Journal of Cosmetics, Dermatological Sciences and Applications
Vol.1 No.2(2011), Article ID:5312,5 pages DOI:10.4236/jcdsa.2011.12004
Vitamin B12 Activates the Wnt-Pathway in Human Hair Follicle Cells by Induction of β-Catenin and Inhibition of Glycogensynthase Kinase-3 Transcription
![]()
Moser Medical Group, Clinic Vienna, Vienna, Austria.
Email: walter.krugluger@aon.at
Received April 19th, 2011; revised May 20th, 2011; accepted May 30th, 2011.
Keywords: Hair Follicle, Growth Factors, Cell Signaling
ABSTRACT
Background and Objectives: Micrograft transplantation is accompanied by a transient induction of telogen in transplanted hair follicles (HF), which might be avoided by supporting the metabolic pathways of the micrograft during the ex vivo period. Vitamin B12 (cobalamin) has been suggested to influence HF growth and cycling in humans, but the mechanisms are unclear. Method: HFs were obtained from patients undergoing routine micrograft transplantation and were cultured for 5 days in Dulbecco’s modified Eagles Medium, supplemented with different amounts of vitamin B12. Hair shaft elongation (HSE) of the isolated HFs as well as quantitative changes of mRNA for b-catenin, glykogensynthase kinase-3 (GSK-3) and TCF/Lef-1 in HF cells were determined. Results: In vitro HSE demonstrated a dose dependent induction of HSE after stimulation with 2.5 mg/ml and 25 mg/ml vitamin B12 (6.2 ± 2.1% and 15.4 ± 3.8% respectively). A dose dependent induction of b-catenin-mRNA could be demonstrated in cultured HFs after stimulation with 2.5 mg/ml and 25 mg/ml vitamin B12 (fold change compared to DMEM: 9.5 ± 2.7, p < 0.05 and 23.1 ± 7.4, p < 0.01; respectively). Concomitantly the amounts of GSK-3 were significantly reduced after stimulation with 25 mg/ml vitamin B12 (fold change compared to DMEM: 0.76 ± 0.12, p < 0.05). Conclusions: Our data demonstrate a hair growth promoting effect of vitamin B12 in vitro. This effect is accompanied by the modulation of intracellular signal transduction molecules of the wnt-pathway and might promote hair growth after micrograft transplantation.
1. Introduction
During hair restoration surgery, transplanted hair follicles (HF) undergo cycling after transplantation, resulting in a period of reduced hair growth immediately after transplantation. Recent progress in studying the biology of isolated micrografts during hair restoration procedures identified key factors for high viability of the micrografts, which has opened new ways of treating the isolated micrografts for better grafting efficiency. However, the posttransplantational effluvium is not completely prevented by improved storage conditions and other factors has to be taken into account.
Vitamin B12 (cobalamin) is an essential cofactor for two enzymes: methionin synthase (MS) and methylmalonylCoA mutase. MS provides methionine for protein synthesis, but also interacts with the folate cyclewhich provides methyl groups for the conversion of dUMP to dTMP during nucleotide synthesis and therefore influences DNA replication. Both actions of vitamin B12 are essential for cell proliferation [1,2]. Deficiency of vitamin B12 is therefore associated with severe changes and abnormalities in rapidly dividing tissues [3]. The mechanisms involved in vitamin B12 associated diseases are not clearly understood in many of the pathologic conditions, but rely on disturbed DNA synthesis in megaloblastic anemia [2,4] and/or on altered methylation of genomic DNA and intracellular proteins in demyelinating diseases [4].
The association of vitamin B12 with diseases of the skin and its appendages is much less characterized. It has been shown, that vitamin B12 deficiency is accompanied by hyperpigmentation of skin [5] and hair [6,7]. This effect of vitamin B12 seems to be due to altered melatonin production. It has been demonstrated in the agouti mouse model, that dietary supplementation favor the production of eumelanin and decrease the production of pheomelanin [7]. In addition, inhibition of hair growth was repeatedly reported, but experimental data are rare. Most recently, it has been demonstrated that topical administration of cyanocobalamin suppressed the effect of the potassium channel inhibitor tolbutamide and induced anagen phase in hair follicles in a mouse model [8].
The wnt-pathway controls both morphogenesis and cycling of different compartments of the hair follicle [9]. It has been shown, that forced expression of Wnt-5a in epithelial wounds induced formation of rudimentary hair follicles and sebaceous gland in an b-catenin dependent manner [10]. The action of Wnt signaling in hair follicle development seems further is closely associated to Notch signaling, and stimulation of Notch signaling is induced by b-catenin dependent Jag1 transcription [11]. Wnt/ b-catenin signaling serves also as a crucial proximal signal for the telogen-anagen transition [9,12]. Chronic activation of b-catenin in telogen hair follicles resulted in changes consistent with induction of an exaggerated, aberrant growth phase (anagen), and transient activation of b-catenin produced normal anagen hairs [12].
In this study, we investigated the postulated growth promoting effect of vitamin B12 in an in vitro hair shaft elongation model. In addition, the influence of vitamin B12 stimulation of hair follicles on transcription of molecules of the wnt-pathway was investigated.
2. Materials and Methods
Sample preparation:
Human hair follicles (HF) were prepared by standard surgical techniques used in hair restoration surgery. Micrografts were taken from the occipital region of patients undergoing hair restoration surgery and had given informed consent for the usage of micrografts for this in vitro study.
Stimulation of HFs:
To evaluate the specific effect of vitamin B12 on hair shaft elongation (HSE) and gene expression, 5 HFs were cultured for 5 days in 1.5 ml of Dulbecco’s modified Eagle’s medium (Sigma-Aldrich Co, StLouis, MO), supplemented with 10% fetal bovine serum (Sigma) and antibiotics (Sigma).
Stimulation with vitamin B12 (Sigma) was performed at concentrations of 2.5 mg/ml and 25 mg/ml vitamin B12.
Evaluation of in vitro hair shaft elongation (HSE):
To evaluate the effect of vitamin B12 on in vitro HSE, hair shaft length was measured at day 0 and day 5 of culture using an invert microscope. Data are expressed as percent change compared to HFs cultured in DMEM.
Real time RT—polymerase chain reaction:
After 5 days of HF culture, mRNA was prepared using the Quiagen RNeasy kit (Quiagen, Hilden, Germany), followed by reversed transcription into cDNA with oligo-dT primers (Clontech, Palo Alto, CA). 5 µl of the cDNA was amplified with specific primer for GADPH, b-catenin, glykogensynthase kinase-3 (GSK-3) or Lef1/TCF (Table 1). PCR reactions containing SYBRgreen were amplified on a Corbett Real Time PCR machine (Rotor gene 2000, Corbett research). For each sample, Ct (cycle threshold)-values obtained for each target gene was normalized to the internal control. Standard curves were constructed from standard reactions for each target gene and internal control by plotting Ctvalues vs. log cDNA dilution. Because the amplification efficiencies of target genes and internal control were equal, the relative change of target gene expression compared to the unstimulated control could be calculated using the equation 2-DDCt, where DDCt = DCt(stimulated) − DCt(control). The DCt values were determined by subtracting the average GADPH Ct-value from the average target gene Ct-value.
After each real time RT PCR, a melting profile as well as agarose gel electrophoresis of each sample was performed to rule out non-specific PCR products and primer dimers.
Statistics:
Data are expressed as mean ± SD of at least five independent experiments. Data were analyzed using standard statistical software and tests.
3. Results
HSE of vitamin B12 stimulated HFs:
To evaluate the influence of vitamin B12 on hair shaft
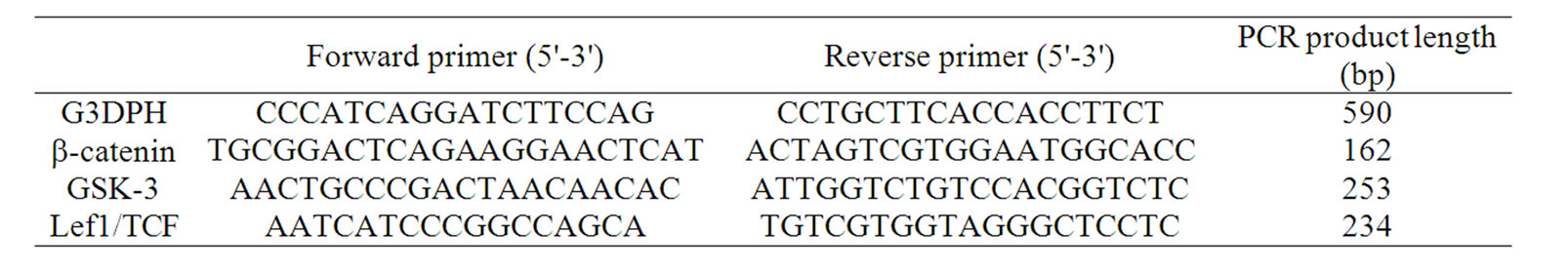
Table 1. Primer pairs used for real time RT-PCR.
growth in vitro, HF were cultured in DMEM and vitro hair shaft elongation HSE was measured on day 0 and day 5. In these experiments HSE reveals increased elongation in HFs containing vitamin B12 as compared to HFs cultured in DMEM (Figure 1). Supplementation of DMEM with 2.5 mg/ml vitamin B12 showed a significant increase of HSE (6.2% ± 2.1%, p < 0.05). Furthermore, a dose dependent increase could be observed, reaching a maximum at 25 mg/ml vitamin B12 (15.4% ± 3.8%, p < 0.01). No signs of induction of catagen (as determined by thinning of the hair shaft at the dermal papilla/hair shaft border) were observed in any of the cultures.
Modulation of b-catenin, GSK-3 and Lef1/TCF by vitamin B12:
Analysis of mRNA transcription in cultured HFs by real time-RT-PCR revealed a dose dependent induction of b-catenin transcription after vitamin B12 stimulation. We found, that addition of 2.5 mg/ml vitamin B12 lead to a significant induction of b-catenin transcription (9.5 ± 2.7fold; p < 0.05; Figure 2) as compared to HFs cultured in DMEM only. Higher doses of vitamin B12 further enhanced the transcription of b-catenin. showing a 23.1 ± 7.4 fold increase at 25 mg/ml vitamin B12 (compared to DMEM; p < 0.01; Figure 2).
Transcription of GSK-3 showed a slight but significant decrease after stimulation with 25 mg/ml of vitamin B12 (fold change compared to DMEM: 0.96 ± 0.06, p = n.s. and 0.76 ± 0.12, p < 0.05; respectively; Figure 2). With lower concentrations, no significant changes in GSK-3 transcription could be observed.
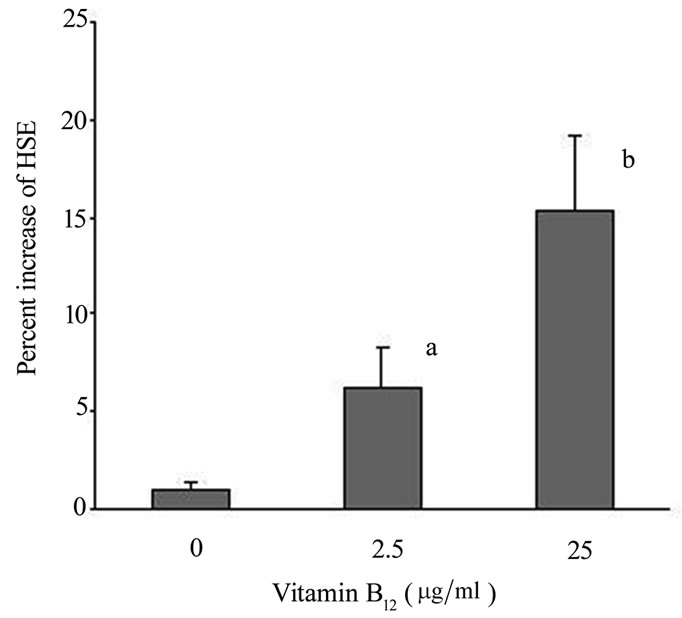
Figure 1. HF of 4 different patients were cultured in quadruplicate for 5 days at 37˚C in DMEM, 10% FBS supplemented with different concentrations of vitamin B12. Hair shaft length was measured at day 0, and day 5 and HSE was given as percent increase compared to unstimulated HF. a: p < 0,05, b: p < 0,01.
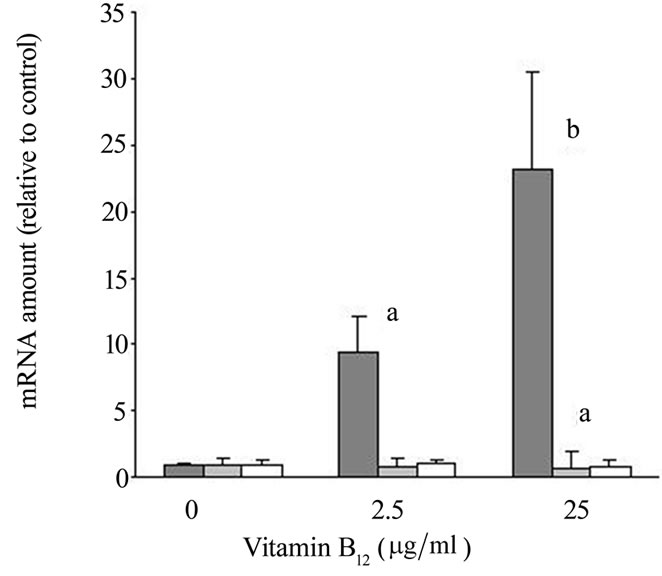
Figure 2. HF of 4 different patients were cultured in quadruplicate for 5 days at 37˚C in DMEM, 10% FBS supplemented with different concentrations of vitamin B12. After culture, specific mRNA for b-catenin (![]() ), GSK-3 (
), GSK-3 (![]() ) and Lef1/TCF (
) and Lef1/TCF (![]() ) was quantified by rT-PCR. Data represent fold change ± SD of specific mRNA levels. a: p=0,05, b: p<0.01.
) was quantified by rT-PCR. Data represent fold change ± SD of specific mRNA levels. a: p=0,05, b: p<0.01.
No changes in the transcription of Lef1/TCF were found in HFs stimulated with 2.5 mg/ml vitamin B12 or 25 mg/ml vitamin B12 (Figure 2).
4. Discussion
Cells of the HF are rapidly dividing cells, and proliferation of the cells is dependent upon synthesis of DNA and therefore on sufficient supply with vitamin B12 and folic acid [3]. It has been shown, that many growth factors are able to induce cell proliferation and DNA synthesis in various compartments of the HF [13,14]. In addition, disturbed DNA synthesis, induced by cytokines or growth factors, lead to transition of the HF from anagen into telogen [15]. On the other hand, transition of the HF from telogen into anagen is also accompanied by cell proliferation and DNA synthesis and is therefore dependent on vitamin B12 and folic acid.
A key pathway in hair follicle morphogenesis and hair follicle cycling is the wnt-pathway. This pathway is regulated by a variety of soluble factors, which are known to influence hair growth and hair follicle cycling. Signaling via the wnt-pathway results in release of membrane bound b-catenin in activation of a down stream cascade which, at least activates the transcription factor Lef1/TCF. As a result of wnt-pathway activation in hair follicle cells, cell proliferation is increased in different compartments of the hair follicle leading organogenesis in hair follicle placodes or the induction of anagen in mature hair follicles (reviewed in [9]). The importance of wnt-pathway in hair follicle biology was further supported by the finding that activation of this pathway results in the formation of ectopic follicles from existing follicles, interfollicular epidermis and sebaceous glands [16].
In this study, we have evaluated the effect of different concentrations of vitamin B12 in an in vitro HSE model. In this model, elongation of the hair shaft occurs under standard tissue culture conditions [17].
We found, that supplementation of medium with vitamin B12 resulted in enhanced HSE in this system. This finding demonstrates the hair shaft growth promoting effect of vitamin B12. The cellular actions of vitamin B12 are dependent on vitamin B12 uptake into the cell either by internalization of free cobalamin or, more important, on receptor mediated uptake of the transcobalamin II (TCII)/vitamin B12 complex into the cells via the TCII-receptor [18]. It has been shown, that cultured cells produce endogenous TCII and the amount of internalized cobalamin correlates directly to the capacity of the cells to produce TCII [19]. Our data suggests that in the in vitro model of HSE cellular expression of TCII and TCII-receptor occurs due to the lack of vitamin B12 in the tissue culture medium. A similar decrease of vitamin B12 can be postulated for the storage period of micrografts during hair restoration surgery, and this might be causative for the observed posttransplantational effluvium.
It has been demonstrated recently, that topical administration of vitamin B12 results in induction of anagen in the mouse model [8]. Similarly, our data suggest that vitamin B12 supports the transition of the HF into anagen by increased transcription of b-catenin and reduced transcription of GSK-3. Although clinical trials have to confirm the role of vitamin B12, the present data support the hypothesis that vitamin B12 stabilize and/or initiate the anagen phase of the HF and might reduce posttransplantational effluvium in hair restoration surgery.
REFERENCES
- R. H. Allen, S. P. Stabler, D. G. Savage and J. Lindenbaum, “Metabolic Abnormalities in Cobalamin (Vitamin B12) and Folate Deficiency,” Journal of the Federation of American Societies for Experimental Biology, Vol. 7, No. 14, 1993, pp. 1344-1352.
- P. R. Walker, B. Smith, C. Carson, J. Le Blanc, M. Sikorska, C. S. Woodhouse and S. C. Morgan, “Induction of Apoptosis in Neoplastic Cells by Depletion of Vitamin B12,” Cell Death and Differentiation, Vol. 4, No. 3, 1997, pp. 233-241. doi:10.1038/sj.cdd.4400225
- I. Volkov, Y. Press and I. Rudoy, “Vitamin B12 Could Be A ‘Master Key’ in the Regulation of Multiple Pathologic Processes,” Journal of Nippon Medical School, Vol. 73, No. 2, 2006, pp. 65-69. doi:10.1272/jnms.73.65
- E. Reynolds, “Vitamin B12, Folic Acid, and the Nervous System,” Lancet Neurology, Vol. 5, No. 11, 2006, pp. 949-960. doi:10.1016/S1474-4422(06)70598-1
- N. Srivastava, S. Chand, M. Bansal, K. Srivastava and S. Singh, “Reversible Hyperpigmentation as the First Manifestation of Dietary Vitamin B12 Deficiency,” Indian Journal of Dermatology Venereology and Leprology, Vol. 72, No. 5, 2006, pp. 389-930. doi:10.4103/0378-6323.27766
- R. Carmel, “Hair and Fingernail Changes in Acquired and Congenital Pernicious Anemia,” Archives of Internal Medicine, Vol. 171, No. 13, 1985, pp. 484-485. doi:10.1001/archinte.145.3.484
- G. L. Wolff, R. L. Kodell, S. R. Moore and C. A. Cooney, “Maternal Epigenetics and Methyl Supplements Affect Agouti Gene Expression in Avy/A Mice,” Journal of the Federation of American Societies for Experimental Biology, Vol. 12, No. 11, 1998, pp. 949-957.
- N. H. Park, “Topical Administration of Cyanocobalamin (Vitamin B12) Showed Suppression of Potassium Channel Inhibitor (Tolbutamide) and Induction of Murine Hair Anagen Phase and Synergistic Effect with Minoxidil,” Dermatology, Vol. 213, No. 1, 2006, pp. 53-80.
- K. S. Stenn and R. Paus, “Controls of Hair Follicle Cycling,” Physiological Reviews, Vol. 8, No. 1, 2001, pp. 449-494.
- C. Fathke, L. Wilson, K. Shah, B. Kim, A. Hocking, R. Moon and F. Isik, “Wnt Signaling Induces Epithelial Differentiation during Cutaneous Wound Healing,” BMC Cell Biology, Vol. 7, No. 4, 2006, p. 4. doi:10.1186/1471-2121-7-4
- S. Estrach, C. A. Ambler, C. Lo Celso, K. Hozumi and F. M. Watt, “Jagged 1 Is a b-Catenin Target Gene Required for Ectopic Hair Follicle Formation in Adult Epidermis,” Development, Vol. 133, 2006, pp. 4427-4438. doi:10.1242/dev.02644
- D. Van Mater, F. T. Kolligs, A. A. Dlugosz and E. R. Fearson, “Transient Activation of Beta-Catenin Signaling in Cutaneous Keratinocytes Is Sufficient to Trigger the Active Growth Phase of the Hair Cycle in Mice,” Genes & Development, Vol. 17, No. 10, 2003, pp. 1219-1224. doi:10.1101/gad.1076103
- M. P. Philpott and T. Kealey, “Effects of EGF on the Morphology and Patterns of DNA Synthesis in Isolated Human Hair Follicles,” Journal of Investigative Dermatology, Vol. 102, 1994, pp. 186-191. doi:10.1111/1523-1747.ep12371760
- C. S. Harmon and T. D. Nevins, “Evidence that Activation of Protein Kinase a Inhibits Human Hair Follicle Growth and Hair Fibre Production in Organ Culture and DNA Synthesis in Human and Mouse Hair Follicle Organ Culture,” British Journal of Dermatology, Vol. 136, No. 6, 1997, pp. 853-858. doi:10.1111/j.1365-2133.1997.tb03924.x
- R. Hirota, S. Tajima, Y. Yoneda T. Tamayama, M. Watanabe, K. Ueda, T. Kubota and R. Yoshida, “Alopecia of IFN-Gamma Knockout Mouse as a Model for Disturbance of the Hair Cycle: A Unique Arrest of the Hair Cycle at the Anagen Phase Accompanied by Mitosis,” Journal of Interferon and Cytokine Research, Vol. 22, No. 9, 2002, pp. 935-945. doi:10.1089/10799900260286641
- C. M. Baker, A. Verstuyf, K. B. Jensen and F. M. Watt, “Differential Sensitivity of Epidermal Cell Subpopulations to Beta-Catenin-Induced Ectopic Hair Follicle Formation,” Developmental Biology, Vol. 343, No. 1-2, 2010, pp. 40-50.doi:10.1016/j.ydbio.2010.04.005
- O. S. Kwon, J. K. Oh, M. H. Kim, S. H. Park, H. K. Pyo, K. H. Kim, K. H. Cho and H. C. Eun, “Human Hair Growth ex Vivo is Correlated with in Vivo Hair Growth: Selective Categorization of Hair Follicles for More Reliable Hair Follicle Organ Culture,” Archives of Dermatological Research, Vol. 297, No. 8, 2006, pp. 367-371. doi:10.1007/s00403-005-0619-z
- B. Seetharam, “Receptor-Mediated Endocytosis of Cobalamin (Vitamin B12),” Annual Review of Nutrition, Vol. 19, No. 1, 1999, pp. 173-195. doi:10.1146/annurev.nutr.19.1.173
- C. A. Hall and P. D. Colligen, “The Function of Cellular Transcobalamin II in Cultured Human Cells,” Experimental Cell Research, Vol. 183, No. 1, 1989, pp. 159-167. doi:10.1016/0014-4827(89)90426-6

