Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2009, 2, 617-620 doi: 10.4236/jbise.2009.28089 Published Online December 2009 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online December 2009 in SciRes. http://www.scirp.org/journal/jbise Effects of ultra-high hydrostatic pressure on foaming and physical-chemistry properties of egg white Rui-Xiang Yang, Wen-Zhao Li, Chun-Qiu Zhu, Qiang Zhang Key Laboratory of Food Nutrition and Safety, Tianjin University of Science & Technology, Tianjin, China. Email: yrxsky@126.com Received 11 July 2009; revised 1 September 2009; accepted 2 September 2009. ABSTRACT The influences of ultra-high hydrostatic pressure treat- ment on foaming and physical properties (solubility, hydrophobicity and sulfhydryl content) of egg white were investigated. A pressure range of 0-500 MPa, time range of 0-20 min and pH range of 7.5-8.5 were se- lected. The foaming property of egg white is improved by 350Mpa and 10min. The treatment resulted in in- crease of sulfhydryl content of egg white, while solu- bility and hydrophobicity were significantly decreased. Keywords: Ultra-High Hydrostatic Pressure; Egg White; Foaming Property 1. INTRODUCTION Egg white is well known for their high nutritional quality, foaming, gelling and emulsifying characteristics, which can give the foods unique color, flavor, and texture char- acteristics. Therefore, as an important ingredient, egg white has been wildly applied in the food industry, such as cakes, biscuits, breads, ice cream, and other protein products [1]. At present, for convenience and safety, liquid egg white products are widely used by home and producer. However, decrease of the foaming property after pasteurization is found in practice, which seriously affects the application and development of the egg white products. Ultra-high static pressure technology is a new sterili- zation technology, which can be used to resolve the problems brought by pasteurization. Ultra-high pressure can improve the function of biological macromolecules by modifying their structure [2]. The high pressure does not affect the primary structure of protein molecules, but can cause agglutination of protein by changing the hy- drogen bonds, disulfide bonds and hydrophobic groups among the protein molecules. In addition, ultra-high hy- drostatic pressure can cause viscosity and surface ten- sion of egg white increased [3]. The relationship be- tween foaming property and solubility, hydrophobicity, sulfhydryl content under ultra-high hydrostatic pressure is also investigated in this paper, and only the irreversible changes in the properties are taken into account. 2. MATERIALS AND METHODS 2.1. Materials and Equipments Fresh eggs were obtained from supermarket. Eggshell was washed by clean water and exposed to ultraviolet light for 30 min to disinfect. The egg white was separated from egg yolk and the chalazae were removed. The al- bumin was gently mixed and stored at 4 until use. ℃ The protein content of the egg white was determined to be 11.23 ± 0.56 % (w/v). 2.2. Ultra-High Hydrostatic Pressure Treatment A volume of 200 mL of egg white was packed in poly- ethylene plastic packs (170×120mm) and sealed. The ultra- high hydrostatic pressure treatment was performed in high pressure equipment (Hpp-M1, Senmiao, China) and a pressure range of 0-600 MPa, time range of 0-20 min were selected in the experiment. All samples were ana- lyzed at room temperature after 24-hour storage at 4.℃ 2.3. Determination of Foaming Property A volume of 35 mL (18~25) egg white was placed in ℃ a graduated cylinder of 250 mL (diameter =398 mm) and whipped for 5 min (which is an optimal time for whip- ping egg white in this measurement system in previous study) with a rotating anchor (275 mm diameter, rotor from the bottom of the graduated cylinder 15 mm) at 2100 rpm, using a laboratory stirrer with controlled con- stant speed (OJ-100, Onuo, China). The volume of foam (V0) was recorded immediately after the whipping stopped. The foaming capacity (FC) [4] was defined by %(%) 100 35 0 V FC (1) The volume of drainage of liquid (Vd) was recorded at 10 min, The foam stability was defined by 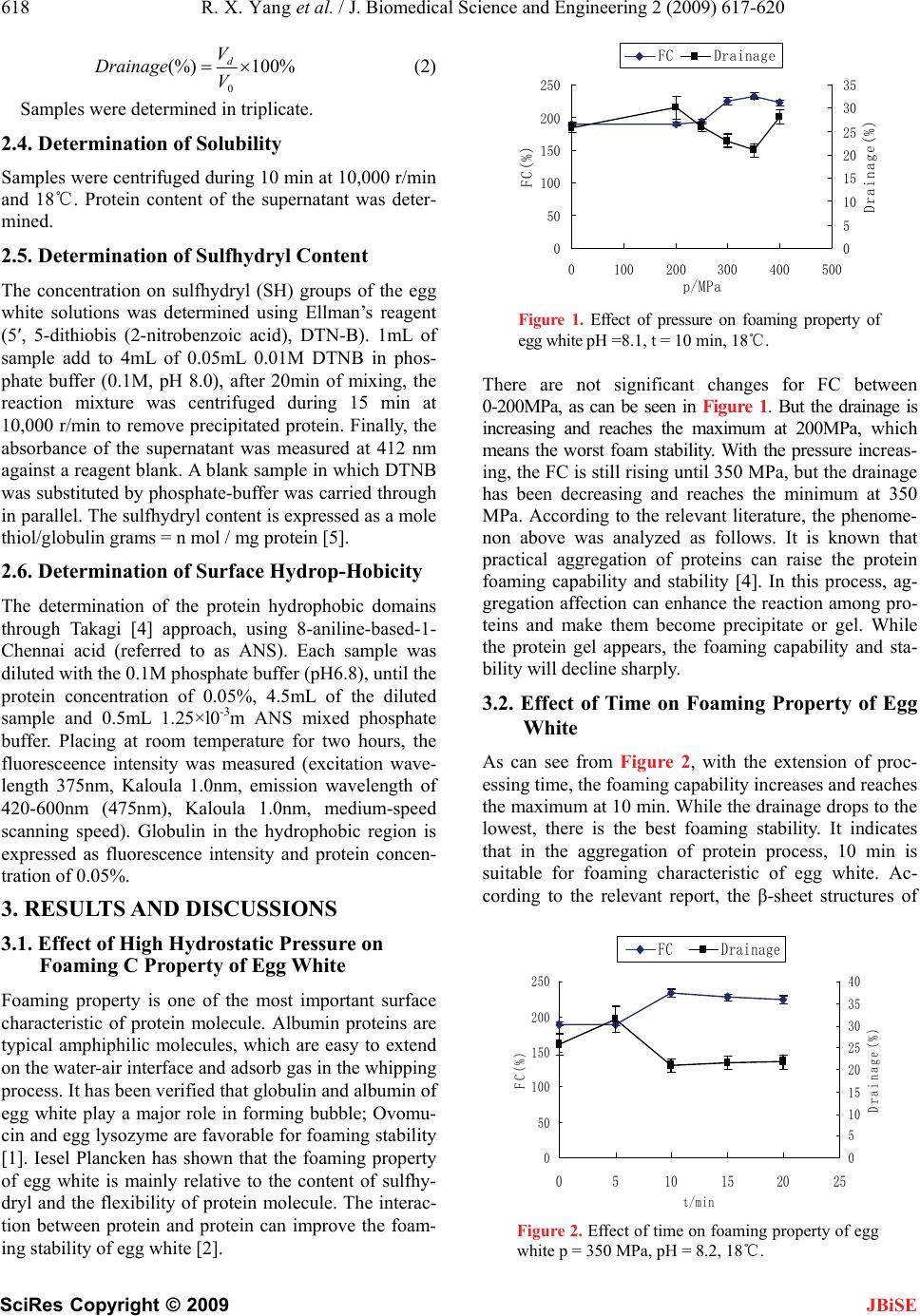 618 R. X. Yang et al. / J. Biomedical Science and Engineering 2 (2009) 617-620 SciRes Copyright © 2009 JBiSE 0 (%) 100% d V Drainage V (2) Samples were determined in triplicate. 2.4. Determination of Solubility Samples were centrifuged during 10 min at 10,000 r/min and 18. Protein content of th℃e supernatant was deter- mined. 2.5. Determination of Sulfhydryl Content The concentration on sulfhydryl (SH) groups of the egg white solutions was determined using Ellman’s reagent (5′, 5-dithiobis (2-nitrobenzoic acid), DTN-B). 1mL of sample add to 4mL of 0.05mL 0.01M DTNB in phos- phate buffer (0.1M, pH 8.0), after 20min of mixing, the reaction mixture was centrifuged during 15 min at 10,000 r/min to remove precipitated protein. Finally, the absorbance of the supernatant was measured at 412 nm against a reagent blank. A blank sample in which DTNB was substituted by phosphate-buffer was carried through in parallel. The sulfhydryl content is expressed as a mole thiol/globulin grams = n mol / mg protein [5]. 2.6. Determination of Surface Hydrop-Hobicity The determination of the protein hydrophobic domains through Takagi [4] approach, using 8-aniline-based-1- Chennai acid (referred to as ANS). Each sample was diluted with the 0.1M phosphate buffer (pH6.8), until the protein concentration of 0.05%, 4.5mL of the diluted sample and 0.5mL 1.25×l0-3m ANS mixed phosphate buffer. Placing at room temperature for two hours, the fluoresceence intensity was measured (excitation wave- length 375nm, Kaloula 1.0nm, emission wavelength of 420-600nm (475nm), Kaloula 1.0nm, medium-speed scanning speed). Globulin in the hydrophobic region is expressed as fluorescence intensity and protein concen- tration of 0.05%. 3. RESULTS AND DISCUSSIONS 3.1. Effect of High Hydrostatic Pressure on Foaming C Property of Egg White Foaming property is one of the most important surface characteristic of protein molecule. Albumin proteins are typical amphiphilic molecules, which are easy to extend on the water-air interface and adsorb gas in the whipping process. It has been verified that globulin and albumin of egg white play a major role in forming bubble; Ovomu- cin and egg lysozyme are favorable for foaming stability [1]. Iesel Plancken has shown that the foaming property of egg white is mainly relative to the content of sulfhy- dryl and the flexibility of protein molecule. The interac- tion between protein and protein can improve the foam- ing stability of egg white [2]. 0 50 100 150 200 250 0100 200300 400 500 p/MPa FC(%) 0 5 10 15 20 25 30 35 Drainage(%) FC Drainage Figure 1. Effect of pressure on foaming property of egg white pH =8.1, t = 10 min, 18℃. There are not significant changes for FC between 0-200MPa, as can be seen in Figure 1. But the drainage is increasing and reaches the maximum at 200MPa, which means the worst foam stability. With the pressure increas- ing, the FC is still rising until 350 MPa, but the drainage has been decreasing and reaches the minimum at 350 MPa. According to the relevant literature, the phenome- non above was analyzed as follows. It is known that practical aggregation of proteins can raise the protein foaming capability and stability [4]. In this process, ag- gregation affection can enhance the reaction among pro- teins and make them become precipitate or gel. While the protein gel appears, the foaming capability and sta- bility will decline sharply. 3.2. Effect of Time on Foaming Property of Egg White As can see from Figure 2, with the extension of proc- essing time, the foaming capability increases and reaches the maximum at 10 min. While the drainage drops to the lowest, there is the best foaming stability. It indicates that in the aggregation of protein process, 10 min is suitable for foaming characteristic of egg white. Ac- cording to the relevant report, the β-sheet structures of 0 50 100 150 200 250 0510 1520 25 t/min FC(%) 0 5 10 15 20 25 30 35 40 Drainage(%) FC Drainage Figure 2. Effect of time on foaming property of egg white p = 350 MPa, pH = 8.2, 18.℃ 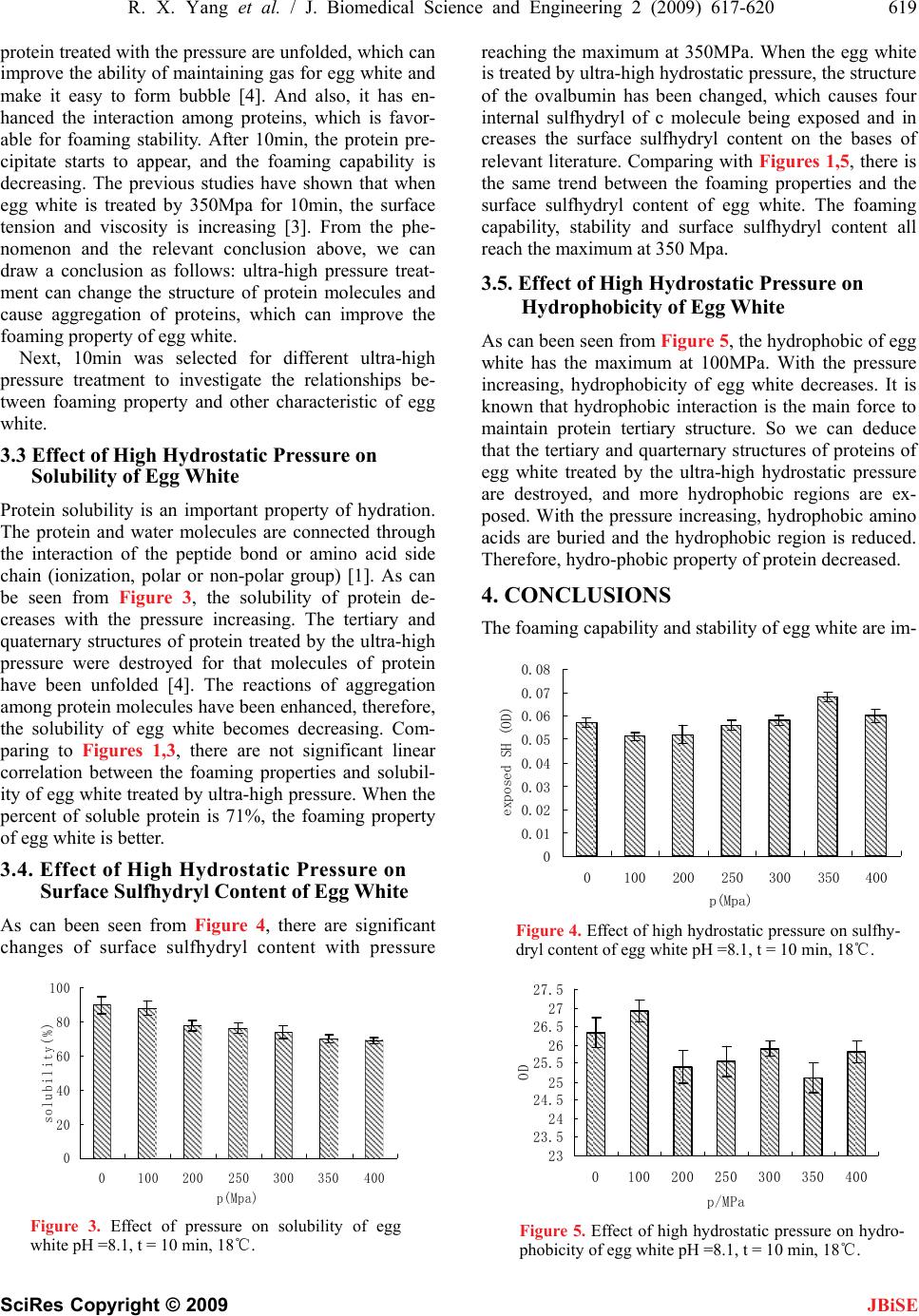 R. X. Yang et al. / J. Biomedical Science and Engineering 2 (2009) 617-620 619 SciRes Copyright © 2009 JBiSE protein treated with the pressure are unfolded, which can improve the ability of maintaining gas for egg white and make it easy to form bubble [4]. And also, it has en- hanced the interaction among proteins, which is favor- able for foaming stability. After 10min, the protein pre- cipitate starts to appear, and the foaming capability is decreasing. The previous studies have shown that when egg white is treated by 350Mpa for 10min, the surface tension and viscosity is increasing [3]. From the phe- nomenon and the relevant conclusion above, we can draw a conclusion as follows: ultra-high pressure treat- ment can change the structure of protein molecules and cause aggregation of proteins, which can improve the foaming property of egg white. Next, 10min was selected for different ultra-high pressure treatment to investigate the relationships be- tween foaming property and other characteristic of egg white. 3.3 Effect of High Hydrostatic Pressure on Solubility of Egg White Protein solubility is an important property of hydration. The protein and water molecules are connected through the interaction of the peptide bond or amino acid side chain (ionization, polar or non-polar group) [1]. As can be seen from Figure 3, the solubility of protein de- creases with the pressure increasing. The tertiary and quaternary structures of protein treated by the ultra-high pressure were destroyed for that molecules of protein have been unfolded [4]. The reactions of aggregation among protein molecules have been enhanced, therefore, the solubility of egg white becomes decreasing. Com- paring to Figures 1,3, there are not significant linear correlation between the foaming properties and solubil- ity of egg white treated by ultra-high pressure. When the percent of soluble protein is 71%, the foaming property of egg white is better. 3.4. Effect of High Hydrostatic Pressure on Surface Sulfhydryl Content of Egg White As can been seen from Figure 4, there are significant changes of surface sulfhydryl content with pressure 0 20 40 60 80 100 0100 200250 300 350400 p(Mpa) solubility(%) Figure 3. Effect of pressure on solubility of egg white pH =8.1, t = 10 min, 18℃. reaching the maximum at 350MPa. When the egg white is treated by ultra-high hydrostatic pressure, the structure of the ovalbumin has been changed, which causes four internal sulfhydryl of c molecule being exposed and in creases the surface sulfhydryl content on the bases of relevant literature. Comparing with Figures 1,5, there is the same trend between the foaming properties and the surface sulfhydryl content of egg white. The foaming capability, stability and surface sulfhydryl content all reach the maximum at 350 Mpa. 3.5. Effect of High Hydrostatic Pressure on Hydrophobicity of Egg White As can been seen from Figure 5, the hydrophobic of egg white has the maximum at 100MPa. With the pressure increasing, hydrophobicity of egg white decreases. It is known that hydrophobic interaction is the main force to maintain protein tertiary structure. So we can deduce that the tertiary and quarternary structures of proteins of egg white treated by the ultra-high hydrostatic pressure are destroyed, and more hydrophobic regions are ex- posed. With the pressure increasing, hydrophobic amino acids are buried and the hydrophobic region is reduced. Therefore, hydro-phobic property of protein decreased. 4. CONCLUSIONS The foaming capability and stability of egg white are im- 0 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0100 200 250300 350400 p(Mpa) exposed SH (OD) Figure 4. Effect of high hydrostatic pressure on sulfhy- dryl content of egg white pH =8.1, t = 10 min, 18℃. 23 23.5 24 24.5 25 25.5 26 26.5 27 27.5 0100 200 250 300 350 400 p/MPa OD Figure 5. Effect of high hydrostatic pressure on hydro- phobicity of egg white pH =8.1, t = 10 min, 18℃.  620 R. X. Yang et al. / J. Biomedical Science and Engineering 2 (2009) 617-620 SciRes Copyright © 2009 proved by the ultra-high hydrostatic pressure (100-400 MPa), and play the best performance by 350Mpa and 10min. The findings of the experiment of physical prop- erties of egg white with ultra-high hydrostatic pressure show that with increasing pressure, the solubility of egg white will decrease. There is positive relation between the surface sulfhydryl content and the foaming proper- ties of egg white. When the hydrophobicity of egg white is the lowest, the foaming capacity and foam stability of egg white will reach the best performance. JBiSE 5. ACKNOWLEDGEMENTS This research is support by the research fund of personnel of Tianjin University of Science & Technology. REFERENCES [1] X. D. Li and L. W. Zhang. (2005) Egg Science and Technology. Chemical Industry Press. [2] E. V. Plancken, A. VanLoey, and M. E. Hendricks. (2007) Foaming properties of egg white affected by heat or high pressure treatment, Journal of Food Engineering, 78, 1410–1426. [3] W. Wang and W. Z. Li. (2009) Effect of ultra-high hydro- static pressure on foaming and physical properties of the egg white. Journal of Tianjin University of Science and Technology, 24, 35–38. [4] H. Wang and Z. C. Tu. (2008) Egg white protein dynamic modification of ultrahigh-pressure micro jet and mecha- nism of, Nanchang University. [5] K. Lomakina. (2005) A study of the factors affecting the foaming properties of egg white-a review. Food Science, 24, 110–118. [6] J. Liu and B. Jiang. (2007) Effects of ultra-high pressure on foaming property of chickpea protein isolated. Anhui Agricultural Science, 35, 9012–9013. |

