previously surrounded by other minerals. When air and water reach previously inaccessible parts of the rock matrix, chemical reaction such as oxidation and leaching can change the rock’s mineral composition [12,13].
Uranium and thorium elements occur in the 4+ oxidation state in primary igneous rocks and minerals. As such, both elements are almost chemically immobile in the near surface environment at low temperature. In the oxidized zone of the terrestrial near-surface environment, uranium and thorium may be mobilized, but in different ways. According to Gascoyne [14], thorium is mainly transported in insoluble resistate minerals or is adsorbed on the surface of clay minerals. But uranium can be oxidized to 5+ and 6+ states in the near surface environment. The most probable oxidation reaction at normal environment is [15]:
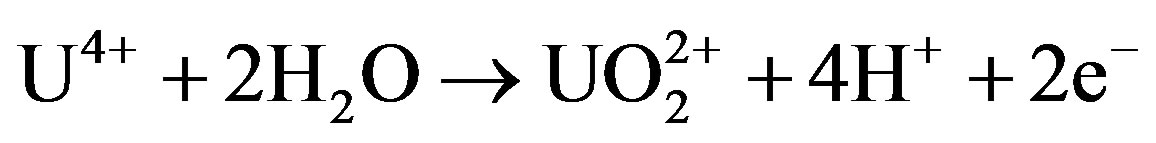
The 6+ oxidation state is the most stable and forms soluble uranyl complex ions  which play an important role in uranium transport during weathering [14]. Finch and Ewing [16] reported that in a highly altered uraninite-bearing ore body of Koongara, Australia, partially exposed to meteoric water, alteration at depth has resulted from interaction with groundwater having a reduced Eh compared to the surface. Uraninite, Pb-uranyl oxide hydrates and uranyl silicates control uranium solubility at depth; uranyl phosphates and uranium adsorption onto clays and FeMn-oxides control uranium solubility near the surface. So, uranium may either move in solution as complex ion, or like thorium, in a sorbed or detrital phase.
which play an important role in uranium transport during weathering [14]. Finch and Ewing [16] reported that in a highly altered uraninite-bearing ore body of Koongara, Australia, partially exposed to meteoric water, alteration at depth has resulted from interaction with groundwater having a reduced Eh compared to the surface. Uraninite, Pb-uranyl oxide hydrates and uranyl silicates control uranium solubility at depth; uranyl phosphates and uranium adsorption onto clays and FeMn-oxides control uranium solubility near the surface. So, uranium may either move in solution as complex ion, or like thorium, in a sorbed or detrital phase.
Most of the studies report mobilization in terms of oxidation state. To further support the risk analysis related to the dispersion of uranium and thorium from solid phases to the environment, crystallographic structure of uranium-thorium compounds in pyroxenite minerals from the Tranomaro uranium-thorium province, southeast Madagascar, is investigated. The sample characterization has been conducted using X-ray powder diffraction analytical technique (XRD).
3. Experimental Methodology
Prior to the study, radiometric measurement within the study area had been done by the Pan African Mining Corporations-Madagascar team. Using Exploranium scintillator counters, high radioactivity spots had been localized. Sampling for the current study was subsequently conducted in 2009, and sampling points were then selected according to counting level previously found. Only samples with count rate greater than 1500 counts per second (cps) have been selected. They were usually from abandoned mining quarries, with details shown in Table 1. Most of the samples are crude rocks, but one of them is a pure uranothorianite mineral carefully picked from a pyroxenite mineral sample.
Mineral sample is ground and sieved in order to get 10 µm to 20 µm size fine and homogeneous powder. For low diffraction background, the powder is packed into a sample holder made of silicon single crystal and smeared uniformly onto a glass slide. When packing into a sample container, care is taken to create a flat upper surface and to achieve a random distribution of the crystalline lattice orientations.
The XRD analysis is carried out using a Bruker D8 Advance diffractometer equipped with a copper anode, powered at (40 kV, 40 mA), and controlled by the XRD Commander software. The sample is illuminated by the Cu-Kα radiation (λ = 1.5406 Å) and the diffracted beam
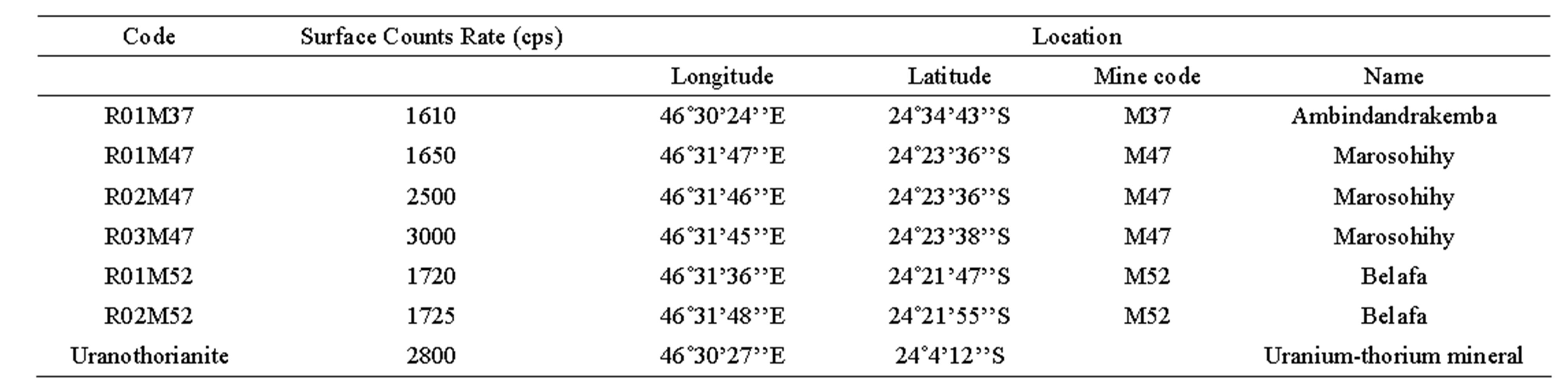
Table 1. Collected mineral samples and their localization.
is detected with a Sol-XE energy dispersive detector. The diffractogram is collected for 2θ varying from 5˚ to 80˚ with steps of 0.01˚ and 2 seconds counting time per step, and the sample spinning at 15 rotations per minute. The diffractogram displaying the diffracted intensity as a function of the diffraction angle is processed using the Bruker Eva software.
Qualitative analysis is carried out by comparison with the JCPDS database (Joint Committee for Powder Diffraction Studies). The more peaks match the data from the database, the higher the confidence in phase identification. For samples containing more than one single phase, the position of the characteristic peaks can overlap. Since each pure crystalline phase is characterized by a specific diffraction pattern, the identification of the phases present in the sample is enhanced by using the Rietweld method. Essentially, it involves fitting the observed diffraction pattern with a synthetic pattern which is a sum of patterns calculated for each phase in the sample [17]. As such this method is known as a full-pattern fitting method [18]. The difference between the synthetic pattern and the observed pattern is minimized by an interactive refinement/optimization procedure. The amounts of the phases present in the sample are obtained from the final value of the refined scale factor for each phase [19].
To improve the accuracy, only compounds which are likely to be found in the studied samples are selected for identification. From previous studies [9,20], Table 2 shows major minerals and uranium-thorium compounds in pyroxenite minerals from the study site. Additionally, all uranium-thorium phase diffraction patterns are added from the JCPDS database for identification.
4. Results and Discussion
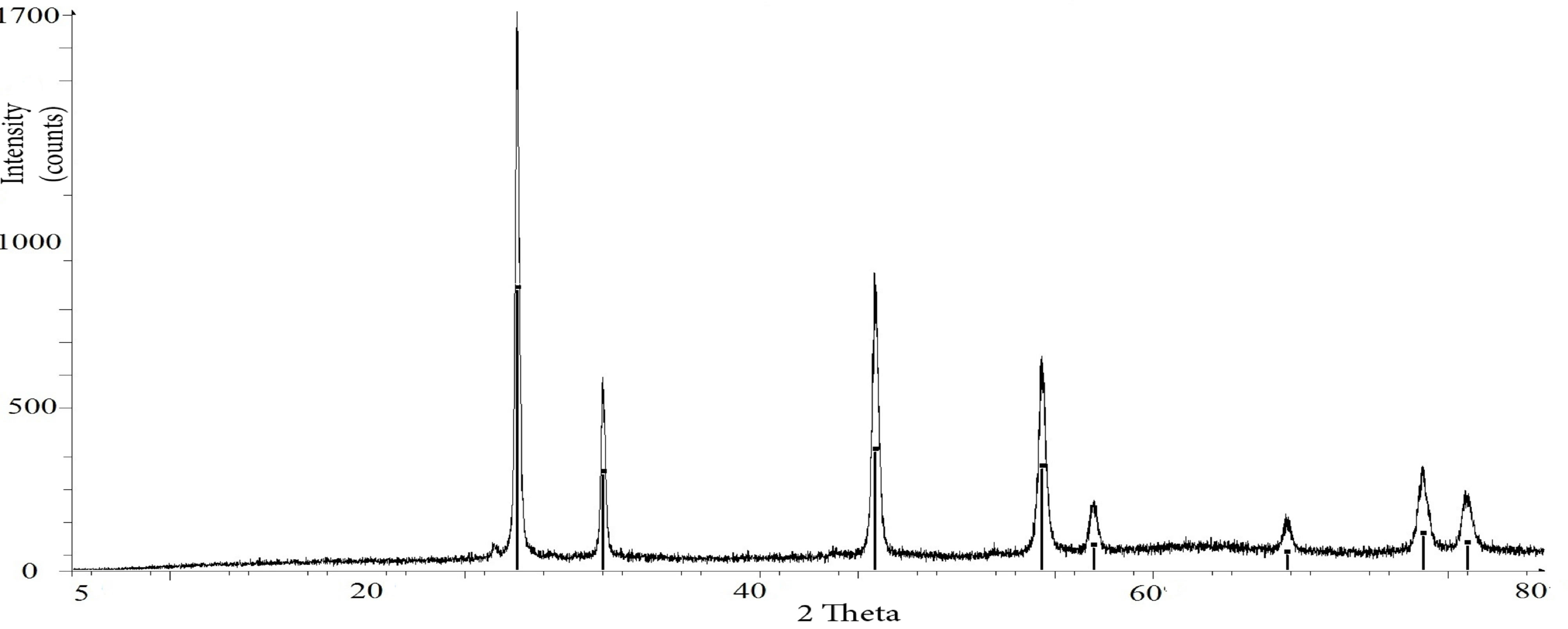
Examples of the graphical output from the Bruker Eva software are shown in Figure 2. Two typical diffractograms are presented: Figure 2(a) shows a simple spectrum from pure uranium-thorium minerals, whereas Figure 2(b) shows a complex spectrum from the R01M37 sample, which is a natural uranium-thorium ore.
A summary of the processed result is presented in Table 3. For natural ores, common major and minor mineral
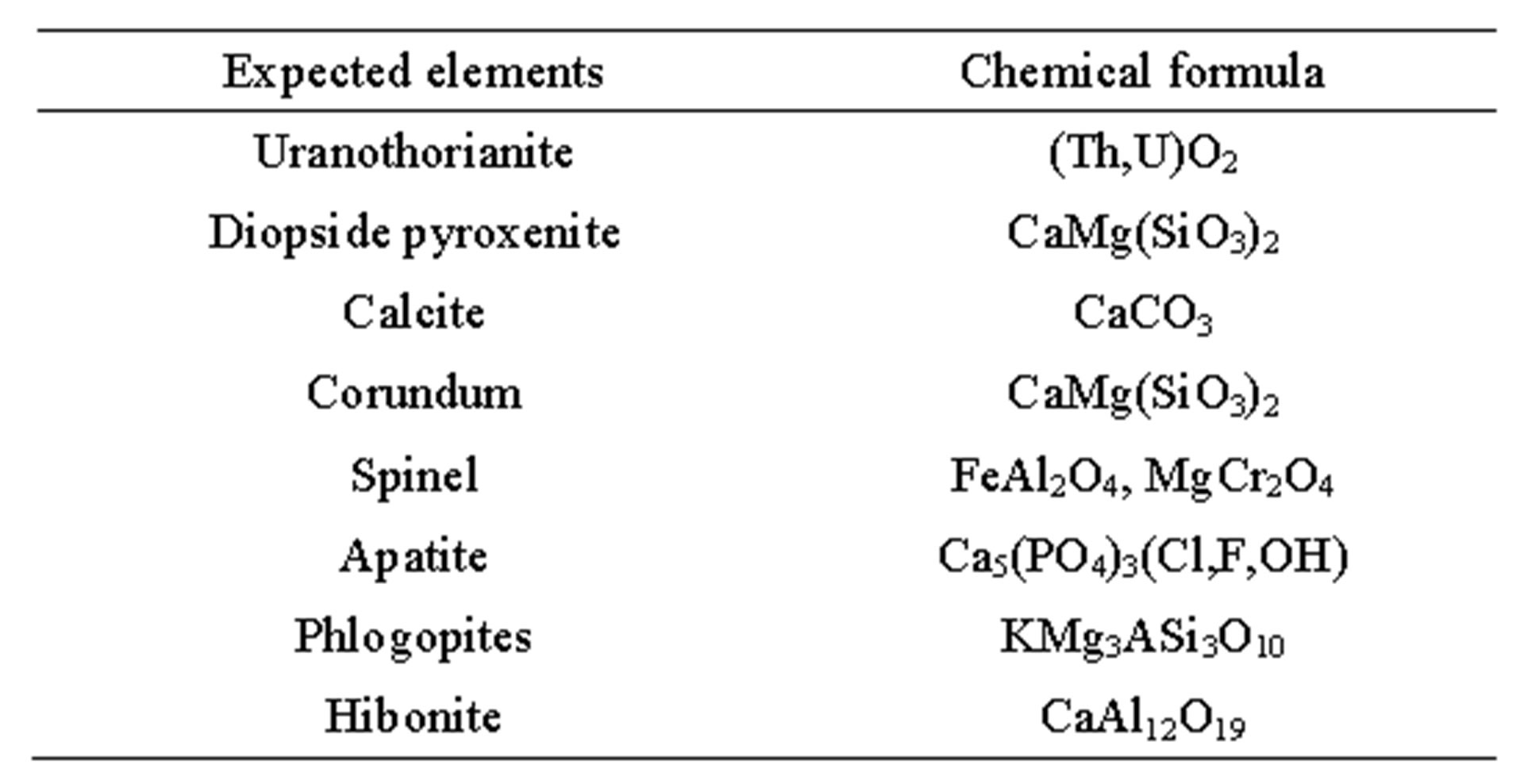
Table 2. Minerals expected to be found in the studied uranium-thorium bearing rocks.
phases such as corundum, spinel, diopside, soddyite, calcium silicate and calcite are usually present in the samples. Interestingly, uranium-thorium compounds are present in multiple crystalline phases and systems.
• The uranothorianite sample contains thorianite as ThO2 in cubic system.
• The R01M37 sample contains 1) Th0.5U0.5O2.04 in cubic system, which can be considered as a mixture of UO2 and ThO2, and 2) Fe2UO6 in hexagonal system, which can be considered as a mixture of Fe2O3 and UO3.
• The R01M47 sample contains only UO3 in monoclinic system.
• The R02M47 sample contains 1) UO3 in monoclinic system and 2) U2O5 in orthorhombic system.
• The R03M47 sample contains 1) UO3 in monoclinic system and 2) U2P2O10 as a complex system, which can be considered as a mixture of U2O5 and P2O5.
• The R01M52 sample contains 1) thorianite as ThO2 in cubic system, 2) uranium oxide as U3O7 in tetragonal system and 3) uraninite as UO2 in orthorhombic system.
• The R02M52 sample contains 1) thorianite as ThO2 in cubic system and 2) uranium oxide as UO3.
The presence of both thorium and uranium oxides is expected. According to Frondel [21], uraninite is commonly present in pegmatites. Moreau explained that previously, thorium and uranium were rather concentrated in the granitic and charnocktic zones. Precambrian granite-
 (a)
(a)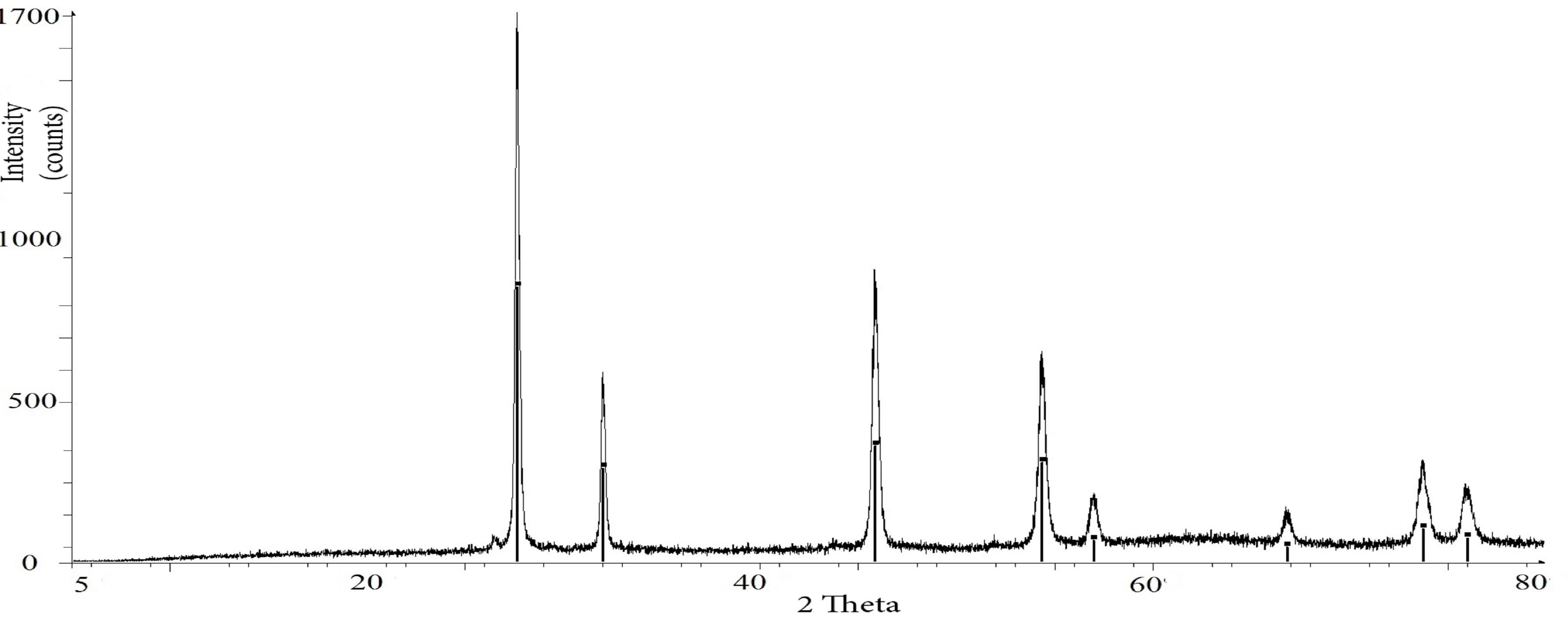 (b)
(b)
Figure 2. Examples of the graphical output from the Bruker Eva software. (a) Pure XRD spectrum from uranothorianite minerals; (b) Complex XRD spectrum from R01M37 sample.
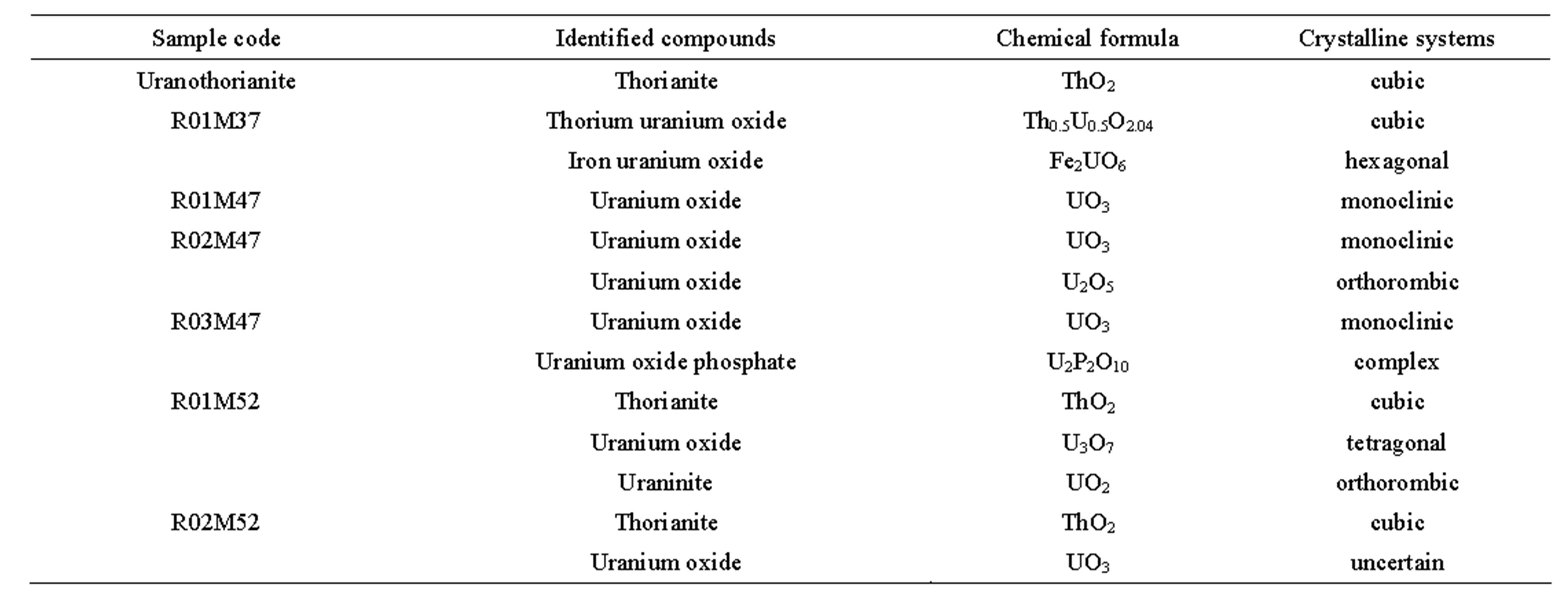
Table 3. Crystalline systems of uranium-thorium minerals identified in the studied samples by XRD analysis.
zation is followed by the formation of the main pegmatitic areas in Madagascar with thorium-uranium niobotantalates, uraninite and beryl. In the same time, a large uranium and thorium provinces with uranothorianite deposits appear within the calcomagnesian series of the southern area of Madagascar [22]. Thorianite is an isometric ThO2 crystal (found in R01M37, R01M52, R02M52 and uranothorianite samples) in the hexoctahedral class
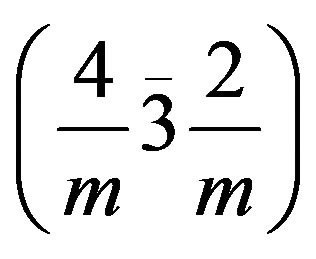
sometimes modified to {111} or {113} [21]. The uranothorianite diffractogram (Figure 2(a)) particularly shows the dominance of ThO2 in uranothorianite sample. This demonstrates that thorianite is the main component of the Tranomaro uranothorianite mineral, and that uranium may be contained in other co-existing phases.
If thorium oxide exists only as cubic system, by contrast, the uranium oxides are present in several and more complex crystalline systems, generally called uraninite. In fact, various definitions of uraninite are proposed by different authors. According to Rogers [23], uraninite is described as the crystalline mineral with higher specific gravity and lower water content and pitchblende for the massive mineraloid with lower specific gravity and higher water content. Following the Dana classification, uraninite is defined as an oxide form of uranium and thorium and has an isometric crystalline structure. According to Wasserstein [24,25] who proposed a classification based on the radiogenic lead content and the redox conditions, α-uraninite corresponds to UO2 (found in R01M37 and R01M52 samples), β-uraninite to U3O7 (found in R01M52 sample) and τ-uraninite to U4O9. It is also known that UO2 is transformed into U3O8 and UO3 (found in R01M37, R01M47, R02M47, R03M47 and R02M52 samples) under oxidizing conditions. Ebelmen reported also that U2O5 (found in R02M47 and R03M47 samples) is an essential constituent of uraninite [26].
In the Tranomaro area, the presence of the uraninite is closely linked to that of the thorianite. This is because uranium and thorium are isometric and both present in minerals at different concentrations (88.15% of uranium in uraninite and 70% of thorium in thorianite). Some authors have proposed a solid solution model to explain the possibility of uranium-thorium exchange between the uraninite and thorianite systems, but their oxidation behaviours differ from each another [27-29]. On one hand, this results in a simple crystal system for thorianite: it only exists in a cubic system. On the other hand, the uranium component of the Tranomaro uranothorianite samples are oxide based and is a mixture of complex oxidations states and crystalline systems. The finding is in agreement with published results, showing that uraninite is a complex uranium oxide compounds [24,25,27].
5. Conclusion
Due to the presence of radioactive minerals, populations living in the Tranomaro area, south-east Madagascar may be exposed to increased environmental radiation. The study of seven mineral samples collected from the site, using X-ray diffraction, confirms the existence of uranium-thorium bearing minerals in the Tranomaro deposit, and moreover, gives an insight on the crystalline structure and combination of the uranium-thorium minerals. The uranium and thorium are present in a complex multiple mineral phases. If the thorium oxide is identified in a simple crystalline system, on the opposite, the uranium oxides, classified as uraninite, are present in more than eight phases and show multiple oxidation states and crystalline systems, including α-uraninites and β-uraninite. As each crystalline system is more or less subject to dissolution or dispersion in the environment, further investigation must be done to investigate the details of their mobilization and transport in the environment, in terms of crystallographic phases.
6. Acknowledgements
This paper was completed within the framework of a cooperation between the Institut National des Sciences et Techniques Nucléaires (Madagascar-INSTN) and the Institute of Nuclear Waste and Disposal (INE). The German Academic Exchange Service (Deutscher Akademischer Austausch Dienst: DAAD) financed a fellowship. The Pan African Mining-Madagascar (PAM) facilitated the access to the Tranomaro sites. Technical supports from Madagascar-INSTN and INE are gratefully acknowledged.
REFERENCES
- C. Grossman, “Uranium Ores in Madagascar,” Journal of the Franklin Institute, Vol. 179, No. 5, 1915, p. 595.
- W. Bourret, “Uranium-Bearing Pegmatites of the Antsirabe-Kitsamby District, Madagascar,” In: J. W. Gabelman Ed., Unconventional Uranium Deposits, Ore Geology Reviews, Vol. 3, 1988, pp. 177-191.
- M. Moreau, “Cycles Uranifères et Thorifères à Madagascar, ” Rapport C.E.A, n° 1685, Commissariat à l’Energie Atomique, 1960.
- Raoelina Andriambololona, G. Rakotoson and G. Paic, “Measurement of the Escape Rate of Radon in Uranium Minerals from Madagascar,” International Journal of Applied Radiation and Isotopes, Vol. 34, No. 7, 1983, pp. 1017-1018. http://dx.doi.org/10.1016/0020-708X(83)90084-4
- G. Paic, Raoelina Andriambololona and G. Rakotoson, “Estimation du Pouvoir d’Échappement du Rn-222 des Roches Uranifères de Madagascar à l’aide d’un Détecteur Ge(Li),” Annales de l’Université de Madagascar, Série Sciences de la Nature et Mathématique, No. 16, 1979, pp. 79-91.
- G. Paic, Raoelina Andriambololona and M. Razanajatovo, “Détermination du Spectre Énergétique Gamma de la Monazite de Fort-Dauphin à l’aide d’un Détecteur Ge(Li),” Annales de l’Université de Madagascar, Série Sciences de la Nature et Mathématique, No. 16, 1979, pp. 79-91.
- Raoelina Andriambololona, “Etude de la Monazite Brute d’Antete et de Vohibarika Manantenina. Mesure des Teneurs en Uranium et Thorium,” Annales de l’Univesité de Madagascar, Sciences de la Nature et Mathématique, No. 17, 1980, pp. 15-32.
- N. Rabesiranana, M. Rasolonirina, F. Terina, A. F. Solonjara and Raoelina Andriambololona, “Top Soil Radioactivity Assessment in a High Natural Radiation Background Area: The Case of Vinaninkarena, AntsirabeMadagascar,” Applied Radiations and Isotopes, Vol. 66, No. 11, 2008, pp. 1619-1622. http://dx.doi.org/10.1016/j.apradiso.2007.12.012
- C. Rakotondratsima, “Les Pyrexonites à Uranothorianite du Sud-Est de Madagascar,” Ph.D Thesis, University of Claude Bernard-Lyon, 1983.
- B. Moine, C. Rakotondratsima and M. Cuney, “Les Pyroxénites à Urano-Thorianite du Sud-Est de Madagascar: Conditions Physico-Chimiques de la Métasomatose,” Bulletin de Minéralogie, Vol. 108, 1985, pp. 325-340.
- A. F. M. Rakotondrazafy, “La Hibonite du Sud-Est de Madagascar,” Ph.D Thesis, University of Antananarivo, 1995.
- J. K. Osmond and M. Ivanovich, “Uranium-Series Mobilization and Surface Hydrology,” In: M. Ivanovich and R. S. Harmon, Eds., Uranium Series Disequilibrium: Applications to Earth, Marine and Environmental Sciences, 2nd Edition, Clarendon Press, Oxford, 1992, pp. 259-289.
- J. N. Rosholt, “Mobilization and Weathering,” In: M. Ivanovich and R. S. Harmon, Eds., Uranium Series Disequilibrium: Applications to Environmental Problems, Clarendon Press, Oxford, 1982, pp. 167-180.
- M. Gascoyne, “Geochemistry of the Actinides and Their Daughters,” In: M. Ivanovich and R. S. Harmon, Eds., Uranium Series Disequilibrium: Applications to Earth, Marine and Environmental Sciences, 2nd Edition, Clarendon Press, Oxford, 1992, pp. 34-61.
- D. Langmuir, “Uranium Solution-Minerals Equilibria at Low Temperatures with Application to Sedimentary Ore Deposits,” Geochimica Cosmochimica Acta, Vol. 42, No. 6, 1978, pp. 547-569. http://dx.doi.org/10.1016/0016-7037(78)90001-7
- R. Finch and R. Ewing, “Uraninite Alteration in an Oxydizing Environment and Its Relevance to the Disposal of Spent Nuclear Fuel,” Department of Geology, University of New Mexico, 1990.
- H. M. Rietveld, “A Profile Refinement Method for Nuclear and Magnetic Structures,” Journal of Applied Crystallography, Vol. 2, 1969, pp. 65-71. http://dx.doi.org/10.1107/S0021889869006558
- R.L. Snyder and D. L. Bish, “Quantitative Analysis,” In: D. L. Bish and J. E. Post, Eds., Modern Powder Diffraction. Reviews in Mineralogy, Vol. 20, Mineralogical Society of America, Washington DC, 1989, pp. 101-144.
- L. B. Mc. Cusker, R. B. Von Dreele, D. E. Cox and P. Louër Scardi, “Rietveld Refinement Guidelines,” Journal of Applied Crystallography, Vol. 32, 1999, pp. 36-50. http://dx.doi.org/10.1107/S0021889898009856
- M. A. F. Rakotondrazafy, B. Moine and M. Cuney, “Mode of Formation of Hibonite (CaAl12O19) within the U-Th Skarns from the Granulites of S-E Madagascar,” Contributions to Mineralogy and Petrology, Vol. 123, No. 2, 1996, pp. 190-201. http://dx.doi.org/10.1007/s004100050150
- C. Frondel, “Systematic Mineralogy of Uranium and Thorium,” US Geological Survey Bulletin, Vol. 1064, 1958, 400 p.
- M. Moreau, “Le Gisement des Minéralisations de Thorianite à Madagascar,” Annales de Géologie de Madagascar, Fasc. 33, 1963, pp. 175-178.
- A. F. Rogers, “Uraninite and Pitchblende,” Proceedings of the 27th Annual Meeting of the Mineralogical Society of America, Stanford University, Chicago, 26-28 December 1946, pp. 90-91.
- B. Wasserstein, “Age of Uraninite by a New Method,” Nature, Vol. 174, No. 4439, 1954, pp. 1004-1005. http://dx.doi.org/10.1038/1741004b0
- B. Wasserstein, “Cube-Edges of Uraninite as a Criterion of Age,” Nature, Vol. 168, No. 4270 1951, p. 380. http://dx.doi.org/10.1038/168380a0
- J. J. Ebelman, “Note on Chemical Composition of Pitchblende,” Journal für Praktische Chemie, Vol. 30, 1943, p. 414.
- K. B. Alberman and J. S. Anderson, “The Oxides of Uranium,” Journal of the Chemical Society, 1949, pp. S303- S311. http://dx.doi.org/10.1039/jr949000s303
- W. Biltz and H. Muller, “Systematic-Affinity Principle; XLI, Uranium Oxides,” Zeitschrift fuer Anorganisch und Allgemeine Chemie, Vol. 163, No. 1, 1927, pp. 257-296. http://dx.doi.org/10.1002/zaac.19271630126
- V. M. Goldschmidt and L. Thomassen, “Die Kristallstruktur Natürlicher und Synthetischer Oxyde von Uran,” Thorium und Cerium: Norske Vidensk, Kristiana Skr Math-Naturvidensk. Kl, No. 2, 1923, pp. 1-48.
NOTES
*Corresponding author.

