Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2009, 2, 632-636 doi: 10.4236/jbise.2009.28092 Published Online December 2009 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online December 2009 in SciRes. http://www.scirp.org/journal/jbise Normobaric hypoxia-induced brain damage in wistar rat Ding-Yu Hu1,2, Qin Li1, Bo Li3, Rong-Ji Dai1, Li-Na Geng1, Yu-Lin Deng1* 1School of Life Science and Technology, Beijing Institute of Technology, Beijing, China; 2Department of Fire Engineering, The Chinese People’s Armed Police Force Academy, Langfang, Hebei, China; 3Beijing Vocational College of Electronic Science, Beijing, China. Email: chem_hdy@yahoo.com.cn Received 2 July 2009; revised 20 August 2009; accepted 28 August 2009. ABSTRACT The biochemical indicators of wistar rat under low oxy- gen concentration, such as brain water content, necro- sis, lactic acid and Na+-K+-ATPase, was detected to evaluate normobaric hypoxia-induced brain damage and to investigate the mechanism of wistar rat brain injury. Histopathological changes in brain tissue in- duced by hypoxia were investigated via hematoxylin and eosin stain (HE). Hypoxia induced factor-1α (HIF-1α) expression in brain was confirmed using im- munohistochemistry. The results showed that the level of lactic acid was positively correlated with the degree of hypoxia, while concentration-dependent decrease in total Na+-K+-ATPase activity was observed. Compared with the control group, hypoxia group had a significant difference on brain water content under severe hypoxic conditions, the rate of brain necrosis increased obvi- ously, followed by the increase of lactic acid level and the decrease of Na+-K+-ATPase activity. Histopa- thological analysis of brain confirmed that there was neuronal cell death in hippocampal gyrus. HIF-1α ex- pression enhanced the hypoxia adaptation capability of the rat model through regulating the expressions of multiple genes. Lactic acid, Na+-K+-ATPase and HIF- 1α played an important role in brain injury as a possi- ble mechanism. Keywords: Hypoxia; Brain Damage; HIF-1α; Rat 1. INTRODUCTION Hypoxia is an important pathobiological process in ma- ny diseases and causes changing of body functions easily [1,2]. Under airtight or demi-airtight environment, due to the effects of organism metabolic and impairment of gas exchange between the organization and environment, quality of the air in the cabin gets worse gradually, con- centration of oxygen drops rapidly and concentration of carbon dioxide heightens rapidly. Hypoxia environment emerges quickly after appreciably long time. Hypoxia might lead to functional impairment, disturbance of consciousness, reaction dullness, retardation at action, damage of learning-memory function. Serious hypoxia might cause pathological damage or even death. Study on hypoxia mostly concentrated on hypoxic-ischemic encephalopathy (HIE) [3,4], plateau hypoxia [5,6], learn- ing-memory [7]; therapy of various diseases induced by hypoxia and mechanisms [8,9], etc. Some studies had upgraded to cell and molecular level. Hypoxia-induced brain damage is a hot research area of brain research. Brain damage may be induced by en- ergy exhaustion in brain cell, overexpression of excita- tory amino acids, oxygen free radical damage,apoptosis and inflammation, etc. The brain is susceptible to oxida- tive stress. This is due to the high content of polyun- saturated fatty acids, high rate of oxygen consumption, regional high concentrations of iron, and relatively low antioxidant capacity. These factors may predispose the premature infant, apoplexy patients to brain damage. Some of the mechanisms of hypoxia-induced brain da- mage were tried to be elucidated but not clearly com- pletely nowadays. More experimental data would be needed. The investigation of the changes in energy me- tabolites and brain damage during hypoxia and brain hypoxic preconditioning might lead to the finding of an effective way to protect the brain from hypoxia injury. The goal of this study was to investigate the bio- chemical effects of hypoxia on brain damage of rat model in the airtight cabin and provide more data for understanding the mechanism of brain damage. Brain water content, necrosis area, the levels of lactic acid and Na+-K+-ATPase activity were detected. HIF-1α (hypoxia induced factor-1α) expression was confirmed using im- munohistochemistry method. Histopathological changes of brain in rat model induced by hypoxia were investi- gated via hematoxylin and eosin stain (HE). All of rat models were exposed to hypoxia for 2h at various con- centrations of oxygen. 2. MATERIALS AND METHODS 2.1 Animals Male wistar rats weighing 180–200g (provided by Insti- tute of Laboratory Animal Science, Chinese Academy of Medical Science) were used in this study. Animals were  D. Y. Hu et al. / J. Biomedical Science and Engineering 2 (2009) 632-636 633 SciRes Copyright © 2009 JBiSE allowed to acclimatize for at least 7 days prior to ex- periment. Animals were housed at a room temperature of 22±2°C and a relative humidity of 50±10% with con- trolled light (12-h light/12-h dark cycle, with the light switched on at 7 a.m.). Food and water were available ad libitum. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Ani- mals published by Beijing Administration Office of Lab- oratory Animal. 2.2. Normobaric Hypoxia Equipment Animals were placed in a custom-made 16-liter plastic normobaric hypoxia chamber. Fresh soda lime was put on the bottom of chamber. O2 and N2 cylinders were linked with the chamber. The concentration of O2 was controlled by infusing N2 at flow rate of 7.5L/min. The concentrations of O2 and CO2 were monitored continu- ously respectively [10]. 18%, 15%, 12%, 10%, 8%, 6% O2 were designed and used in the experiment respec- tively. Compared to hypoxia group, control group, which exposed to normobaric normoxia (21% O2) without food and water, was set up. 2.3. Water Content of Brain Tissue After exposed to hypoxia for 2h, rats were anesthetized with 1% pentobarbital (50 mg/kg of body weight, in- traperitoneally) then killed by cervical dislocation. The brain of each rat was isolated and weighted. Water con- tent of brain tissue detected by lyopyilization was calcu- lated as a measure of hypoxia-induced brain damage, i.e. % water content = 100× ((wet brain weight-dry brain weight) / wet brain weight) %. 2.4. Estimation of Brain Necrosis After exposed to hypoxia for 2h, rats were anesthetized with 1% pentobarbital (50 mg/kg of body weight, in- traperitoneally), then killed by cervical dislocation. The brain of each rat was isolated and coronally sectioned into five slices (2 mm thick), and then those slices were placed in 3% 2, 3, 5-triphenyltetrazolium chloride (TTC) at 37 °C for 30 min. Those slices were dried on filter paper and weighted respectively. Total damage sections (grey section) were isolated and weighted. The relative damage percentage was estimated by calculating the brain damage area percentage by total slice (100×total damage section / total slice). 2.5. Analysis of Lactic Acid and Na+-K+-ATPase The levels of lactic acid and Na+-K+-ATPase activity in rat model tissue were measured with kits (manufactured by Nanjing Jiancheng Bio-engineering Institute) accord- ing to the manufacturer’s instruction. After exposed to hypoxia for 2h, rats were anesthetized with 1% pento- barbital (50 mg/kg of body weight, intraperitoneally), killed by cervical dislocation. The brain tissue was iso- lated for biochemical examinations over an ice cube. After weighting, the isolated tissue were collected in 0.1 M phosphate buffer (pH 7.4) and homogenized. The homogenates were centrifuged at 2000 r min-1 or 1000 r min-1 at 4℃ for 10 min. The supernatants were used for analysis of lactic acid and Na+-K+-ATPase activity re- spectively. The procedures of quantifying lactic acid and Na+-K+-ATPase activity were carried out according to the description of the kits. These indexes were evaluated by means of measurement of optical density at 530nm, 636nm with a UV spectrophotometer respectively. 2.6. Histopathological Examination In histopathological examination, rats were exposed to 6% O2 for 2h respectively and sacrificed by decapitation whose brains were taken out and transferred to 4% para- formaldehyde. Hippocampus sections were prepared (5 μm thick) and stained by hematoxylin and eosin. Stained sections were evaluated qualitatively (light microscopy) by an examiner blinded to experimental conditions. 2.7. HIF-1α Immunohistochemistry After exposed to 6% O2 for 2h, the rats were anesthetized with 1% pentobarbital (50 mg/kg of body weight, intrap- eritoneally ) and perfused through the ascending aorta with 200 ml of 1% NaCl solution, followed with 200 ml of 4% paraformaldehyde. The brain of each rat was isolated and kept in the 4% paraformaldehyde solution until slicing. The brains were dehydrated in 10% sucrose for 1 day and then 30% sucrose solution for 2 days, till the brain sank to the bottom of the bottle. Hippocampus section were cut at 35μm thickness on a freezing microtome and processed for HIF-1α immunohistochemistry. The sections were rinsed in PBS-T (add 1ml of tween 20 to 2L of phosphate buffer saline), for three times. Then added with 3 ml of 1% H2O2 blocking solution at room temperature for 30 min. After reaction, the slices were rinsed and then added with 2 ml of 5% BSA solution for 20 min. Added 1:200 dilution of rab- bit anti-HIF-1α antibody, and weaved in the refrigerator for 24h. The reaction was followed by adding biotin labeled monoclone mice anti-rabbit antibody. The slices were rinsed, soaked in the SABC solution for 30 min. Then DAB solution was used to stain for 10 min. The sections were dehydrated in ascending alcohol concentrations, cleared and covered in xylene. Rabbit anti-HIF-1α anti- body, biotin labeled monoclone mice anti-rabbit antibody, SABC and DAB solution were purchased from Boster Biological Technology, LTD (Wuhan, Hubei, China). 2.8. Statistical Analysis All results were expressed as mean±SEM. Statistical analysis of data was performed by applying one-way analysis of variance (ANOVA) followed by Tukey test. The p values less than 0.05 were considered as statisti- cally significant difference. 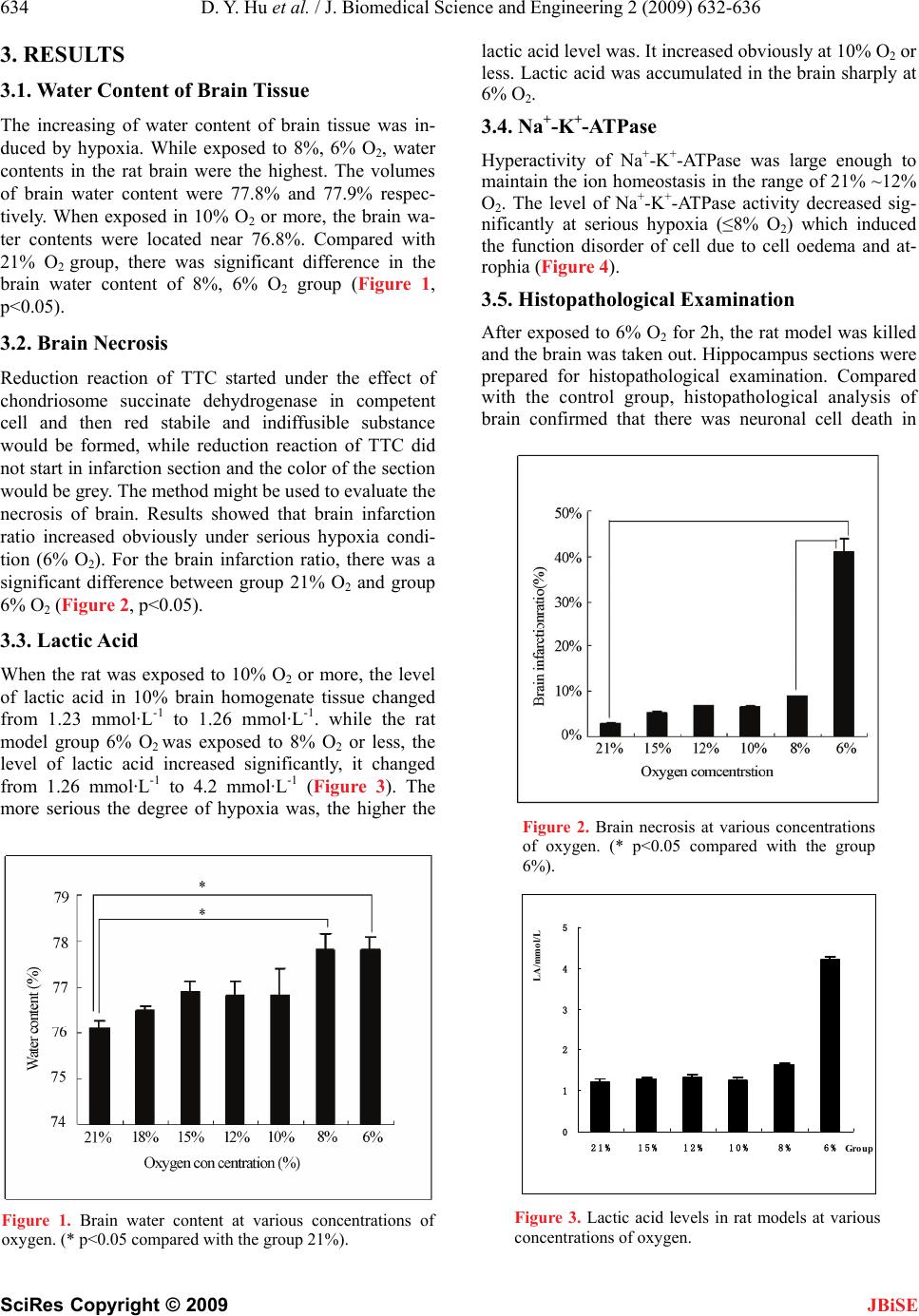 634 D. Y. Hu et al. / J. Biomedical Science and Engineering 2 (2009) 632-636 SciRes Copyright © 2009 JBiSE 3. RESULTS 3.1. Water Content of Brain Tissue The increasing of water content of brain tissue was in- duced by hypoxia. While exposed to 8%, 6% O2, water contents in the rat brain were the highest. The volumes of brain water content were 77.8% and 77.9% respec- tively. When exposed in 10% O2 or more, the brain wa- ter contents were located near 76.8%. Compared with 21% O2 group, there was significant difference in the brain water content of 8%, 6% O2 group (Figure 1, p<0.05). 3.2. Brain Necrosis Reduction reaction of TTC started under the effect of chondriosome succinate dehydrogenase in competent cell and then red stabile and indiffusible substance would be formed, while reduction reaction of TTC did not start in infarction section and the color of the section would be grey. The method might be used to evaluate the necrosis of brain. Results showed that brain infarction ratio increased obviously under serious hypoxia condi- tion (6% O2). For the brain infarction ratio, there was a significant difference between group 21% O2 and group 6% O2 (Figure 2, p<0.05). 3.3. Lactic Acid When the rat was exposed to 10% O2 or more, the level of lactic acid in 10% brain homogenate tissue changed from 1.23 mmol·L-1 to 1.26 mmol·L-1. while the rat model group 6% O2 was exposed to 8% O2 or less, the level of lactic acid increased significantly, it changed from 1.26 mmol·L-1 to 4.2 mmol·L-1 (Figure 3). The more serious the degree of hypoxia was, the higher the Figure 1. Brain water content at various concentrations of oxygen. (* p<0.05 compared with the group 21%). lactic acid level was. It increased obviously at 10% O2 or less. Lactic acid was accumulated in the brain sharply at 6% O2. 3.4. Na+-K+-ATPase Hyperactivity of Na+-K+-ATPase was large enough to maintain the ion homeostasis in the range of 21% ~12% O2. The level of Na+-K+-ATPase activity decreased sig- nificantly at serious hypoxia (≤8% O2) which induced the function disorder of cell due to cell oedema and at- rophia (Figure 4). 3.5. Histopathological Examination After exposed to 6% O2 for 2h, the rat model was killed and the brain was taken out. Hippocampus sections were prepared for histopathological examination. Compared with the control group, histopathological analysis of brain confirmed that there was neuronal cell death in Figure 2. Brain necrosis at various concentrations of oxygen. (* p<0.05 compared with the group 6%). 0 1 2 3 4 5 21%15% 12% 10%8%6%Gro u p LA/mmol/L Figure 3. Lactic acid levels in rat models at various concentrations of oxygen. 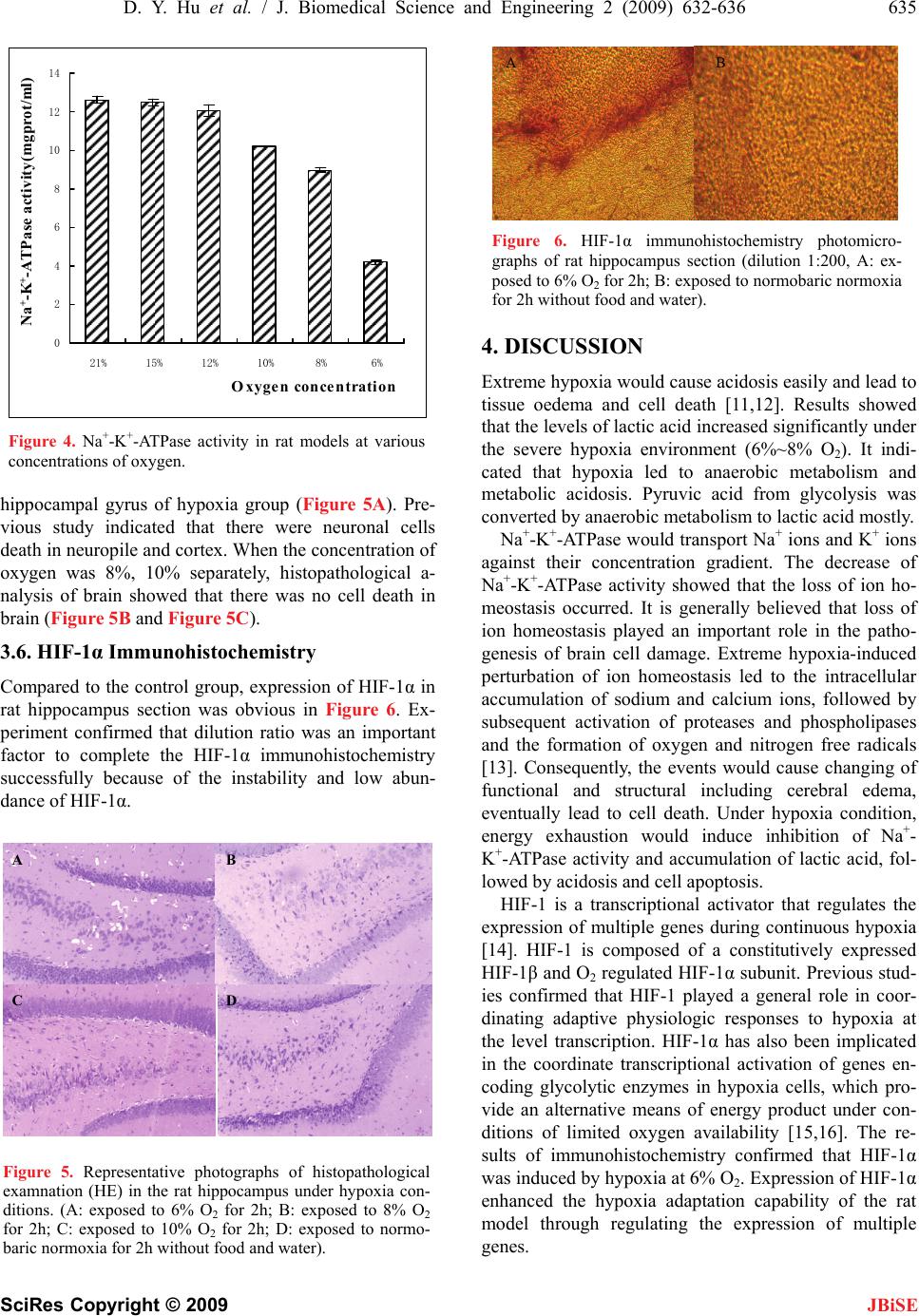 D. Y. Hu et al. / J. Biomedical Science and Engineering 2 (2009) 632-636 635 SciRes Copyright © 2009 JBiSE 0 2 4 6 8 10 12 14 21% 15% 12% 10%8%6% Oxygen concentration Na + -K + -ATPase activity(mgprot/ml) A B Figure 4. Na+-K+-ATPase activity in rat models at various concentrations of oxygen. hippocampal gyrus of hypoxia group (Figure 5A). Pre- vious study indicated that there were neuronal cells death in neuropile and cortex. When the concentration of oxygen was 8%, 10% separately, histopathological a- nalysis of brain showed that there was no cell death in brain (Figure 5B and Figure 5C). 3.6. HIF-1α Immunohistochemistry Compared to the control group, expression of HIF-1α in rat hippocampus section was obvious in Figure 6. Ex- periment confirmed that dilution ratio was an important factor to complete the HIF-1α immunohistochemistry successfully because of the instability and low abun- dance of HIF-1α. Figure 5. Representative photographs of histopathological examnation (HE) in the rat hippocampus under hypoxia con- ditions. (A: exposed to 6% O2 for 2h; B: exposed to 8% O2 for 2h; C: exposed to 10% O2 for 2h; D: exposed to normo- baric normoxia for 2h without food and water). Figure 6. HIF-1α immunohistochemistry photomicro- graphs of rat hippocampus section (dilution 1:200, A: ex- posed to 6% O2 for 2h; B: exposed to normobaric normoxia for 2h without food and water). 4. DISCUSSION Extreme hypoxia would cause acidosis easily and lead to tissue oedema and cell death [11,12]. Results showed that the levels of lactic acid increased significantly under the severe hypoxia environment (6%~8% O2). It indi- cated that hypoxia led to anaerobic metabolism and metabolic acidosis. Pyruvic acid from glycolysis was converted by anaerobic metabolism to lactic acid mostly. Na+-K+-ATPase would transport Na+ ions and K+ ions against their concentration gradient. The decrease of Na+-K+-ATPase activity showed that the loss of ion ho- meostasis occurred. It is generally believed that loss of ion homeostasis played an important role in the patho- genesis of brain cell damage. Extreme hypoxia-induced perturbation of ion homeostasis led to the intracellular accumulation of sodium and calcium ions, followed by subsequent activation of proteases and phospholipases and the formation of oxygen and nitrogen free radicals [13]. Consequently, the events would cause changing of functional and structural including cerebral edema, eventually lead to cell death. Under hypoxia condition, energy exhaustion would induce inhibition of Na+- K+-ATPase activity and accumulation of lactic acid, fol- lowed by acidosis and cell apoptosis. A B HIF-1 is a transcriptional activator that regulates the expression of multiple genes during continuous hypoxia [14]. HIF-1 is composed of a constitutively expressed HIF-1β and O2 regulated HIF-1α subunit. Previous stud- ies confirmed that HIF-1 played a general role in coor- dinating adaptive physiologic responses to hypoxia at the level transcription. HIF-1α has also been implicated in the coordinate transcriptional activation of genes en- coding glycolytic enzymes in hypoxia cells, which pro- vide an alternative means of energy product under con- ditions of limited oxygen availability [15,16]. The re- sults of immunohistochemistry confirmed that HIF-1α was induced by hypoxia at 6% O2. Expression of HIF-1α enhanced the hypoxia adaptation capability of the rat model through regulating the expression of multiple genes. C D  636 D. Y. Hu et al. / J. Biomedical Science and Engineering 2 (2009) 632-636 SciRes Copyright © 2009 5. CONCLUSIONS The values of lactic acid are positively correlated with the degree of hypoxia, while total Na+-K+-ATPase activ- ity shows a concentration-dependent decrease. Com- pared with the control group; hypoxia group has a sig- nificant difference in brain water content under severe hypoxia condition. The area of brain necrosis increases sharply followed by the increase of lactic acid level and the decrease of Na+-K+-ATPase activity, neuronal cell death and HIF-1 expression appear in hippocampal gy- rus obviously. Lactic acid, Na+-K+-ATPase and HIF-1α played an important role as a possible mechanism in brain injury. JBiSE 6. ACKNOWLEDGEMENT This work was supported by Commission of Science Technology and Industry for National Defense (Grant No. A2220060042) and the Na- tional Natural Science Foundation of China (Grant No. 20705005). REFERENCES [1] I. L. Kanstrup, T. D.Poulsen and J. M. Hansen. (1999) Blood pressured and plasma catecholamine in acute and prolonged hypoxia effects of local hypothermia, Apple Phys, 87(6), 2053–8. [2] Q. H. Chen. (2001) The changes of function and mor- phology of pulmonary arterial vessels in the pika at high altitude, Chin. J. Appl Phys, 17(2), 178–81. [3] W. J. Xia. (2005) The effects of hematopoietic growth factors and tanshinone ⅡA on neuro-protection, Doctor Dissertation, The Chinese University of Hong Kong, Hong kong, China. [4] M. Christiane and H. Brahimi. (2007) Harnessing the hypoxia-inducible factor in cancer and ischemic disease, Biochem. Pharmacol, 73, 450–457. [5] S. D. Aramjit and K. Manoj. (2007) cDNA cloning, gene organization and variant specific expression of HIF-1α in high altitude yak (Bos grumiens), Gene, 386(1–2), 73– 80. [6] S. Fau, C. Po, B. Gillet, et al. (2007) Effect of the reper- fusion after cerebral ischemia in neonatal rats using MRI monitoring, Experimental Neurology, 208(2), 297–304. [7] L. Liu, T. van Groen, Inga Kadish, et al. (2009) DNA methylation impacts on learning and memory in aging, Neurobiology of Aging, 30(4), 549–560. [8] G. D. Funk, A. G. Huxtable and A. R. Lorier. (2008) ATP in central respiratory control: A three-part signaling sys- tem, Respiratory Physiology & Neurobiology, 164(1–2), 131–142. [9] M. L. Peter. (2008) Opioidergic and dopaminergic mo- dulation of respiration, Respiratory Physiology & Neu- robiology, 164(1–2), 160–167. [10] J. E. Rice, R. C. Vannucci and J. B. Brierley. (1981) The influence of immaturity on hypoxia-ischemia brain damage in the rat, Ann. Neurol, 9, 131–141. [11] W. M. Bernhardt, C. Warnecke, C. Willam, et al. (2007) Organ protection by hypoxia and hypoxia-inducible factors, Methods Enzymol, 435, 219, 221–245. [12] O. Marta, S. Monika and A. Jan. (2008) Regulation of pH in the mammalian central nervous system under normal and pathological conditions: Facts and hypotheses, Neu- rochem Int, 52(6), 905–919. [13] D. B. Kintner, Y. Wang and D. Sun. (2007) Role of mem- brane ion transport proteins in cerebral ischemic damage, Front. Biosci, 12, 762–770. [14] G. L. Semenza. (2004) O2-regulated gene expression: Transcriptional control of cardiorespiratory physiology by HIF-1, J. Appl. Physiol, 96, 1173–1177. [15] M. W. Charles, B. Greg and L. S. Gregg. (1996) In vivo expression of mRNAs encoding hypoxia-inducible factor 1, Biochem Biophys Res Commun, 225, 485–488. [16] G. L. Semenza. (2001) HIF-1 and mechanisms of hy- poxia sensing, Cell. Biology, 13, 167–171. |

