 Surgical Science, 2011, 2, 468-475 doi:10.4236/ss.2011.210103 Published Online December 2011 (http://www.SciRP.org/journal/ss) Copyright © 2011 SciRes. SS Lithium Associated Hyperparathyroidism: An Evidence Based Surgical Approach Umashankar K. Ballehaninna1,2, Steven M. Nguyen3, Ronald S. Chamberlain1,3,4 1Department o f S urg e ry, Saint Barnabas Medical Center, Livingston, USA 2Department o f S urg e ry, Maimonides Medical Center, Brooklyn, USA 3Department o f S urg e ry, University of Medicine and Dentistry of New Jersey, Newark, USA 4Saint George’s University, School of Medi ci ne , West Indies, Grenada E-mail: rchamberlain@sbhcs.com Received May 17, 2011; revised August 15, 2011; accepted October 17, 2011 Abstract Background: Long-term lithium use in psychiatric patients may lead to lithium associated hyperparathyroi- dism (LAH). Although anecdotal case reports have appeared, an evidence based algorithm for management of LAH is lacking. Methods: A comprehensive literature search was performed (1973-2010) using PubMed with keywords; “lithium” “hypercalcemia” “hyperparathyroidism” “sestamibi” “intra-operative parathyroid hormone (IOPTH) monitoring” “parathyroidectomy” and “medical management”. All English language pub- lications addressing etiology and clinical management issues concerning LAH were critically analyzed. Re- sults: Lithium associated hyperparathyroidism occurs in 4.3% - 6.3% of chronic lithium users compared to the general population which has an incidence of 0.5% - 1%. 194 cases of LAH have been reported which in- cludes 10 patients (5%) treated medically and 170 patients (88%) who underwent parathyroidectomy. No de- tails were available for 14 patients (7%). Among parathyroidectomy patients, 104 (59%) had adenomatous disease and 66 (39%) had multiglandular hyperplasia. Preoperative localization studies were utilized in only 22 patients (13%) and IOPTH monitoring was reported in only 3 studies (32 patients, 19%). Among surgical patients, bilateral neck exploration (BNE) was the most common approach performed in 162 patients (95%); focused neck exploration was utilized in only 8 patients (5%). Parathyroidectomy normalized LAH bio- chemical changes in nearly all patients (90% - 97%) in the early post-operative period, but recurrent hyper- parathyroidism occurred in 8% - 42% of patients. Conclusion: LAH is an under appreciated and poorly un- derstood endocrine disorder. LAH has a higher incidence of multiglandular disease and bilateral neck explo- ration is mandatory in majority for disease control. Nonsurgical approaches may be useful in select patients on short-term lithium therapy. Keywords: Lithium, Hypercalcemia, Hyperparathyroidism, Sestamibi Intra-Operative Parathyroid Hormone (IOPTH) Monitoring, Parathyroidectomy, Medical Management 1. Introduction Lithium Carbonate (Li+) is the preferred and most effi- cacious therapy for acute treatment and maintenance the- rapy for bipolar depressive disorder, and is a useful ad- junct in unipolar depression [1,2]. Long-term lithium the- rapy is associated with multiple endocrine and metabolic alterations such as hyperthyroidism, hypothyroidism, re- duced bone mineral density (BMD), osteopenia, as well as several gastrointestinal (nausea, constipation), cardio- vascular (bradyarrythmias), renal (nephrocalcinosis, re- duced glomerular filtration rate (GFR), polyuria, nephro- genic diabetes insipidus) and psychosomatic adverse eff- ects (weakness, fatigue, depression). Chronic lithium the- rapy is also associated with hyperparathyroidism (LAH) characterized by hypercalcemia, hypermagnesemia, re- duced urinary calcium, and elevated serum PTH levels often with enlarged parathyroid gland(s) [3,4]. Rare case reports and a limited number of reviews have yielded in- adequate and conflicting data concerning the ideal treat- ment of LAH [4-6]. This article provides a systematic review of LAH, addresses current controversies, and pro- 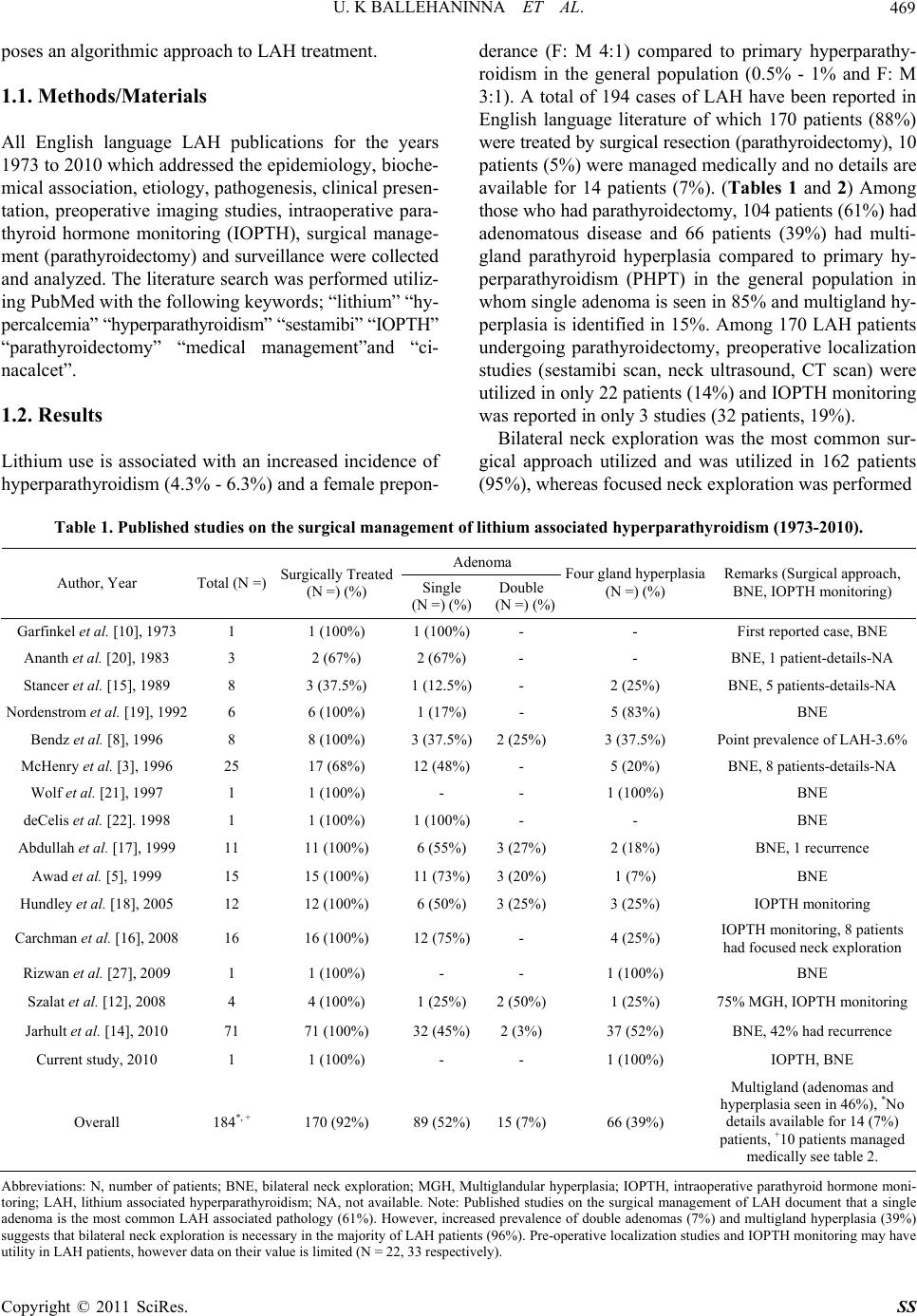 U. K BALLEHANINNA ET AL.469 poses an algorithmic approach to LAH treatment. 1.1. Methods/Materials All English language LAH publications for the years 1973 to 2010 which addressed the epidemiology, bioche- mical association, etiology, pathogenesis, clinical presen- tation, preoperative imaging studies, intraoperative para- thyroid hormone monitoring (IOPTH), surgical manage- ment (parathyroidectomy) and surveillance were collected and analyzed. The literature search was performed utiliz- ing PubMed with the following keywords; “lithium” “hy- percalcemia” “hyperparathyroidism” “sestamibi” “IOPTH” “parathyroidectomy” “medical management”and “ci- nacalcet”. 1.2. Results Lithium use is associated with an increased incidence of hyperparathyroidism (4.3% - 6.3%) and a female prepon- derance (F: M 4:1) compared to primary hyperparathy- roidism in the general population (0.5% - 1% and F: M 3:1). A total of 194 cases of LAH have been reported in English language literature of which 170 patients (88%) were treated by surgical resection (parathyroidectomy), 10 patients (5%) were managed medically and no details are available for 14 patients (7%). (Tables 1 and 2) Among those who had parathyroidectomy, 104 patients (61%) had adenomatous disease and 66 patients (39%) had multi- gland parathyroid hyperplasia compared to primary hy- perparathyroidism (PHPT) in the general population in whom single adenoma is seen in 85% and multigland hy- perplasia is identified in 15%. Among 170 LAH patients undergoing parathyroidectomy, preoperative localization studies (sestamibi scan, neck ultrasound, CT scan) were utilized in only 22 patients (14%) and IOPTH monitoring was reported in only 3 studies (32 patients, 19%). Bilateral neck exploration was the most common sur- gical approach utilized and was utilized in 162 patients (95%), whereas focused neck exploration was performed Table 1. Published studies on the surgical management of lithium associated hyperparathyroidism (1973-2010). Adenoma Author, Year Total (N =) Surgically Treated (N =) (%) Single (N =) (%) Double (N =) (%) Four gland hyperplasia (N =) (%) Remarks (Surgical approach, BNE, IOPTH monitoring) Garfinkel et al. [10], 1973 1 1 (100%) 1 (100%)- - First reported case, BNE Ananth et al. [20], 1983 3 2 (67%) 2 (67%)- - BNE, 1 patient-details-NA Stancer et al. [15], 1989 8 3 (37.5%) 1 (12.5%)- 2 (25%) BNE, 5 patients-details-NA Nordenstrom et al. [19], 1992 6 6 (100%) 1 (17%)- 5 (83%) BNE Bendz et al. [8], 1996 8 8 (100%) 3 (37.5%)2 (25%) 3 (37.5%) Point prevalence of LAH-3.6% McHenry et al. [3], 1996 25 17 (68%) 12 (48%)- 5 (20%) BNE, 8 patients-details-NA Wolf et al. [21], 1997 1 1 (100%) - - 1 (100%) BNE deCelis et al. [22]. 1998 1 1 (100%) 1 (100%)- - BNE Abdullah et al. [17], 1999 11 11 (100%) 6 (55%)3 (27%) 2 (18%) BNE, 1 recurrence Awad et al. [5], 1999 15 15 (100%) 11 (73%)3 (20%) 1 (7%) BNE Hundley et al. [18], 2005 12 12 (100%) 6 (50%)3 (25%) 3 (25%) IOPTH monitoring Carchman et al. [16], 2008 16 16 (100%) 12 (75%)- 4 (25%) IOPTH monitoring, 8 patients had focused neck exploration Rizwan et al. [27], 2009 1 1 (100%) - - 1 (100%) BNE Szalat et al. [12], 2008 4 4 (100%) 1 (25%)2 (50%) 1 (25%) 75% MGH, IOPTH monitoring Jarhult et al. [14], 2010 71 71 (100%) 32 (45%)2 (3%) 37 (52%) BNE, 42% had recurrence Current study, 2010 1 1 (100%) - - 1 (100%) IOPTH, BNE Overall 184*, + 170 (92%) 89 (52%)15 (7%) 66 (39%) Multigland (adenomas and hyperplasia seen in 46%), *No details available for 14 (7%) patients, +10 patients managed medically see table 2. Abbreviations: N, number of patients; BNE, bilateral neck exploration; MGH, Multiglandular hyperplasia; IOPTH, intraoperative parathyroid hormone moni- toring; LAH, lithium associated hyperparathyroidism; NA, not available. Note: Published studies on the surgical management of LAH document that a single adenoma is the most common LAH associated pathology (61%). However, increased prevalence of double adenomas (7%) and multigland hyperplasia (39%) suggests that bilateral neck exploration is necessary in the majority of LAH patients (96%). Pre-operative localization studies and IOPTH monitoring may have utility in LAH patients, however data on their value is limited (N = 22, 33 respectively). Copyright © 2011 SciRes. SS  U. K BALLEHANINNA ET AL. 470 Table 2. Published studies on the medical management of lithium associated hyperparathyroidism (1973-2010). Author, Year N = Management Gama et al. [3], 1999 1 Resolution of LAH after lithium was discontinued Sloand et al. [23], 2006 2 Modest response while lithium was continued in one patient Khandawala et al. [24], 2006 1 Resolution of LAH after lithium was discontinued Rifai et al. [25], 2006 1 Discontinuation of Li+, normocalcemia achieved by cinacalcet Duggal et al. [26], 2007 1 Resolution of LAH after lithium was discontinued Gregoor et al. [34], 2007 3 Cinacalcet therapy ameliorated LAH Szalat et al. [12], 2009 1 Postoperative recurrence—hypercalcemia corrected with cinacalcet, however elevated PTH persisted. Abbreviations: LAH, lithium associated hyperparathyroidism, Li+, Lithium, PTH, parathyroid hormone. Note: Medical management (withdrawal of lithium and/or use of cinacalcet) has been successfully used to treat a small number of LAH patients. in 8 (5%) patients. The latter approach was utilized signi- ficantly less often in LAH when compared to PHPT pa- tients in whom the published unilateral focused neck ex- ploration rate is 80% - 95% [7]. This change likely re- flects the increased incidence of four-gland hyperplasia in LAH. Parathyroidectomy normalized LAH associated biochemical changes in nearly all patients (67% - 100%) in the early post-operative period, however at a median long-term follow-up of 6.3 years 8% - 42% patients ex- perienced recurrence. 2. Case Review A 64 year-old female with a past medical history of type II diabetes mellitus, hypertension, hypercholesterolemia, and bipolar depression was referred for surgical evalua- tion due to persistent hypercalcemia. She had been tre- ated with lithium (300 mg BID) for four years. Over the prior three years, the patient had mild elevations of her serum calcium level (normal range; 10 - 11 mg/dL) which rose to 11.7 mg/dL and 12.3 mg/dL over a two months duration. Symptomatically she complained of decreased memory and mental clarity. Her diabetes was well con- trolled (hemoglobin A1C level of 6%) with no evidence of retinopathy or nephropathy. A sestamibi parathyroid scan localized a parathyroid adenoma at the mid pole of the left lobe of the thyroid gland. The patient was sched- uled for a PTH directed left parathyroidectomy under local anesthesia. Pre-operative PTH level was 147 pg/ml (normal range; 10 - 55 pg/ml). At exploration a large cy- stic structure was identified on the anterior surface of the left lower thyroid lobe which obscured the surgical field. The procedure was converted to general anesthesia and exploration was expanded to the upper pole of the thy- roid where an enlarged left superior parathyroid gland was identified and removed. On pathologic review the gland was hypercellular and weighed 286 mg. A post- excision intact PTH level measured 15 minutes after ex- cision remained high (89 pg/ml), and a four-gland ex- ploration of the neck was performed. Enlarged right su- perior (866 mg) and inferior parathyroid glands (256 mg) were identified and removed. The left inferior parathy- roid was partially resected and intraoperative pathologic evaluation identified this gland as also hypercellular. The remaining portion of the left inferior parathyroid was autotransplanted into the left sternocleidomastoid muscle. The post-operative course was uncomplicated and the patient was discharged home on day 2 with a serum cal- cium level of 9.1 mg/dl and a PTH level of 15.8 pg/mL. 3. Discussion A causal association between chronic lithium therapy and hyperparathyroidism has been conclusively demon- strated. As noted earlier, patients on long-term lithium therapy have a 4 - 6 fold increased incidence of hyper- parathyroidism compared to the general population [5,7]. Bendz et al. identified a 2.7% increased point prevalence of hyperparathyroidism among 124 patients on long-term lithium therapy (>15 years), while Mallette et al. revi- ewed studies involving 309 patients on chronic lithium therapy and identified 37 patients (12%) with hypercal- cemia and 18 patients (16%) with elevated PTH levels [8-10]. McHenry et al. have reported that 80% of pa- tients treated with lithium for 6 to 24 months experience a 10% increase in serum calcium levels. Christiansen et al. re- ported that both serum calcium and PTH levels increased by 30% in the same population, and several other authors have also reported a 10% - 60% increased prevalence of hypercalcemia and hyperparathyroidism in patients taking lithium [11-14]. LAH is associated with characteristic biochemical changes consisting of an elevated serum levels of calci- um, magnesium and parathyroid hormone and decreased urinary calcium and cyclic adenosine monophosphate (CAMP) levels. These changes are similar to familial hypocalciuric hypercalcemia (FHH), but distinct from PHPT in which urinary calcium excretion is increased Copyright © 2011 SciRes. SS  U. K BALLEHANINNA ET AL.471 [15]. A large number of mechanisms have been proposed to account for the biochemical changes seen in LAH. (Table 3) To briefly summarize, lithium competitively antagonizes calcium sensing receptors (CaSR) and raises the threshold of serum calcium necessary to inhibit PTH secretion. This action not only increases parathyroid hor- mone secretion, but also exerts a multitude of systemic effects on the parathyroid glands, renal tubules and bone metabolism resulting in parathyroid gland hyperplasia and biochemical changes. Lithium is also known to ac- centuate adenoma formation by increasing PTH gene transcription, which may unmask hyperparathyroidism in patients with sub-clinical pre-existing adenomas once lithium is initiated. As a consequence of the aforementioned systemic ef- fects, it seems reasonable to conclude that lithium use should be associated with multiglandular parathyroid hy- perplasia. However in most series, a solitary parathyroid adenoma was the most common LAH associated pathol- ogy. Awad et al. reported parathyroid adenomas in 14 out of 15 patients (93%) with LAH, and Carchman et al. identified adenomas in 12 out of 16 LAH patients (75%) [5,16]. Similarly, in a series of 12 LAH patients, Hun- dley et al. noted parathyroid adenomas in 9 patients (75%), while Bendz et al. reported adenoma in 72.5% of LAH patients (5 out of 8) [8,18]. Of note however, many au- thors have also described a higher incidence of multi- glandular hyperplasia. Nordenstrom et al. identified mul- tiglandular hyperplasia (MGH) in 83% of LAH patients (5 out of 6) [19]. Likewise, Jarhult et al. studied 71 pa- tients with LAH and reported MGH in 37 patients (52%) [14]. Table 1 summarizes all reported LAH cases and demonstrates that parathyroid adenoma was the most common pathology identified in 104 out of 170 patients (61%) following parathyroidectomy [3,5,8,23-26]. (Ta- ble 1) In comparison, PHPT is associated with a single adenoma 85%, multigland hyperplasia (MGH) 15% and parathyroid carcinoma in 1% of patients [7]. It is impor- tant to point out that there is also an increased incidence rate of multigland hyperplasia (MGH) in LAH (39%, 66 of 170 patients) compared to the general population with PHPT (15%). This increased MGH incidence is likely attributable to the systemic action of lithium, and as a result may effect interpretation of localization studies (increased false negative rate, poor sensitivity) and sur- gical planning (increased need of bilateral neck explora- tion) [3,4,6,18]. The initial management of LAH is medical intervene- tion which includes discontinuation of lithium or alterna- tive treatment such as atypical antipsychotics and cal- cimimmetics. (Table 2) Data relating to the efficacy of LAH medical management is available for only 10 of 194 reported LAH patients (5%). As such it is difficult to make any meaningful conclusions [12,23-26,34]. In many LAH patients, discontinuation of lithium carbonate is not medically feasible due to increased propensity of relapse [35,36]. Although serum Ca2+ and PTH levels tend to normalize within 4 - 6 weeks after lithium dis- continuation, this change is mostly seen in patients re- ceiving lithium for a relatively short-term defined as 5 - 10 years, or in those with only mild hypercalcemia [4]. Khandawala et al. reported on one patient who was tak- ing lithium for 5 years, discontinuation of Li+ resulted in normocalcemia and a normal PTH level which was maintained at 5 months follow-up [24]. Dugal et al. re- ported on a single patient who had been on lithium ther- apy for 10 years, in whom discontinuation of lithium and simultaneous saline diuresis with furosemide also achi- Table 3. Proposed mechanisms of action responsible for Lithium associated hyperparathyroidism (LAH). Mechanism of action Explanatation Antagonism of cations [28,29] Li+ competitively antagonizes cations at calcium receptor at several locations—kidney, bone and parathyroid gland—causing acute hypercalcemia and hyper-magnesemia ↑ Set point or reset “calci-stat” [4,12,30, 31] Li+ antagonizes calcium sensing receptor (CASR) causing a shift in inhibitory set point for PTH secretion, which then requires a higher levels of calcium level to block PTH secretion. This results in an overproduction of PTH despite elevated serum calcium levels Mitogenic action [16,32,33] Li+ exacerbates a pre-existing adenoma or PHPT—this occurs especially in patients presenting with psychiatric symptoms—the lithium therapy worsens hypercalcemia by promoting PTH overproduc- tion. Nearly 55% of LAH have parathyroid adenomas (single or double). ↑ Parathyroid volume and parathyroid hyperplasia [2,5,11] LAH is associated with multiglandular hyperplasia in 25% - 55% cases (Table 1) compared to 15% in PHPT, which is attributed to direct stimulation of parathyroid gland by lithium. In patients on chronic lithium therapy neck ultrasound demonstrates increased parathyroid gland volume. Mechanism: 1) Inhibits inosital monophosphate (IMP) which regulates intracellular calcium exposure to nuclear CASR in PTH cells, thus altering the set point to turn-off PTH gene transcription. 2) Li+ inhibits glycogen synthase kinase-3b (GSK-3b) responsible for inhibition of PTH gene tran- scription, thereby leading to overproduction of PTH. Abbreviations: PHPT-primary hyperparathyroidism; PTH, parathyroid hormone; Li+, lithium; CASR, calcium sensing receptor; IMP, inosital monophosphate; GSK-3b, glycogen synthase kinase-3b. Note: Several different mechanisms have been proposed by which lithium may cause hypercalcemia, ↑ PTH levels, ↑ magnesium and ↓ urinary calcium levels. Copyright © 2011 SciRes. SS  U. K BALLEHANINNA ET AL. Copyright © 2011 SciRes. SS 472 eved normocalcemia [26]. In contrast, drug withdrawal from patients on chronic lithium therapy (>10 years), has a lower rate of hypercalcemia resolution [4]. Carchman et al. noted that even after discontinuation of lithium for more than 5 months, 8 out of 16 patients on chronic lith- ium therapy experienced persistent hypercalcemia [16]. In LAH patients who have mild hypercalcemia or were asymptomatic, alternative medical therapy such as val- proate, carabamazepine or atypical antipsychotics (rise- peridone) may be treatment alternatives, however at pre- sent there is very limited data to assess their efficacy in LAH patients [2,4]. Recently, a new class of drugs (calcimimmetics, ex- ample: cinacalcet) has been successfully used to treat LAH with or without discontinuation of lithium [34]. (Table 3) Calcimimmetics activate calcium-sensing re- ceptors (blocked by lithium) which in turn reduce PTH secretion and prevent parathyroid hyperplasia. To date, a biochemical remission was achieved in 6 cases using cinacalcet among patients on chronic lithium therapy [12, 23,25,34]. Gregoor et al. reported resolution of LAH in 3 patients using cinacalcet while lithium was continued [34]. Szalat et al. used cinacalcet in an LAH patient who had a post-operative recurrence of hyperparathyroidism and reported resolution of hypercalcemia despite persis- tently elevated PTH levels [12]. Although above results are intriguing, the consistently reproducible results of parathyroidectomy have made surgical therapy the main- stay of LAH management, particularly among sympto- matic patients with moderate to severe hypercalcemia, and those in whom discontinuation of Li+ is not feasible or has failed [4]. The most common indication for parathyroidectomy in LAH patients include worsening psychosomatic (50% - 60%) (fatigue, weakness, decreased concentration, short- term memory loss, depression), bone (30% - 40%) (bone pain, reduce bone mineral density, osteopenia or osteo- porosis), gastrointestinal symptoms (20% - 30%) (con- stipation, nausea or pancreatitis), cardiac dysrhythmias and renal symptoms (1% - 5%) (decreased GFR, decreased creatinine clearance or nephrocalcinosis) [3,5,12,15,18]. Today, preoperative localization studies (ultrasound of neck, sestamibi scan and computerized tomography (CT) scan of neck) have been routinely incorporated into the management algorithm of PHPT patients, and resulted in the near universal use of focused neck exploration (90% - 95%) for this group. However, there is a paucity of data supporting the utility of preoperative localization studies in LAH patients. Among 195 LAH patients who under- went parathyroidectomy, preoperative localization stu- dies were utilized in only 22 patients (13%). In the larg- est series involving 18 patients, Carchman et al. used se- stamibi scan or ultrasound in 16 patients (94%). Imaging studies predicted a single adenoma in 10 patients, 2 (20%) of which had MGH at exploration [19]. In the same study, pre-operative localization studies identified MGH in 4 patients, however 2 of these patients subsequently were found to have only a single adenoma at exploration. Li- mited or focused neck exploration was possible in only 8 of 16 patients (50%) aided by both pre-operative loca- lization studies and IOPTH monitoring. Szalat et al. have also reported using preoperative neck ultrasound and parathyroid scan which identified solitary uptake in 3 of 4 cases. However, at exploration IOPTH testing failed to return to baseline or decrease by >50% (positive re- sponse), and all patients required bilateral neck explora- tion (BNE) [12]. Despite the small number of patients included in these studies, they nevertheless highlight the poor sensitivity and limited utility of pre-operative loca- lization studies for precise localization of pathogically enlarged parathyroid glands in LAH and in planning sur- gical approach [6]. Parathyroidectomy is the main stay of treatment for LAH patients. Given the reported higher incidence of multiglandular hyperplasia (39%) and double adenomas (7%), it is not surprising that bilateral neck exploration (BNE) is the most commonly reported surgical approach in LAH. Among 170 patients with LAH, BNE was used in 162 patients (95%), whereas focused neck exploration was performed in only 8 patients (5%). In the largest reported LAH series, Jarhult et al. utilized BNE in all but 2 of 71 patients who underwent parathyroidectomy [14]. As noted earlier, intraoperative parathyroid hormone monitoring (IOPTH) has been extensively validated in patients with PHPT, and prompted more limited surgical exploration [7]. To date, only 3 studies involving LAH patients (N = 32) have assessed the usefulness of IOPTH monitoring [12,18,19]. Hundley et al. utilized IOPTH monitoring in 12 patients with LAH, of which 6 patients underwent MGH resection prompted by IOPTH moni- toring [18]. In a study of 16 patients, Carchman et al. performed focused parathyroidectomy in 8 patients, while 8 patients underwent BNE guided by IOPTH mo- nitoring [19]. Of note, these authors were able to perform a limited surgical exploration in 50% of LAH patients, compared to a 5% rate in other LAH series [12,14]. Parathyroidectomy results in biochemical remission leading to normocalcemia and normal PTH levels in the immediate post-operative period and improves psycho- somatic symptoms in 90% - 97% of LAH patients. Awad et al. observed eucalcemia in 15 out of 16 patients who had parathyroidectomy for LAH; however, one patient subsequently developed recurrence at 2 years and under- went neck re-exploration with removal of an additional parathyroid adenoma [5]. At a median follow-up of 6 months (5 - 50 months range), Carchman et al. noted that 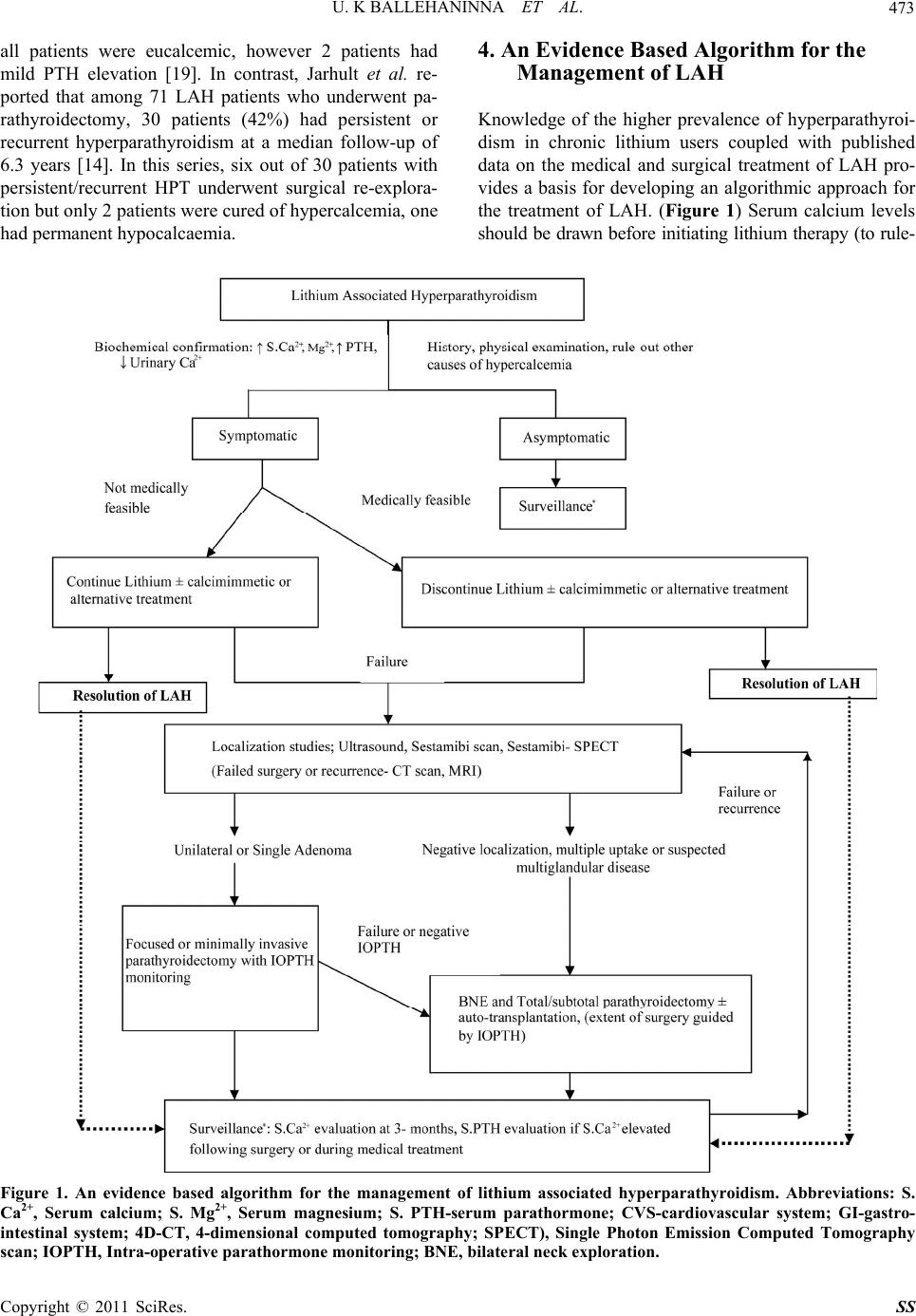 U. K BALLEHANINNA ET AL.473 all patients were eucalcemic, however 2 patients had mild PTH elevation [19]. In contrast, Jarhult et al. re- ported that among 71 LAH patients who underwent pa- rathyroidectomy, 30 patients (42%) had persistent or recurrent hyperparathyroidism at a median follow-up of 6.3 years [14]. In this series, six out of 30 patients with persistent/recurrent HPT underwent surgical re-explora- tion but only 2 patients were cured of hypercalcemia, one had permanent hypocalcaemia. 4. An Evidence Based Algorithm for the Management of LAH Knowledge of the higher prevalence of hyperparathyroi- dism in chronic lithium users coupled with published data on the medical and surgical treatment of LAH pro- vides a basis for developing an algorithmic approach for the treatment of LAH. (Figure 1) Serum calcium levels should be drawn before initiating lithium therapy (to rule- Figure 1. An evidence based algorithm for the management of lithium associated hyperparathyroidism. Abbreviations: S. Ca2+, Serum calcium; S. Mg2+, Serum magnesium; S. PTH-serum parathormone; CVS-cardiovascular system; GI-gastro- intestinal system; 4D-CT, 4-dimensional computed tomography; SPECT), Single Photon Emission Computed Tomography scan; IOPTH, Intra-operative parathormone monitoring; BNE, bilateral neck exploration. Copyright © 2011 SciRes. SS  U. K BALLEHANINNA ET AL. Copyright © 2011 SciRes. SS 474 out pre-existent primary HPT) and monitored within 4 - 6 weeks of beginning lithium therapy, and every 3 months thereafter. Serum PTH levels should be measured when- ever hypercalcemia is identified. A confirmatory diagno- sis of LAH necessitates ruling-out other causes of HPT. Asymptomatic patients with mild to moderate hypercal- cemia may be monitored at regular intervals. The Natio- nal Institute of Health (NIH) guidelines for the treatment of PHPT are also useful in LAH patients in regards to pa- tient selection for parathyroidectomy [37]. (Figure 1) When medically feasible lithium should be discontinued or alternative therapy substituted (valproate, carbamaze- pine or atypical antipsychotics) in selected LAH patients. Cinacalcet hydrochloride, a calcimimmetic agent, may have modest efficacy in treating LAH, and be beneficial to patients in whom discontinuation of lithium is not me- dically feasible or when surgery is contraindicated. In the vast majority of symptomatic patients, and in those in whom either discontinuation of lithium is not possible or when alternative therapy has failed to correct hypercal- cemia, parathyroidectomy is required. While preopera- tive localization studies may provide useful information regarding the number and location of enlarged parathy- roid glands, the increased incidence of multiglandular disease in LAH, as well as a higher risk of recurrent or persistent HPT, mandates meticulous surgical explora- tion to visualize and remove all pathologically enlarged gland(s). Whereas a single parathyroid adenoma is found in the majority (61%) of LAH patients, limited or fo- cused surgical exploration may be attempted when pre- operative localization studies suggest feasibility, but must be further confirmed with IOPTH monitoring. A very high incidence of persistent and recurrent disease in LAH patients necessitates long-term follow-up and strict surveillance (Figure 1). In summary, lithium use in psychiatric patients may be associated with hypercalcemia and elevated PTH levels referred to as lithium associated hyperparathyroidism (LAH). An algorithmic approach and strict post-opera- tive surveillance protocols may improve treatment suc- cess and identify recurrent or persistent disease (Figure 1). 5. References [1] A. M. Nivoli, A. Murru and E. Vieta, “Lithium: Still a Cornerstone in the Long-Term Treatment in Bipolar Dis- order?” Neuropsychobiology, Vol. 62, No. 1, 2010, pp. 27-35. doi:10.1159/000314307 [2] W. Coryell, “Maintenance Treatment in Bipolar Disorder: A Reassessment of Lithium as the First Choice,” Bipolar Disorders, Vol. 11, No. 2, 2009, pp. 77-83. doi:10.1111/j.1399-5618.2009.00712.x [3] C. McHenry and K. Lee, “Lithium Therapy and Disorders of the Parathyroid Glands,” Endocrine Practice, Vol. 2, No. 2, 1996, pp. 103-109. [4] B. D. Saunders, E. F. Saunders and P. G. Gauger, “Lith- ium Therapy and Hyperparathyroidism: An Evidence- Based Assessment,” World Journal of Surgery, Vol. 33, No. 11, 2009, pp. 2314-2323. doi:10.1007/s00268-009-9942-4 [5] S. S. Awad, J. Miskulin and N. Thompson, “Parathyroid Adenomas versus Four-Gland Hyperplasia as the Cause of Primary Hyperparathyroidism in Patients with Pro- longed Lithium Therapy,” World Journal of Surgery, Vol. 27, No. 4, 2003, pp. 486-488. doi:10.1007/s00268-002-6824-4 [6] N. Perera, L. Gluch and B. A. Crawford, “Misleading Parathyroid Sestamibi Scan in Lithium Users,” Internal Medicine Journal, Vol. 39, No. 8, 2009, pp. 556-557. doi:10.1111/j.1445-5994.2009.01972.x [7] J. I. Lew and C. C. Solorzano, “Surgical Management of Primary Hyperparatyroidism: State of the Art,” Surgical Clinics of North America, Vol. 89, No. 5, 2009, pp. 1205-1225. doi:10.1016/j.suc.2009.06.014 [8] H. Bendz, I. Sjödin, G. Toss and K. Berglund, “Hyper- parathyroidism and Long-Term Lithium Therapy: A Cross-Sectional Study and the Effect of Lithium With- drawal,” Journal of Internal Medicine, Vol. 240, No. 6, 1996, pp. 357-365. doi:10.1046/j.1365-2796.1996.28864000.x [9] L. E. Mallette, K. Khouri, H. Zengotita, B. W. Hollis and S. Malini, “Lithium Treatment Increases Intact and Midregion Parathyroid Hormone and Parathyroid Vol- ume,” The Journal of Clinical Endocrinology & Metabo- lism, Vol. 68, No. 3, 1989, pp. 654-660. doi:10.1210/jcem-68-3-654 [10] P. E. Garfinkel, C. Ezrin and H. C. Stancer, “Hypothy- roidism and Hyperparathyroidism Associated with Lith- ium,” Lancet, Vol. 2, 1973, pp. 331-332. doi:10.1016/S0140-6736(73)90846-5 [11] C. Christiansen, P. C. Baastrup and I. Transbol, “Devel- opment of Primary Hyperparathyroidism during Lithium Therapy: Longitudinal Study,” Neuropsychobiology, Vol. 6, No. 5, 1980, pp. 280-283. doi:10.1159/000117770 [12] A. Szalat, H. Mazeh and H. R. Freund, “Lithium-Associ- ated Hyperparathyroidism: Report of Four Cases and Re- view of the Literature,” European Journal of Endocri- nology, Vol. 160, No. 2, 2009, pp. 317-323. doi:10.1530/EJE-08-0620 [13] C. R. McHenry, F. Racke, M. Meister, P. Warnaka, M. Sarasua, E. F. Nemeth, et al., “Lithium Effects on Dis- persed Bovine Parathyroid Cell Grown in Tissue Cul- ture,” Surgery, Vol. 110, No. 6, 1991, pp. 1061-1066. [14] J. Järhult, S. Ander, B. Asking, S. Jansson, A. Meehan, A. Kristoffersson, et al., “Long-Term Results of Surgery for Lithium-Associated Hyperparathyroidism,” British Jour- nal of Surgery, Vol. 97, No. 11, 2010, pp. 1680-1685. [15] W. Khairallah, A. Fawaz, E. M., Brown and G. El-Hajj Fuleihan, “Hypercalcemia and Diabetes Insipidus in a Pa- tient Previously Treated with Lithium,” Nature Clinical  U. K BALLEHANINNA ET AL.475 Practice Nephrology, Vol. 3, No. 7, 2007, pp. 397-404. doi:10.1038/ncpneph0525 [16] E. Carchman, J. Ogilvie, J. Holst, J. Yim and S. Carty, “Appropriate surgical Treatment of Lithium-Associated Hyperparathyroidism,” World Journal of Surgery, Vol. 32, No. 10, 2008, pp. 2195-2199. doi:10.1007/s00268-008-9616-7 [17] H. Abdullah, R. Bliss, A. I. Guinea and L. Delbridge, “Pathology and Outcome of Surgical Treatment for Lith- ium-Associated Hyperparathyroidism,” British Journal of Surgery, Vol. 86, No. 1, 1999, pp. 91-93. doi:10.1046/j.1365-2168.1999.00977.x [18] J. C. Hundley, D. T. Woodrum, B. D. Saunders, G. M. Doherty and P. G. Gauger, “Revisiting Lithium-Associ- ated Hyperparathyroidism in the Era of Intraoperative Parathyroid Hormone Monitoring,” Surgery, Vol. 138, No. 6, 2005, pp. 1027-1031. doi:10.1016/j.surg.2005.09.028 [19] J. Nordenström, M. Elvius, M. Bågedahl-Strindlund, B. Zhao and O. Törring, “Biochemical Hyperparathyroidsm and Bone Mineral Status in Patients Treated Long-Term with Lithium,” Metabolism, Vol. 43, No. 12, 1994, 1563- 1567. doi:10.1016/0026-0495(94)90017-5 [20] J. Ananth and S. E. Dubin, “Lithium and Symptomatic Hyperparathyroidism,” Journal of the Royal Society of Medicine, Vol. 76, No. 12, 1983, pp. 1026-1029. [21] M. E. Wolf, M. Moffat, J. Mosnaim and S. Dempsey, “Lithium Therapy, Hypercalcemia, and Hyperparathy- roidism,” American Journal of Therapeutics, Vol. 4, No. 9-10, 1997, pp. 323-325. doi:10.1097/00045391-199709000-00007 [22] G. de Celis, M. Fiter, X. Latorre, and C. Llebaria, “Oxy- philic Parathyroid Adenoma and Lithium Therapy,” Lan- cet, Vol. 352, No. 9133, 1998, p. 1070. doi:10.1016/S0140-6736(05)60117-1 [23] J. A. Sloand, M. A and M. A. Shelly, “Normalization of Lithium-Induced Hypercalcemia and Hyperparathyroid- ism with Cinacalcet Hydrochloride,” America n J ournal of Kidney Diseases, Vol. 48, No. 5, 2006, pp. 832-837. doi:10.1053/j.ajkd.2006.07.019 [24] H. M. Khandwala and S. Van Uum, “Reversible hyper- calcemia and Hyperparathyroidism Associatead with Lithiuim Therapy: Case Report and Review of Litera- ture,” Endocrine Practice, Vol. 12, No. 1, 2006, pp. 54- 58. [25] M. Rifai, J. K., Moles and D. P. Harrington, “Lithium- Induced Hypercalcemia and Parathyroid Dysfunction,” Psychosomatics, Vol. 42, No. 4, 2001, pp. 359-361. doi:10.1176/appi.psy.42.4.359 [26] H. S. Duggal and I. Singh, “Lithium-Induced Hypercal- cemia and Hyperparathyroidism Presenting with Delir- ium,” Prog Neuropsychopharmacol Biol Psychiatry, Vol. 32, No. 3, 2008, pp. 903-904. doi:10.1016/j.pnpbp.2007.12.014 [27] M. M. Rizwan and N. D. Perrier, “Long-Term Lithium Therapy Leading to Hyperparathyroidism: A Case Re- port,” Perspect Psychiatr Care, Vol. 45. No. 1, 2009, pp. 62-65. [28] S. Carney and P. Jackson, “Acute Lithium Administration Impairs the Action of Parathyroid Hormone on Rat Renal Calcium Magnesium and Phosphate Transport,” Clinical and Experimental Pharmacology and Physiology, Vol. 25, No. 10, 1998, pp. 795-799. doi:10.1111/j.1440-1681.1998.tb02155.x [29] D. Riccardi and G. Gamba, “The Many Roles of the Cal- cium-Sensing Receptor in Health and Disease,” Archives of Medical Research, Vol. 30, No. 6, 1999, pp. 436-448. doi:10.1016/S0188-4409(99)00071-5 [30] C. Livingstone and H. Rampes, “Lithium: A Review of Its Metabolic Adverse Effects,” Journal of Psychophar- macology, Vol. 20, No. 3, 2006, pp. 347-355. doi:10.1177/0269881105057515 [31] F. H. Shen and D. J. Sherrard, “Lithium-Induced Hyper- parathyroidism: An Alteration of the ‘Set Point’,” Annals of Internal Medicine, Vol. 96, No. 1, 1982, pp. 63-65. [32] E. M. Grandiean and J. M. Aubry, “Lithium: Updated Human Knowledge Using an Evidence-Based Approach: Part III: Clinical Safety,” CNS Drugs, Vol. 23, No. 5, 2009, pp. 397-418. doi:10.2165/00023210-200923050-00004 [33] A. W. Saxe and G. Gibson, “Lithium Increases Tritiated Thymidine Uptake by Abnormal Human Parathyroid Tissue,” Surgery, Vol. 110, No. 6, 1991, pp. 1067-1076 [34] P. S. Gregoor and G. M. de Jong, “Lithium Hypercalce- mia, Hyperparathyroidism, and Cinacalcet,” Kidney Int, Vol. 71, No. 5, 2007, p. 470. doi:10.1038/sj.ki.5002065 [35] L. A. Smith, V. Cornelius, A. Warnock, A. Bell and A. H. Young, “Effectiveness of Mood Stabilizers and Antipsy- chotics in the Maintenance Phase of Bipolar Disorder: A Systematic Review of Randomized Controlled Trials,” Bipolar Disorders, Vol. 9, No. 4, 2007, pp. 394-412. doi:10.1111/j.1399-5618.2007.00490.x [36] A. H. Young and J. M. Hammond, “Lithium in Mood Disorders: Increasing Evidence Base, declining Use?” The British Journal of Psychiatry, Vol. 191, 2007, pp. 474-476. doi:10.1192/bjp.bp.107.043133 [37] M. A. Kouvaraki, M. Greer, S. Sharma, D. Beery, R. Armand, J. E. Lee, et al., “Indications for Operative In- tervention in Patients with Asymptomatic Primary Hy- perparathyroidism: Practice Patterns of Endocrine Sur- gery,” Surgery, Vol. 139, No. 4, 2006, pp. 527-534. doi:10.1016/j.surg.2005.09.006 Copyright © 2011 SciRes. SS
|