 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 500-509 doi:10.4236/jbnb.2011.225061 Published Online December 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes for Protein Delivery (A Review Article) Masayuki Ishihara1*, Satoko Kishimoto1,2, Megumi Takikawa3, Yasutaka Mori1,4, Shingo Nakamura5, Masanori Fujita1 1Research Institute, National Defense Medical College, Tokorozawa, Japan; 2Research Fellow of the Japan Society for the Promotion of Science, Tokyo, Japan; 3Department of Plastic Surgery, National Defense Medical College, Tokorozawa, Japan; 4Aeromedical Laboratory, Japan Air Self-Defense Force, Sayama, Japan; 5Department of Surgery, National Defense Medical College, Tokoro- zawa, Japan. E-mail: *ishihara@ndmc.ac.jp Received October 1st, 2011; revised November 14th, 2011; accepted November 28th, 2011. ABSTRACT We produced low-molecular-weight heparin/protamine micro (nano) particles (LMW-H/P MPs·NPs) as a carrier for heparin-binding growth factors (GFs), such as fibroblast growth factor (FGF)-2 and various GFs in platelet-rich plasma (PRP). A mixture of LMW-H (MW: approximately 5000 Da, 6.4 mg/ml) and protamine (MW: approximately 3000 Da, 10 mg/ml) at a ratio of 7:3 (vol:vol) yields a dispersion of micro (nano) particles (200 nm - 3 µm in diameter). The diluted LMW-H solution in saline (0.32 mg/ml) mixed with diluted protamine (0.5 mg/ml) at a ratio at 7:3 (vol:vol) resulted in soluble nanoparticles (approximately 100 nm in diameter). The generated NPs could be then stabilized by adding 2 mg/ml dextran (MW: 178-217 kDa) and remained soluble after lyophilization of dialyzed LMW-H/P NPs so- lution. The LMW-H/P MPs·NPs adsorb GFs, control their release, protect GFs and activate their biological activities. Furthermore, administration of GFs-containing F/P MPs·NPs exhibited significantly higher inductions of vasculariza- tion and fibrous tissue formation in vivo than GFs alone. LMW-H/P MPs·NPs can also efficiently bind to tissue culture plates and retain the binding of GFs. The LMW-H/P MPs·NP-coated matrix with various GFs or cytokines provided novel biomaterials that could control cellular activity such as proliferation and differentiation. Thus, LMW-H/P MPs·NPs are an excellent carrier for GFs and are a functional coating matrix for various kinds of cell cultures. Keywords: Polyelectrolyte Complexes, Nanoparticles, Heparin-Binding Growth Factors, Platelet-Rich Plasma, Drug Delivery 1. Introduction Polyelectrolyte complexes (PECs) are produced by elec- trostatic interactions between oppositely charged polye- lectrolytes. When this interaction occurs at non-equiva- lent ratios, nonstoichiometric PECs are produced, caus- ing each PECs to carry an excess charge. Proteins inter- act with both synthetic and natural PECs [1,2]. These binding characteristics, along with a simple preparation, allow PECs to be an excellent model for studying the in vivo behavior of charged biopolymers as well as having potential applications in medicine and biotechnology [3]. Reported data indicate that polyanions and polycations can bind to proteins below and above their isoelectric points, respectively. These interactions can result in so- luble complexes, complex coacervation and/or the for- mation of amorphous precipitates [1,2]. Main aspects studied by different authors are compositions of PECs obtained under various experimental conditions, such as the strength and position of ionic sites, charge density, and rigidity of polymer chains as well as chemical prop- erties such as solubility, pH, temperature, and concentra- tion [3]. Those electrostatic interactions are also important be- cause of their similarity to biological systems [4]. Inter- actions between proteins and nucleic acids, for example, play a role in the transcription process [1]. DNA/chitosan PECs [5], chitosan/chondroitin sulfate PECs and chito- san/hyaluronate PECs [6] were described as gene and drug micro-carriers. Moreover, PECs that are insoluble  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 501 for Protein Delivery (A Review Article) also have potential applications as membranes, micro- capsules, micro (nano) particles, and scaffolds for tissue engineering [7]. Basic protamine molecules complexed with acidic mo- lecules such as heparin form a microcomplexes through ionic interactions. We previously have reported the a low-molecular-weight heparin/protamine micro (nano) particles (LMW-H/P MPs·NPs) which we originally pre- pared as PECs [8,9]. LMW-H/P MPs·NPs are specifi- cally bound to FGF-2 and other various heparin-binding growth factors (GFs) in platelet-rich plasma (PRP) [10] through interaction between LMW-H molecules in LMW- H/P MPs·NPs and those GFs. LMW-H/P MPs·NPs are able to protect those GFs from heat and proteolytic inac- tivation and to enhance those biological activities. Since those GFs-containing LMW-H/P MPs·NPs are 200 nm - 3 µm in diameter, those can be easily injected [8-10]. Moreover, the GFs-containing the LMW-H/P MPs·NPs showed a substantial effect to induce vascularization and fibrous tissue formations due to stabilizating, activating, and gradually releasing GFs molecules from GFs-con- taining the LMW-H/P MPs·NPs [8-10]. In this review articles, we described on the LMW-H/P MPs·NPs which we originally prepared as PECs, its characterizations and its potential medical applications as carriers for GFs such as FGF-2 and GFs in PRP. Fur- thermore, as a coating matrix, LMW-H/P MPs·NPs were efficiently bound to tissue culture plates. With the ability of LMW-H/P MPs·NPs to retain GFs, the LMW-H/P MPs·NPs could serve as a useful coating matrix for cul- ture of various types of cells. 2. Preparation of LMW-H/P MPs·NPs Heparin interacts with a variety of functional proteins, including heparin-binding growth factors (GFs), cyto- kines, extracellular matrix components, and adhesion molecules [11-13]. Thus, heparin may be useful as a the- rapeutic agent in various pathological conditions that involve functional proteins. However, high-dose heparin cannot be used because of the excessive risk of bleeding [14]. In contrast, low-molecular-weight heparin (LMW- H, MW: approximately 5000 Da) which has much lower anti-coagulant activity, has pharmacological and practi- cal advantages compared with native heparin. The lower protein binding activity of LMW-H produces a low, sta- ble, and predictable anticoagulant response, thereby by- passing the need for laboratory monitoring of drug levels to adjust the dosage [14]. In addition, one or two subcu- taneous injections per day are sufficient to maintain therapeutic concentrations because of its longer plasma half-life [14]. On the other hand, protamine, a purified mixture of proteins obtained from fish sperm, neutralizes heparin and LMW-H by forming a stable complex that lacks an- ticoagulant activity [15]. Protamine is also in clinical use to reverse the anticoagulant activity of heparin following cardiopulmonary bypass as well as in cases of heparin- induced bleeding [16]. We previously prepared water- insoluble particles (>10 µm in diameter) by mixing non- anticoagulant heparin with chitosan. We then mixed fuc- oidan with chitosan and investigated the ability of the resulting insoluble fucoidan/chitosan microparticles to protect fibroblast growth factor-2 (FGF-2) activity [17, 18]. We also prepared water-insoluble micro (nano) par- ticles (200 nm - 3 µm in diameter) by mixing LMW-H (6.4 mg/ml) with protamine (10 mg/ml) at a ratio of 7:3 (vol:vol), and reported the ability of the resulting in- jectable low-molecular-weight heparin/protamine micro (nano) particles (LMW-H/P MPs·NPs) to protect FGF-2 activity (see Figure 1) [8,9]. Furthermore, GFs released from platelets that were involved in cell proliferation, migration, and angiogenesis were able to adsorb onto LMW-H/P MPs·NPs [10]. In order to produce of the nanoparticles, equally di- luted LMW-H and protamine (100-fold, 50-fold, and 20-fold diluted) were mixed in a ratio at 7:3 (vol:vol) in this study (see Figure 2). The diameter of generated LMW-H/P NPs by mixing 100-fold, 50-fold, and 20-fold diluted protamine to equally diluted LMW-H in the ratio of 3:7 (vol:vol) were 84.6 ± 26.8, 95.0 ± 27.0, and 112.5 ± 46.1 nm, respectively (see Figure 2) [9]. And no mi- croparticles (>1 µm in diameter) were observed in the mixtures. In contrast, generations of small amount of microparticles (approximately 1 µm in diameter) were observed by mixing of 10-fold diluted protamine (1 mg/ml) to PMW-H (0.64 mg/ml) in the ratio of 3:7 (vol/vol). When non-diluted protamine (10 mg/ml) was added to non-diluted LMW-H (6.4 mg/ml) up to ratio of 3:7 (vol:vol), maximal LMW-H/P MPs (200 nm - 3 µm in diameter) was produced and the high turbidity was observed. When 10-fold concentrated protamine (100 mg/ml) was added to the equally 10-fold concentrated LMW-H (64 mg/ml) up to ratio of 3:7 (vol:vol), mixtures of larger LMW-H/P MPs (3 - 10 µm in diameter) and larger cotton-like precipitates (>10 µm) were immedi- ately generated and those products were insoluble [9]. An illustration on generations of micro/nanoparticles and insoluble precipitates by mixing protamine to LMW-H in various concentrations was shown in Figure 3. Cotton-like compounds were generated after lyophili- zations of both LMW-H/P MPs·NPs (200 nm - 3 µm in diameter) and LMW-H/P NPs (approximately 100 nm in diameter) solutions without dextran, and they were hardly re-soluble in water. However, both the freeze- Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes for Protein Delivery (A Review Article) Copyright © 2011 SciRes. JBNB 502 Figure 1. Preparation of LMW-H/P MPs·NPs. Figure 2. LMW-H/P NPs by mixing diluted protamine and LMW-H.  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 503 for Protein Delivery (A Review Article) Figure 3. Generation of LMW-H/P MPs·NPs as PECs. dried LMW-H/P MPs·NPs were easily dessolved in wa- ter by adding 0.5% and 0.2% dextran, respectively, be- fore their lyophilizations. In addition, aggregation of LMW-H/P NPs in solution to LMW-H/P MPs was pro- hibited in the presence of dextran [9]. Thus, the addition of dextran is effective to stabilize the LMW-H/P MPs·NPs and to prepare stable and resoluble freeze-dry LMW-H/P MPs·NPs. As a coating matrix, LMW-H/P MPs·NPs were effi- ciently bound to tissue culture plates. With the ability of LMW-H/P MPs·NPs to adsorb and retain GFs, the LMW-H/P MPs·NPs could be very useful in various types of cell culture as a coating matrix. Human micro- vascular endothelial cells and human dermal fibroblast cells well adhered to LMW-H/P MPs·NPs-coated tissue culture plates [19] and grew optimally in low fetal bovine serum (FBS) (1% - 2%) medium supplemented with FGF-2 (5 ng/ml). This protocol could make it possible to use low autologous human serum (1% - 2%) for the cul- turing of human bone marrow-derived mesenchymal stem cells (BMSCs) and human adipose-derived stromal cells (ASCs) [20]. Furthermore, CD34+ hematopoietic progenitor cells (CD34+ cells) derived from human bone marrow exhibited a comparatively higher proliferation on LMW-H/P MPs·NPs-coated plates in hematopoietic pro- genitor growth medium (HPGM) supplemented with appropriate cytokines than those on uncoated plates [21]. 3. Protein-Delivery Micro (Nano) Particles 3.1. FGF-2 Containing LMW-H/P MPs·NPs FGF-2 binds heparin with high affinity (Kd of 8.6 × 10−9 M). The polysaccharides can prolong the biological half- life of FGF-2 as well as protect FGF-2 from heat, acid, and proteolytic inactivation [22]. Similarly, the LMW- H/P MPs·NPs have high affinity for FGF-2 (Kd = 2.4 × 10−9 M) [8], and this interaction of FGF-2 with the LMW-H/P MPs·NPs can substantially prolong the bio- logical half-life time of FGF-2. The protection of FGF-2 against heat inactivation and trypsin degradation by the LMW-H/P MPs was effective in a concentration-depen- dent manner [8]. FGF-2 molecules were released in vitro from the FGF-2-containing LMW-H/P MPs·NPs with half-releasing time of about 6 days. Those results dem- onstrated that FGF-2 molecules are bound and stabi- lized on the LMW-H/P MPs·NPs, and that the FGF-2 molecules incorporated into the LMW-H/P MPs·NPs will be gradually released upon biodegradation of the hy- drogel in vivo. When the FGF-2-containing LMW-H/P MPs·NPs were subcutaneously injected into the backs of mice, neovas- cularization was induced near the injection site after 3 days. Neovascularization induced by the FGF-2-con- taining LMW-H/P MPs·NPs reached a maximum at 1 week, after which a slight decrease in the neovasculari- zation rate occurred. No significant vascularization was Copyright © 2011 SciRes. JBNB 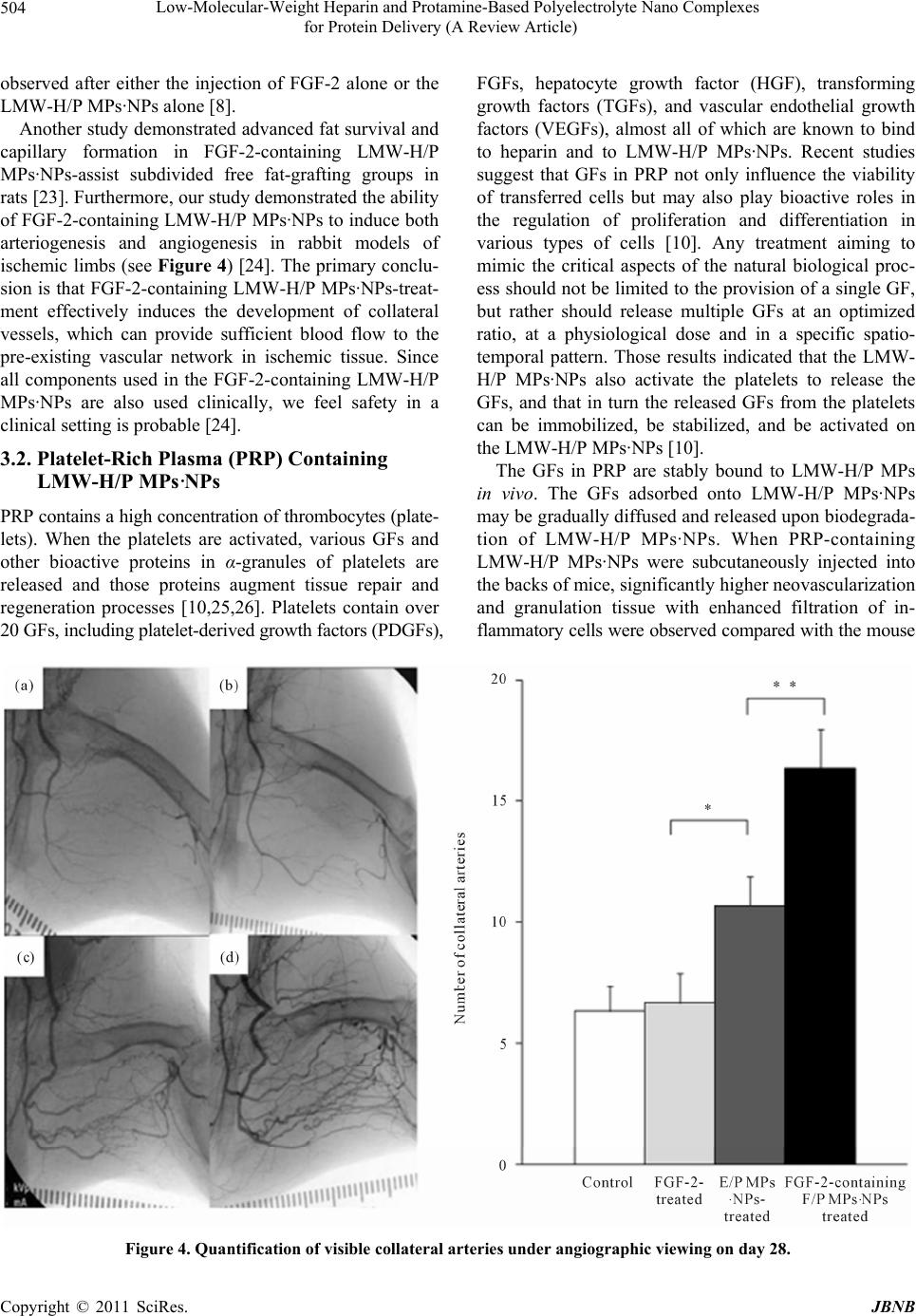 Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 504 for Protein Delivery (A Review Article) observed after either the injection of FGF-2 alone or the LMW-H/P MPs·NPs alone [8]. Another study demonstrated advanced fat survival and capillary formation in FGF-2-containing LMW-H/P MPs·NPs-assist subdivided free fat-grafting groups in rats [23]. Furthermore, our study demonstrated the ability of FGF-2-containing LMW-H/P MPs·NPs to induce both arteriogenesis and angiogenesis in rabbit models of ischemic limbs (see Figure 4) [24]. The primary conclu- sion is that FGF-2-containing LMW-H/P MPs·NPs-treat- ment effectively induces the development of collateral vessels, which can provide sufficient blood flow to the pre-existing vascular network in ischemic tissue. Since all components used in the FGF-2-containing LMW-H/P MPs·NPs are also used clinically, we feel safety in a clinical setting is probable [24]. 3.2. Platelet-Rich Plasma (PRP) Containing LMW-H/P MPs·NPs PRP contains a high concentration of thrombocytes (plate- lets). When the platelets are activated, various GFs and other bioactive proteins in α-granules of platelets are released and those proteins augment tissue repair and regeneration processes [10,25,26]. Platelets contain over 20 GFs, including platelet-derived growth factors (PDGFs), FGFs, hepatocyte growth factor (HGF), transforming growth factors (TGFs), and vascular endothelial growth factors (VEGFs), almost all of which are known to bind to heparin and to LMW-H/P MPs·NPs. Recent studies suggest that GFs in PRP not only influence the viability of transferred cells but may also play bioactive roles in the regulation of proliferation and differentiation in various types of cells [10]. Any treatment aiming to mimic the critical aspects of the natural biological proc- ess should not be limited to the provision of a single GF, but rather should release multiple GFs at an optimized ratio, at a physiological dose and in a specific spatio- temporal pattern. Those results indicated that the LMW- H/P MPs·NPs also activate the platelets to release the GFs, and that in turn the released GFs from the platelets can be immobilized, be stabilized, and be activated on the LMW-H/P MPs·NPs [10]. The GFs in PRP are stably bound to LMW-H/P MPs in vivo. The GFs adsorbed onto LMW-H/P MPs·NPs may be gradually diffused and released upon biodegrada- tion of LMW-H/P MPs·NPs. When PRP-containing LMW-H/P MPs·NPs were subcutaneously injected into the backs of mice, significantly higher neovascularization and granulation tissue with enhanced filtration of in- flammatory cells were observed compared with the mouse Figure 4. Quantification of visible collateral arteries under angiographic viewing on day 28. Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 505 for Protein Delivery (A Review Article) groups injected with PRP alone, LMW-H/P MPs·NPs alone, and the control [18]. Compared to either PRP alone or LMW-H/P MPs·NPs alone, locally administered PRP-containing LMW-H/P MPs·NPs augmented the wound bed and substantially increased viability of rat dorsal paired pedicle skin flaps [27]. The improved flap survival was noted if PRP-containing LMW-H/P MPs·NPs was administered 2 days before the flap elevation [27]. PRP-containing LMW-H/P MPs·NPs may thus represent a promising new biomaterial for improving skin flaps, particularly in the field of reconstructive surgery. Clinical research was performed using autologous PRP-containing LMW-H/P MPs·NPs and PRP alone in 26 patients with thin hair (including 10 women) [28]. Hair growth and thickening following administration of both PRP-containing LMW-H/P MPs·NPs and PRP alone was observed in all patients compared with the control, but PRP-containing LMW-H/P MPs·NPs appeared to provide the most substantial change in the hair (see Fig- ure 5) [28]. Because of the use of autologous materials, this method using PRP-containing LMW-H/P MPs·NPs is simpler, cheaper, and has no side effect compared with conventional methods. 4. Cell Culture System Using Microparticle-Coated Plates 4.1. Various Types of Cell Cultures Using LMW-H/P MPs·NPs-Coated Plates The LMW-H/P MPs·NPs are able to attach to polymeric surfaces such as plastic and glass. The LMW-H/P MPs·NPs generate a stable paste-like coating through complete drying. It is probable that polypeptides, such as FGF-2, interleukin (IL)-3, and granulocyte/macrophage- colony stimulating factor (GM-CSF) once bound to the LMW-H/P MPs·NPs-coated plates, are gradually re- leased from the coated surface in vitro with a half-life of 4 - 6 days [19]. Furthermore, LMW-H/P MPs·NPs- coat- ing could optimally stimulate growth of human mi- cro-vascular endothelial cells (hMVECs) and human dermal fibroblast cells (hDFCs) in low FBS (1% - 2%)- DMEM with FGF-2 and growth of hematopoietic cell line (TF-1) with IL-3 and GM-CSF (see Figure 6) [19]. Heparin and heparinoids bind various GFs and cyto- kines including FGFs, HGF, VEGF, heparin-binding epidermal growth factor (HBEGF), PDGF, TGF-β, GM-CSF, interleukins (i.e., IL-1, IL-2, IL-3, IL-4, IL-6, Figure 5. PRP-containing LMW-H/P MPs·NPs treatment for Alopecia Areata. Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 506 for Protein Delivery (A Review Article) Figure 6. Preparation of LMW-H/P MPs·NPs-coated plates. IL-7, and IL-8), interferon γ, and macrophage inflamma- tory protein-1 [11-13]. These GFs and cytokines can po- tentially be immobilized on the LMW-H/P MPs·NPs- coated plates. Actually, in addition to FGF-2, IL-3 and GM-CSF described above, we have already observed that FGF-1, HGF, HBEGF, TGF-β, human stem cell factor (SCF), thrombopoietin (Tpo), and Flt-3 ligand (Flt-3) could be efficiently immobilized on the LMW-H/P MPs·NPs-coated plates [21]. Furthermore, the bound GFs to the LMW-H/P MPs·NPs-coated plates appeared to enhance and to stabilize those biological activities. Thus, LMW-H/P MPs·NPs-coating provides an excellent bio- material to immobilize and retain GFs and cytokines for optimal growth of various types of cells with low (no) serum medium (see Figure 6). 4.2. Proliferation of BMSCs and ASCs on LMW-H/P MPs·NPs-Coated Plates Cell-based therapies such as tissue engineering will benefit from a source of autologous multipotent stem cells, including bone marrow-derived mesenchymal stem cells (BMSCs) and adipose tissue-derived stromal cells (ASCs). There are two stem cell lineages in bone marrow cell populations, i.e., hematopoietic cells (HCs) and BMSCs. The BMSCs and ASCs are multipotential, indi- cating that in culture [29,30] or after in vivo implantation these cells can differentiate into a variety of cell types including osteoblasts, chondrocytes, adipocytes, myo- blasts [31], and neuronal cells [32]. Furthermore, cul- tured ASCs secreted significant amounts of angiogenic growth factors such as FGF-2, HGF, PDGF, and VEGF at levels that are bioactive [33]. Thus, LMW-H/P MPs·NPs may serve as an effective matrix for cultures of BMSCs and ASCs. The safe and effective expansions of BMSCs and ASCs represent a promising option for tissue engi- neering strategies. Most protocols for the expansion of BMSCs and ASCs include high concentrations (10% - 20%) of animal se- rum such as FBS as a nutritional supplement. In some cell cultures, this involves multiple doses of FBS, which raises concerns over possible contamination as well as immunological reactions caused by medium-derived FBS proteins, sialic acid derivatives, etc. [34,35]. Patients may experience problems when undergoing autologous cell-based therapies if a serum other than an autologous serum is used during the culturing of the cells. However, it would be difficult to obtain large amounts of autolo- gous serum from the patient for large-scale autologous cell culture [20]. It should be noted that the growth of cultured BMSCs or ASCs on LMW-H/P MPs·NP-coated plates in combination with FGF-2 and FBS (1% - 2%) was significantly stimulated, and similar stimulation was Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 507 for Protein Delivery (A Review Article) observed in those cultured cells on LMW-H/P MPs·NPs- coated plates with FGF-2 and 1% - 2% human serum (HS) prepared from adult bloods instead of FBS. 4.3. Proliferation of CD34+ Hematopoietic Progenitor Cells (CD34+ HCs) on LMW-H/P MPs·NPs-Coated Plates Hematopoietic progenitor cells proliferate and mature in semi-solid media when stimulated by exogenous hema- topoietic cell growth factors (HCGFs) such as SCF, Tpo, Flt-3, IL-3, and GM-CSF [36,37]. These cells also pro- liferate in association with bone marrow-derived stromal cells (BMSCs) [38,39], although biologically active amounts of HCGFs cannot be detected in stromal culture supernatants [38,39]. It is possible that HCGFs are syn- thesized by the stromal cells but remain bound to the stromal cells and/or their extracellular matrix. In fact, it was demonstrated that both natural and recombinant HCGFs, such as IL-3 and GM-CSF, could be adsorbed by heparan sulfate, which is the major sulfated glycosa- minoglycan of bone marrow stroma [38,39]. Serum-free medium supplemented with large amounts of SCF, Tpo, and Flt-3 was reported for expansion of CD34+ HCs [40, 41]. Although such medium is commercially avail- able (HPGM, Lonza Japan Corp., Tokyo, Japan), it is prohibi- tively expensive. We demonstrated that recombinant HCGFs such as SCF, Tpo, and Flt-3 were immobilized onto LMW-H/P MPs·NPs-coated plates, and the immobi- lized cytokines were stabilized, were activated, and were gradually released into the medium. Those cytokines, once bound, can be presented in the biologically active form to hematopoietic progenitor cells [38,39]. Further- more, only one-fourth of the concentration of the cyto- kines recommended by the manufacture was required for maximal expansion of CD34+ HCs on the LMW-H/P MPs·NPs-coated plates. These findings may have impor- tant implications for the use of heparinoid as an artificial matrix for ex vivo expansion of hematopoietic progenitor cells with adequate cytokines. The LMW-H/P MPs·NPs- coating matrix in the presence of lower concentrations of SCF, Tpo, and Flt-3 is a convenient and safe material for stable expansion of CD34+ HCs using HPGM without any animal serum. 5. Conclusions It is recognized in polymer chemistry that positively and negatively charged polymers interact ionically [17]. Through these ionic interactions, basic protamine mole- cules can bind with acidic molecules (LMW-H) to form micro (nano) particle complexes. We previously reported that GF-containing LMW-H/P MPs·NPs, which are 200 nm - 3 μm in diameter, can be easily injected [8-10]. Fur- thermore, the LMW-H/P MPs·NPs were observed on the protection of FGF-2 and GFs in PRP activity from heat and proteolytic inactivation. These results indicate that LMW-H/P MPs·NPs may serve as an effective microcar- rier for various GFs, particularly for the local application of GFs. GFs-containing LMW-H/P MPs·NPs show a substantial effect to induce vascularization and fibrous tissue formation because of stabilization, activation, and gradual release of GF molecules from GFs-containing LMW-H/P MPs·NPs [8-10]. The presented method for the optimal proliferation and differentiation of ASCs and BMSCs on LMW-H/P MPs·NPs-coated plates in low concentration human se- rum medium (1% - 2%) supplemented with FGF-2 (5 ng/ml). No animal serum is required in the culture of those cell types. The bound GFs to the LMW-H/P MPs·NPs-coated plates appeared to enhance and to stabi- lize those biological activities. The proliferated cells maintained their potential to differentiate into adipocytes and osteoblasts [20,33]. Furthermore, the LMW-H/P MPs·NPs-coating matrix in the presence of lower con- centrations of SCF, Tpo, and Flt-3 were convenient ma- terials for stable expansion of CD34+ HCs using HPGM without any animal serum. These results suggest a prom- ising cell source, particularly for the preparation of large amounts of ASCs, BMSCs, or CD34+ HCs required for cell-based therapies in several clinical fields. LMW-H, protamine, several GFs and cytokines, and autologous PRP are already in clinical use. Since auto- logous ASCs, BMSCs, or CD34+ HCs are available, the clinical safety of LMW-H/P MPs·NPs as protein-carrier is possible. Furthermore, ASCs, BMSCs, or CD34+ HCs can be efficiently expanded as cell sources for regenera- tive medicines with the use of LMW-H/P MPs·NPs- coated plates as a matrix without animal serum or feeder cells. REFERENCES [1] J. M. Park, B. B. Muhoberac, P. L Dubin and J. Xia, “Ef- fect of Protein Charge Heterogeneity in Protein-Polyelec- trolyte Complexation,” Macromolecules, Vol. 25, No. 1, 1992, pp. 290-295. doi:10.1021/ma00027a047 [2] K. W. Mattison, P. L. Dubin and I. J. Brittain, “Complex Formation between Bovine Serum Albumin and Strong Polyelectrolytes: Effect of Polymer Charge Density,” Journal of Physical Chemistry B, Vol. 102, No. 19, 1998, pp. 3830-3836. doi:10.1021/jp980486u [3] S. Dragan, M. Cristea, C. Luca and B. C. Simionescu, “Polyelectrolyte Complex. I: Synthesis and Characteriza- tion of Some Insoluble Polyanion-Polycation Com- plexes,” Journal of Polymer Science A, Vol. 34, No. 17, 1996, pp. 3487-3495. doi:10.1002/(SICI)1099-0518(199612)34:17<3485::AID- Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes 508 for Protein Delivery (A Review Article) POLA3>3.0.CO;2-U [4] Y. Koyama, M. Yamashita, N. I. Tanaka and T. Ito, “En- hancement of Transcriptional Activity of DNA Com- plexes by Amphoteric PEG Derivertive,” Biomacro- molecules, Vol. 7, No. 4, 2006, pp. 1274-1279. doi:10.1021/bm0504633 [5] L. Webster, M. B. Huglin and I. D. Robb, “Complex For- mation between Poly-Electrolytes in Dilute Aqueous So- lution,” Polymer, Vol. 38, No. 6, 1997, pp. 1373-1380. doi:10.1016/S0032-3861(96)00650-7 [6] M. Hashimoto, Y. Koyama and T. Sato, “In Vitro Gene Delivery by pDNA/Chitosan Complexes Coated with Anionic PEG Derivatives That Have a Sugar Side Chain,” Chemistry Letter, Vol. 37, No. 3, 2008, pp. 266-267. doi:10.1246/cl.2008.266 [7] A. Denuziere, D. Ferrier and A. Domard, “Chitosan- Chondroitin Sulfate and Chitosan-Hyaluronate Polyelec- trolyte Complexes. Physico-Chemical Aspects,” Carbo- hydrate Polymer, Vol. 29, No. 4, 1996, pp. 317-323. doi:10.1016/S0144-8617(96)00035-5 [8] S. Nakamura, Y. Kanatani, S. Kishimoto, M. Nambu, C. Ohno, H. Hattori, B. Takase, Y. Tanaka, H. Yura, T. Ki- yosawa, T. Maehara and M. Ishihara, “Controlled Release of FGF-2 Using Fragmin/Protamine Microparticles and Effect on Neovascularization,” Journal of Biomedical Materials Research A, Vol. 91, No. 3, 2009, pp. 814-823. doi:10.1002/jbm.a.32265 [9] Y. Mori, S. Nakamura, S. Kishimoto, M. Kawakami, S. Satoshi, T. Matsui and M. Ishihara, “Preparation and Characterization of Low-Molecular-Weight Heparin/Pro- tamine Nanoparticles (LMW-H/P NPs) as FGF-2 Car- rier,” International Journal of Nanomedicine, Vol. 5, 2010, pp. 147-155. doi:10.2147/IJN.S8692 [10] M. Takikawa, S.-I. Nakamura, S. Nakamura, M. Nambu, M. Ishihara, M. Fujita, S. Kishimoto, T. Doumoto, S. Yanagibayashi, R. Azuma, N. Yamamoto and T. Kiyo- sawa, “Enhancement of Vascularization and Granulation Tissue Formation by Growth Factors in Human Platelet- Rich Plasma-Containing Fragmin/Protamine Microparti- cles,” Journal of Biomedical Materials Research B, Vol. 97, 2011, pp. 373-380. doi:10.1002/jbm.b.31824 [11] M. Ishihara and K. Ono, “Structure and Function of Heparin and Heparan Sulfate: Heparinoid Library and Modification of FGF-Activities,” Trends in Glycoscience and Glycotechnoogy, Vol. 10, No. 52, 1998, pp. 223-233. doi:10.4052/tigg.10.223 [12] M. Salmivirta, K. Lidhold and U. Lindahl, “Heparan Sul- fate: A Piece of Information,” FASEB Journal, Vol. 10, No. 52, 1996, pp. 1270-1279. [13] U. Lindahl, K. Lidholt, D. Spillmann and L. Kjellen, “More to ‘Heparin’ Than Anti-Coagulation,” Thrombosis Research, Vol. 75, No. 1, 1994, pp. 1-32. doi:10.1016/0049-3848(94)90136-8 [14] J. Hirsh, T. E. Warkentin, S. G. Shaughnessy, S. S. An- and, J. L. Halperin, R. Raschke and C. Granger, “Heparin and Low-Molecular Heparin, Mechanisms of Action, Phormacokinetics, Dosing, Monitoring, Efficacy, and Safety,” Chest, Vol. 119, No. 2, 2001, pp. 64-94. doi:10.1378/chest.119.1_suppl.645 [15] M. Wolzt, A. Wetermann, M. Nieszpaur-Los, B. Schnei- der, A. Fassolt, K. Lechner, H. Eichler and P. A. Kyrle, “Studies on the Neutralizing Effects of Protamine on Un- fractionated and Low Molecular Weight Heparin (Frag- min®) at the Site of Activation of the Coagulation Sys- tem in Man,” Thrombosis and Haemostasis, Vol. 73, No. 3, 1995, pp. 439-443. [16] M. Pan, J. S. Lezo, A. Medina, M. Romero, E. Hernandez, J. Segura, F. Melian, F. Wanguemert, M. Landin, F. Benitez, M. Amay, F. Velasco and A. Torres, “In-Labo- ratory Removal of Femoral Sheath Following Protamine Administration in Patients Having Intracoronary Stent Implantation,” American Journal of Cardiology, Vol. 80, No. 10, 1997, pp. 1336-1338. doi:10.1016/S0002-9149(97)00676-0 [17] M. Fujita, M. Ishihara, M. Shimizu, K. Obara, T. Ishizuka, Y. Saito, H. Yura, Y. Morimoto, B. Takase, T. Matsui, M. Kikuchi and A. Kurita, “Vascularization in Vivo Caused by the Controlled Release of Fibroblast Growth Factor-2 from an Injectable Chitosan/Non-Anticoagulant Heparin Hydrogel,” Biomaterials, Vol. 25, No. 4, 2004, pp. 699- 706. doi:10.1016/S0142-9612(03)00557-X [18] S. Nakamura, M. Nambu, S. Kishimoto, T. Ishizuka, H. Hattori, Y. Kanatani, B. Takase, H. Aoki, T. Kiyosawa, T. Maehara and M. Ishihara, “Effect of Controlled Release of Fibroblast Growth Factor-2 from Chitosan/Fucoidan Micro Complex Hydrogel on in Vitro and in Vivo Vascu- larization,” Journal of Biomedical Materials Research A, Vol. 85, 2008, pp. 619-627. doi:10.1002/jbm.a.31563 [19] S. Kishimoto, S. Nakamura, S.-I. Nakamura, Y. Kanatani, H. Hattori, Y. Tanaka, Y. Harada, M. Tagawa, Y. Mori, T. Maehara and M. Ishihara, “Fragmin/protamine Micropar- ticle-Coated Matrix Immobilized Cytokines to Stimulate Various Cell Proliferations with Low Serum Media,” Ar- tificial Organs, Vol. 33, No. 6, 2009, pp. 431-438. doi:10.1111/j.1525-1594.2009.00745.x [20] S. Kishimoto, H. Hattori, S. Nakamura, Y. Amano, Y. Kanatani, Y. Tanaka, Y. Mori, Y. Harada, M. Tagawa and M. Ishihara, “Expansion and Characterization of Human bone Marrow-Derived Mesenchymal Stem Cells Cultured on Fragmin/Protamine Microparticle-Coated Matrix with Fibroblast Growth Factor-2 in Low Serum Medium,” Tissue Engineering Part C, Vol. 15, No. 3, 2009, pp. 523- 527. doi:10.1089/ten.tec.2008.0492 [21] S. Kishimoto, S. Nakamura, S.-I. Nakamura, H. Hattori, F. Oomuma, Y. Kanatani, Y. Tanaka, Y. Harada, M. Ta- gawa, T. Maehara and M. Ishihara, “Cytokine-Immobi- lized Microparticle-Coated Plates for Culturing Hemato- poietic Progenitor Cells,” Journal of Controlled Release, Vol. 133, 2009, pp. 185-190. doi:10.1016/j/jconrel,2008.10.005 [22] D. Gospodarowicz and J. Cheng, “Heparin Protects Basic and Acidic FGF from Inactivation,” Journal of Cellular Physiology, Vol. 128, No. 3, 1986, pp. 475-484. Copyright © 2011 SciRes. JBNB  Low-Molecular-Weight Heparin and Protamine-Based Polyelectrolyte Nano Complexes for Protein Delivery (A Review Article) Copyright © 2011 SciRes. JBNB 509 doi:10.1002/jcp.1041280317 [23] S.-I. Nakamura, M. Ishihara, M. Takikawa, K. Murakami, S. Kishimoto, S. Nakamura, S. Yanagibayashi, Y. Mori, M. Fujita, S. Kubo, N. Yamamoto and T. Kiyosawa, “Increased Survival of Free Fat Grafts and Vasculariza- tion in Rats with Local Delivery of Fragmin/Protamine Microparticles Containing FGF-2 (F/P MP-F),” Journal of Biomedical Materials Research B, Vol. 96, 2011, pp. 234-241. doi:10.1002/jbm.b.31757 [24] T. Horio, M. Fujita, Y. Tanaka, M. Ishihara, S. Kishimoto, S. Nakamura, M. Shimizu, Y. Nogami, H. Hattori, K. Hase and T. Maehara, “Efficacy of Fragmin/Protamine Microparticles Containing Fibroblast Growth Factor-2 (F/P MP/FGF-2) in a Rabbit Model of Hindlimb Ische- mia,” Journal of Vascular Surgery, Vol. 54, 2011, pp. 791-798. doi:10.1016/j.jvs.2011.02.060 [25] R. E. Marx, “Platelet-Rich Plasma: Evidence to Support Its Use,” Journal of Oral and Maxillofacial Surgery, Vol. 62, 2001, pp. 225-228. doi:10.1016/j.joms.2003.12.003 [26] B. L. Eppley, W. S. Pietrzak and M. Blanton, “Platelet- rich Plasma: A Review of Biology and Application in Plastic Surgery,” Plastic and Reconstructive Surgery, Vol. 118, 2002, pp. 147-159. doi:10.1097/01.prs.0000239606.92676.cf [27] M. Takikawa, Y. Sumi, M. Ishihara, S. Kishimoto, S. Nakamura, S. Yanagibayashi, H. Hattori, R. Azuma, N. Yamamoto and T. Kiyosawa, “PRP&F/P MPs Improved Survival of Dorsal Paired Pedicle Skin Flaps in Rats,” Journal of Surgical Research, Vol. 170, 2011, pp. 189- 196. doi:10.1016/j/jss.2011.05.051 [28] M. Takikawa, S.-I. Nakamura, S. Nakamura, M. Nambu, M. Ishihara, K. Murakami, S. Kishimoto, K. Sasaki, S. Yanagishita, R. Azuma, N. Yamamoto and T. Kiyosawa, “Enhanced Effect of Platelet-Rich Plasma Containing a New Carrier on Hair Growth,” Dermatologic Surgery, Vol. 37, 2011, pp. 1-9. doi:10.1111/j.1524-4725.2011.02123.x [29] D. J. Prockop, “Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues,” Science, Vol. 276, No. 5309, 1997, pp. 71-74. doi:10.1126/science.276.5309.71 [30] M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simon- etti, S. Craig and D. R. Marshak, “Multilineage Potential of Adult Human Mesenchymal Stem Cells,” Science, Vol. 284, No. 5411, 1999, pp. 143-147. doi:10.1126/science.284.5411.143 [31] G. Ferrari, G. Cusella-DeAngelis, M. Coletta, E. Paolucci, A. Stornaiuolo, G. Cossu and F. Mavilio, “Muscle Re- generation by Bone Marrow-Derived Myogenic Progeni- tors,” Science, Vol. 279, No. 5356, 1998, pp. 1528-1530. doi:10.1126/science.279.5356.1528 [32] T. M. Coyne, A. J. Marcus, K. Reynold, I. B. Black and D. Woodbury, “Desparate Host Response and Donor Sur- vival after the Transplantation of Mesenchymal or Neu- roectodermal Cells to the Intact Roden Brain,” Trans- plantation, Vol. 84, No. 11, 2007, pp. 1507-1516. doi:10.1097/01.tp.0000288185.09601.4d [33] S. Nakamura, S. Kishimoto, S.-I. Nakamura, M. Nambu, M. Fujita, Y. Tanaka, Y. Mori, M. Tagawa, T. Maehara and M. Ishihara, “Fragmin/Protamine Microparticles as Cell Carriers to Enhance Viability of Adipose-Derived Stromal Cells and Their Subsequent Effect on in Vivo Neovascularization,” Journal of Biomedical Matererials Research A, Vol. 92, 2010, pp. 1614-1622. doi:10.1002/jbm.a.32506 [34] J. L. Spees, C. A. Gregory, H. Singh, H. A. Tucker, A. Peister, P. J. Lynch, S. C. Hsu, J. Smith and D. J. Prockop, “Internalized Antigens Must Be Removed to Prepare Hypoimmunogenic Mesenchymal Stem Cells for Cell and Gene Therapy,” Molecular Therapy, Vol. 9, No. 5, 2004, pp. 747-756. doi:10.1016/j.ymthe.2004.02.012 [35] M. J. Martin, A. Muotri, F. Gage and A. Varki, “Human Embryonic Stem Cells Express an Immunogenic Nonhu- man Sialic Acid,” Nature Medicine, Vol. 11, No. 2, 2005, pp. 228-232. [36] P. Gupta, T. R. Oegema, J. J. Brazil, A. Z. Dudek, A. Slungaard and C. M. Verfaillie, “Structurally Specific Heparan Sulfates Support Primitive Human Hematopoi- esis by Formation of a Multimolecular Stem Cell Niche,” Blood, Vol. 92, No. 12, 1998, pp. 4641-4651. [37] M. Alvarez-Silva and R. Borojevic, “GM-CSF and IL-3 Activities in Schistosomal Liver Granulomas Are Con- trolled by Stroma-Associated Heparan Sulfate Proteogly- cans,” Journal of Leukocyte Biology, Vol. 59, No. 3, 1996, pp. 435-441. [38] R. Roberts, J. Gallagher, E. Spooncer, T. D. Allen, F. Bloomfield and T. M. Dexter, “Heparan Sulfate Bound Growth Factors: A Mechanism for Stromal Cell Mediated Haemopoiesis,” Nature, Vol. 332, No. 6162, 1988, pp. 376-378. doi:10.1038/332376a0 [39] M. Y. Gordon, G. P. Riley, S. M. Watt and M. F. Greaves, “Compartmentalization of a Haematopoietic Growth Factor (GM-CSF) by Glycosaminoglycans in the Bone Marrow Microenvironment,” Nature, Vol. 326, No. 6111, 1987, pp. 403-405. doi:10.1038/326403a0 [40] A. L. Drayer, S. G. Olthof and E. Vellenga, “Mammalian Target of Rapamycin Is Required for Thrombopoietin-In- duced Proliferation of Megakaryocyte Progenitors,” Stem Cells, Vol. 24, No. 1, 2006, pp. 105-114. doi:10.1634/stemcells-2005-0062 [41] H. Schepers, A. T. Wierenga, D. V. Gasliga, B. J. Eggen, E. Vellenga and J. J. Schuringa, “Reintroduction of C/EBPalpha in Leukemic CD34+ Stem/Progenitor Cells Impairs Self-Renewal and Partially Restores Myelopoi- esis,” Blood, Vol. 110, No. 4, 2007, pp. 1317-1325. doi:10.1182/blood-2006-10-052175
|