 Vol.1, No.3, 176-182 (2009) doi:10.4236/health.2009.13028 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Active volatiles of cabernet sauvignon wine from Changli County Yong-Sheng Tao, Hua Li* College of Enology, Northwest A & F University, Yangling, Shaanxi, China; Corresponding author: lihuawine@nwsuaf.edu.cn Received 6 September 2009; revised 27 September 2009; accepted 28 September 2009. ABSTRACT This study investigated the contribution of vola- tile compounds to the overall aroma of Cabernet Sauvignon wines from Changli County (China). Wine samples were collected from vintages from 2000 to 2005. Volatile compounds were ex- tracted by PDMS solid-phase micro-extraction fi- bers and identified by Gas Chromatography-Ma- ss Spectrometry (GC-MS). A total of 65 volatile compounds were identified and quantified, in- cluding higher alcohols, ethyl and acetate esters, and fatty acids. According to their odor active values (OA-Vs), 21 volatile compounds were con- sidered to be the powerful impact odorants of Cabernet Sauvignon wines from Changli. Odor descriptions of impact volatiles suggested Cab- ernet Sauvignon red wines from Changli County as having a complex aroma, which included not only pleasant floral and fruity odors, but also cheese, clove flavors, and grassy and smoky aromas. Keywords: Cabernet Sauvignon; Red wine; Aroma compounds; OAV; GC-MS 1. INTRODUCTION Cabernet Sauvignon, Cabernet Franc and Cabernet Ger- nischet are known as “the Three Pearls” of wine grapes in China, often used by Chinese wineries to produce pre- mium quality red wines. In contrast to Cabernet Franc and Cabernet Gernischet, Cabernet Sauvignon can be found in almost all wine production districts and has the largest growing area in China. Changli County, a region of North China, has become a famous wine producing district as one of the four districts of Wine Denomination of Origin in China, and the winemaking sector is the principal economy of the county. In Changli County, the main red grape variety used in wine production is Cabernet Sau- vignon. The growing area of Cabernet Sauvignon is 2400 Ha and accounts for 72% of the total grape planting areas. Therefore, it is important to understand the characters of Cabernet Sauvignon red wine made from Changli. Wine aroma is an important aspect of wine quality. In a recent consumer study, the flavor of wine was found to be one of the attributes most important to consumers when buying wine. Volatile compounds influence the organo- leptic characteristics of wines, particularly the aromatic characteristics, and the aroma constituents of different grapes and wines have been extensively studied in the last few years. In the order of 1000 volatile compounds, such as alcohols, esters, organic acids, phenols, thiols, mono- terpenes and norisoprenoids have been found in wines, only several tens of which can be impact odorants. Vola- tile compounds found in wines can reflect the influence of variety, climate and soil, etc. Therefore, these compounds play a decisive role in wine quality and regional charac- teristics [1-3]. Since the contribution of volatile com- pounds to the final aroma depends on whether the con- centration in the wine is above the perception threshold, OAV (odor activity value) was introduced to determine impact odorants [4,5]. OAV calculation depends both on measuring concentration and on odor threshold in the same matrix. Only those odorants with OAV >1 can be perceived. Some studies have indicated that the young red wines of Cabernet Sauvignon, Merlot and Grenache have simi- lar aromatic characteristics [6]. The most active odorants of these three varietal young red wines suggested by aroma extract dilution analysis (AEDA) were isopentyl and β-phenylethyl alcohols, the ethyl esters of butyric, isobutyric, 2-methyl butyric and hexanoic acids, γ-nona- lactone and eugenol. Data showed that differences be- tween these varieties are quantitative rather than qualita- tive [7,8]. In past decades, the unique characteristics of Chinese wine began to attract notice with the rapid de- velopment of wine production in China. However, sen- sory data for Chinese wine are scarce, especially for wines with denomination of origin. A study of aromatic compounds of the Cabernet Sauvignon red wine Sha- cheng (China) showed that ethyl octanoate, ethyl hexa- noate and isopentyl acetate jointly contributed to more than 97% of the global aroma according to OAVs [9].  Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 177 177 However, this result may be misleading; further studies are necessary to understand the nature of aromatic com- pounds found in premium Chinese wine. Quantitative assessment of volatile compounds in win- es has met with some difficulty, mainly due to their com- plexity and large concentration variations from 1 ng/L to several g/L. Therefore, sample preparation essentially consists of extraction and concentration of the compou- nds of interest. In this study, volatile compounds were extracted by solid-phase micro-extraction and detected by GC-MS, which has been published [10]. This work re- ported the results of the first study profiling of the major volatile compounds and the impact odorants in Cabernet Sauvignon wines from the Changli County region of China. 2. MATERIALS AND METHODS 2.1. Wines Changli Cabernet Sauvignon wines from vintages be- tween 2000 and 2005 (Each year has two samples which were supplied by Huaxia Winemaking Company and Yueqiannian Winemaking Company respectively, Chan- gli County.) were used to analyze the composition of volatile compounds. Wine samples were collected six months after winemaking and then stored at 5-10 ℃ before analysis. Wine making: Sound grapes of Cabernet Sauvignon were obtained from the vineyard. Grapes were de- stemmed and crushed on a commercial grape destem- mer-crusher, the output of which was pumped to stain- less steel tanks. The must was treated with sulfur dioxide (45 mg/L) and soaked for approximately 24 h. Alcohol fermentation was going on at 25-30℃. After fermenta- tion, the wines were racked and subjected to malo-lactic fermentation. The wines were then racked and sulfur dioxide (75mg/L) was added. The wines were stored at 15℃ in stainless-steel tanks. Racking and stabilizing processes were carried out prior to analysis. Reducing sugars, density, ethanol, extract, titratable acidity, pH, volatile acidity, total and free SO2 were ana- lyzed with the methods provided by the Office Interna- tional de la Vigne et du Vin (OIV, 1990) [11]. 2.2. Reagents All reagents used were analytical grade. Absolute ethanol, tartaric acid, and sodium chloride were purchased from Xi’an chemical factory (Xi’an, China). Water was obtained from a Milli-Q purification system (Millipore). Solvents did not require additional distillation. 32 pure reference compounds were from Sigma–Aldrich (China sector): ethyl acetate, ethyl butyrate, 1-propanol, 2-methyl thio- phene, 2-methyl-1-propanol, isopentyl acetate, 1-butanol, 2,5-dimethyl-tetrahydro-furan, isopentyl alcohol, ethyl hexanoate, ethenyl benzene, ethyl lactate, 1-hexanol, 3- octanol, ethyl octanoate, furfural, decanal, cis-geraniol, β- ionone, linalool, β-damascenone, ethyl decanoate, phe- nethyl acetate, 1-decanol, hexanoic acid, benzyl alcohol, 2- phenyl-ethanol, ethyl dodecanoate, ethyl hexadecanoate, octanoic acid, decanoic acid, and p-ethyl-phenol. 2.3. Standard Solutions Exact volumes of the standard chemical compounds were dissolved in synthetic wines to prepare the calibration data. These standard compounds were dissolved in syn- thetic wines at concentrations three orders of magnitude higher than typically found in wines. For quantification, five-point calibration curves were prepared for each compound using the method described by Ferreira et al. (2000) [8]. The final alcohol content of the synthetic wine was 11% (v/v). The synthetic wine had 6 g/L of tartaric acid and its pH was 3.3–3.4 adjusted with 1M NaOH (synthetic wine matrix). Octan-3-ol was employed as an internal standard because it was not the typical volatile compound in wine and it had a perfect ion peak shape and peak place in the TIC. Exact volumes of octan-3-ol were dissolved in absolute ethanol. All these solutions were stored at 4 ℃ in darkness [1,12]. 2.4. Solid Phase Micro-Extraction (SPME) Sampling Conditions SPME was performed following the methods described previously [13]. Both wine samples and model solutions were analyzed in 15-ml glass vials, filled with 10 ml of each sample and 2 g NaCl. For SPME analyses, the vials were dipped in a thermostatic water bath. A magnetic stirring bar was placed in the vial to agitate the sample. PDMS (100 µm Polydimethylsiloxane) was used as the solid-phase fiber for micro-extraction. The vial was equi- librated at 40℃ for 10 min, and the power magnetic stirrer was then added. SPME was performed at 40℃ for 30 min, and was immediately followed by the desorption of the analytes into the gas chromatograph injector. The solid-phase fiber remained into the injector for about 3 min. 2.5. GC–MS Analysis GC–MS apparatus: TRACE DSQ (Thermo-Finnigan, USA). Analytical column: DB-Wax capillary column (30m×0.32mm i.d., 0.25 µm film thickness), (J&W, Folsom, USA). Carrier: He at 1ml/min. The temperature program used was 40 ℃ for 3 min, raised to 160 ℃ at 4 ℃/min, then raised to 230℃ at 7 ℃/min for 8 min. The transfer line temperature was 230℃, and the injection temperature was 250 ℃. Mass spectra were recorded in electron impact (EI) ionization mode. Mass spectrometry:  Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 http://www.scirp.org/journal/HEALTH/Openly accessible at 178 Table 1. General composition of cabernet sauvignon must and wine. Ranges Must composition Titratable aciditya(g/L) 9.3-9.7 pH 3.2-3.4 Reducing sugars (g/L) 191-200 Wine composition Density (20℃) 0.991-0.994 Ethanol (%, v/v) 10.4-12.1 Reducing sugars (g/L) 0.78-1.82 Extract (g/L) 21-25 Titratable aciditya (g/L) 3.6-4.5 pH 3.3-3.6 Volatile acidity (g/L) 0.46-0.71 Free SO2 (mg/L) 11-19 Total SO2 (mg/L) 90-121 (a) As tartaric acid. (b) As acetic acid. mass range 33-450 amu, scanned at 1 s intervals. The ion source temperature was 230℃. 2.6. Qualitative Analysis and Quantification Identification of volatile compound was achieved by comparing mass spectra obtained from the sample with those from pure standards injected in the same conditions, and by comparing the Kov’ats index or the mass spectra found in the NIST2.0 MS library Database or found in the literature. An internal standard quantification method using oc- tan-3-ol was employed. Quantitative data of the identified compounds were obtained by interpolation of the relative areas versus the internal standard area using calibration graphs built for pure reference compounds. The concen- tration of volatile compounds, for which there was no pure reference, was obtained by using the same calibra- tion graphs as the compounds with the most similar chemical structure according to the formula and chemical character [3,14]. 3. RESULTS AND DISCUSSION Those general compositions of sample wines were dis- played in Table 1. There is no significant difference among these samples. Volatile compounds found in Cabernet Sauvignon red wines from Changli County detected by SPME-GC-MS are shown in Table 2. There are 65 aroma compounds and their concentrations vary from 0.5μg/L to 2.23 g/L. The majority of the compounds were higher alcohols, esters, and fatty acids. Other compounds identified were ter- penes, norisoprenoids, volatile phenols and furans. The OAV of each compound was obtained using concentra- tion divided by odor threshold. Twenty-one compounds had OAV values greater than one. Impact odorants of the Chardonnay white wine from Changli had previously been identified using the same method. Thirteen of the 41 volatile compounds detected had aroma activity and contributed to the pleasant fruity and floral aroma of the Chardonnay wine [14]. The active aroma compounds identified in that study were approximately half of the total volatiles detected in Cabernet Sauvignon red wines identified in this study, indicating the aroma of the red wine may be more complex. 3.1. Esters Esters found in wine include acetates, ethyl esters and other esters of fusels and fatty acids. In the sample wines, 21 esters were identified with concentrations ranging from 62 to 390 mg/L. Contents of esters accounted for about 20-30% of the total aroma compounds. Five ace- tates, 13 ethyl esters and three others were found in this chemical group. In acetates, the OAVs of ethyl acetate and isopentyl acetate were higher than one. Ethyl acetate may contribute a pleasant, fruity fragrance to the general wine aroma at concentrations lower than 150 mg/L. However, at higher concentrations, ethyl acetate can contribute a sour-vinegar odor [21]. Isopentyl acetate contributes a fresh fruity odor, reminiscent of banana flavors. Among 13 ethyl esters, ethyl butyrate, ethyl isovaler- ate, ethyl hexanoate, ethyl lactate and ethyl octanoate have OAVs over one. Ethyl butyrate has the favor of sour fruit, strawberry and sweet fruit. Ethyl isovalerate smells of banana and sweet fruit. Ethyl hexanoate has the flavor of green apple, fruit, strawberry and anise. Ethyl octanoate gives pineapple, pear and floral aromas. Ethyl lactate contributes lactic and raspberry odors. These ac- tive ethyl esters are responsible for the full-bodied fruity and floral aroma of wine. Results also confirmed most of the wines rich in these compounds showed elevated lev- els of higher alcohol acetates, thus adding to the sweet and soapy odors, and pleasant floral and fruity aroma. Esters of fusel and fatty acids had lower concentra- tions, but their odor thresholds were also lower. In this study, isopentyl lactate had OAVs over one, and influ- ences the overall aroma of the wine. Isopentyl lactate contributes cream and nut flavors. This compound is produced by malo-lactic fermentation [2]; therefore ma- lo-lactic fermentation may be occurring in the wine as well. 3.2. Higher Alcohols Higher major alcohols were the most abundant volatiles in all the studied wines. They are formed mainly during the first two stages of alcoholic fermentation [3,21]. In our work, 25 higher alcohols were identified and quanti fied, forming the largest group of volatile compounds. Their concentrations varied from 248 to 886 mg/L and 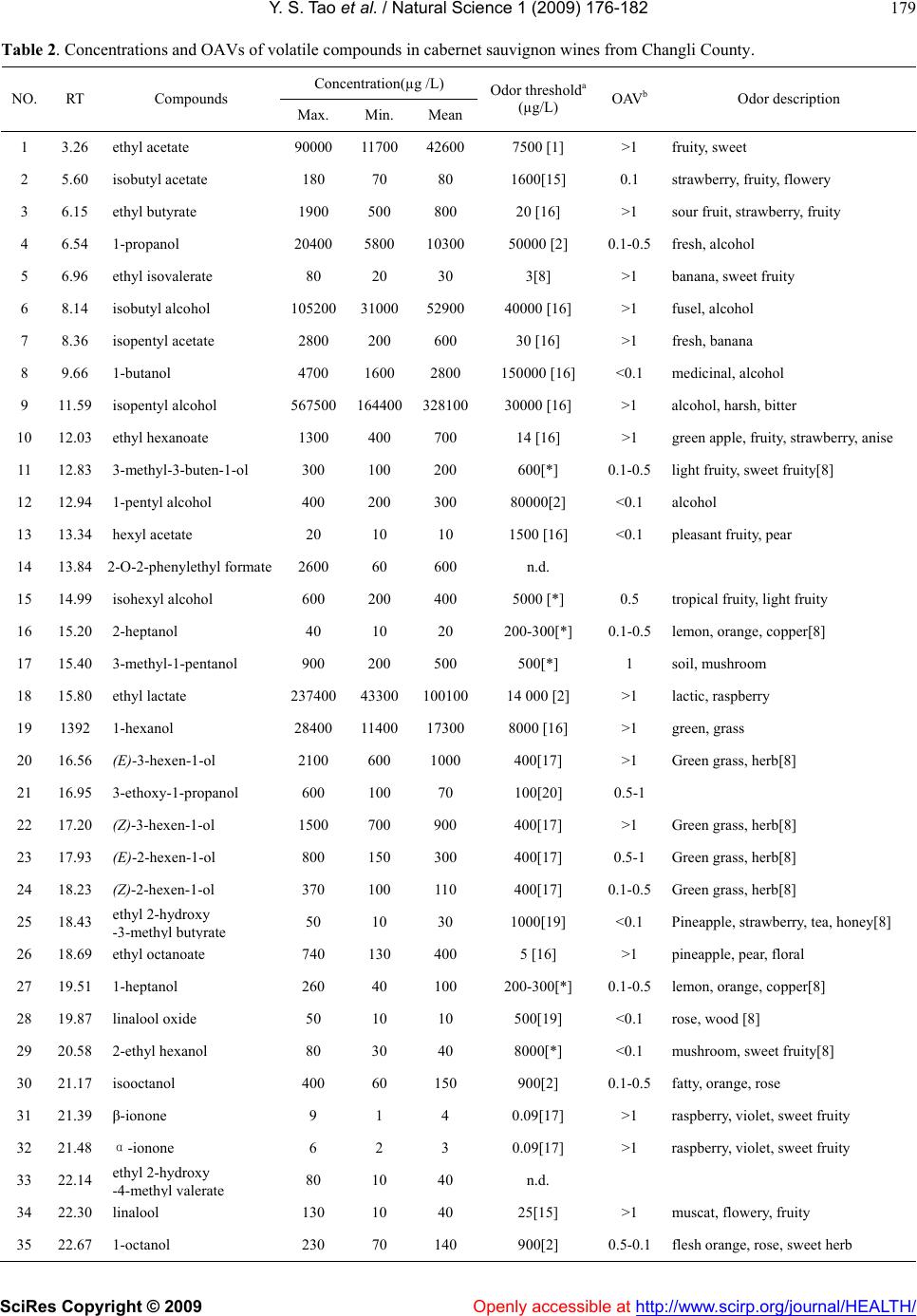 Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 179 Table 2. Concentrations and OAVs of volatile compounds in cabernet sauvignon wines from Changli County. Concentration(µg /L) NO. RT Compounds Max. Min. Mean Odor thresholda (µg/L) OAVbOdor description 1 3.26 ethyl acetate 90000 11700426007500 [1] >1 fruity, sweet 2 5.60 isobutyl acetate 180 70 80 1600[15] 0.1 strawberry, fruity, flowery 3 6.15 ethyl butyrate 1900 500 800 20 [16] >1 sour fruit, strawberry, fruity 4 6.54 1-propanol 20400 5800 1030050000 [2] 0.1-0.5fresh, alcohol 5 6.96 ethyl isovalerate 80 20 30 3[8] >1 banana, sweet fruity 6 8.14 isobutyl alcohol 105200 310005290040000 [16] >1 fusel, alcohol 7 8.36 isopentyl acetate 2800 200 600 30 [16] >1 fresh, banana 8 9.66 1-butanol 4700 1600 2800 150000 [16] <0.1 medicinal, alcohol 9 11.59 isopentyl alcohol 567500 16440032810030000 [16] >1 alcohol, harsh, bitter 10 12.03 ethyl hexanoate 1300 400 700 14 [16] >1 green apple, fruity, strawberry, anise 11 12.83 3-methyl-3-buten-1-ol 300 100 200 600[*] 0.1-0.5light fruity, sweet fruity[8] 12 12.94 1-pentyl alcohol 400 200 300 80000[2] <0.1 alcohol 13 13.34 hexyl acetate 20 10 10 1500 [16] <0.1 pleasant fruity, pear 14 13.84 2-O-2-phenylethyl formate 2600 60 600 n.d. 15 14.99 isohexyl alcohol 600 200 400 5000 [*] 0.5 tropical fruity, light fruity 16 15.20 2-heptanol 40 10 20 200-300[*] 0.1-0.5lemon, orange, copper[8] 17 15.40 3-methyl-1-pentanol 900 200 500 500[*] 1 soil, mushroom 18 15.80 ethyl lactate 237400 4330010010014 000 [2] >1 lactic, raspberry 19 1392 1-hexanol 28400 11400 173008000 [16] >1 green, grass 20 16.56 (E)-3-hexen-1-ol 2100 600 1000 400[17] >1 Green grass, herb[8] 21 16.95 3-ethoxy-1-propanol 600 100 70 100[20] 0.5-1 22 17.20 (Z)-3-hexen-1-ol 1500 700 900 400[17] >1 Green grass, herb[8] 23 17.93 (E)-2-hexen-1-ol 800 150 300 400[17] 0.5-1 Green grass, herb[8] 24 18.23 (Z)-2-hexen-1-ol 370 100 110 400[17] 0.1-0.5Green grass, herb[8] 25 18.43 ethyl 2-hydroxy -3-meth l but rate 50 10 30 1000[19] <0.1 Pineapple, strawberry, tea, honey[8] 26 18.69 ethyl octanoate 740 130 400 5 [16] >1 pineapple, pear, floral 27 19.51 1-heptanol 260 40 100 200-300[*] 0.1-0.5lemon, orange, copper[8] 28 19.87 linalool oxide 50 10 10 500[19] <0.1 rose, wood [8] 29 20.58 2-ethyl hexanol 80 30 40 8000[*] <0.1 mushroom, sweet fruity[8] 30 21.17 isooctanol 400 60 150 900[2] 0.1-0.5fatty, orange, rose 31 21.39 β-ionone 9 1 4 0.09[17] >1 raspberry, violet, sweet fruity 32 21.48 α-ionone 6 2 3 0.09[17] >1 raspberry, violet, sweet fruity 33 22.14 ethyl 2-hydroxy -4-meth l valerate 80 10 40 n.d. 34 22.30 linalool 130 10 40 25[15] >1 muscat, flowery, fruity 35 22.67 1-octanol 230 70 140 900[2] 0.5-0.1flesh orange, rose, sweet herb 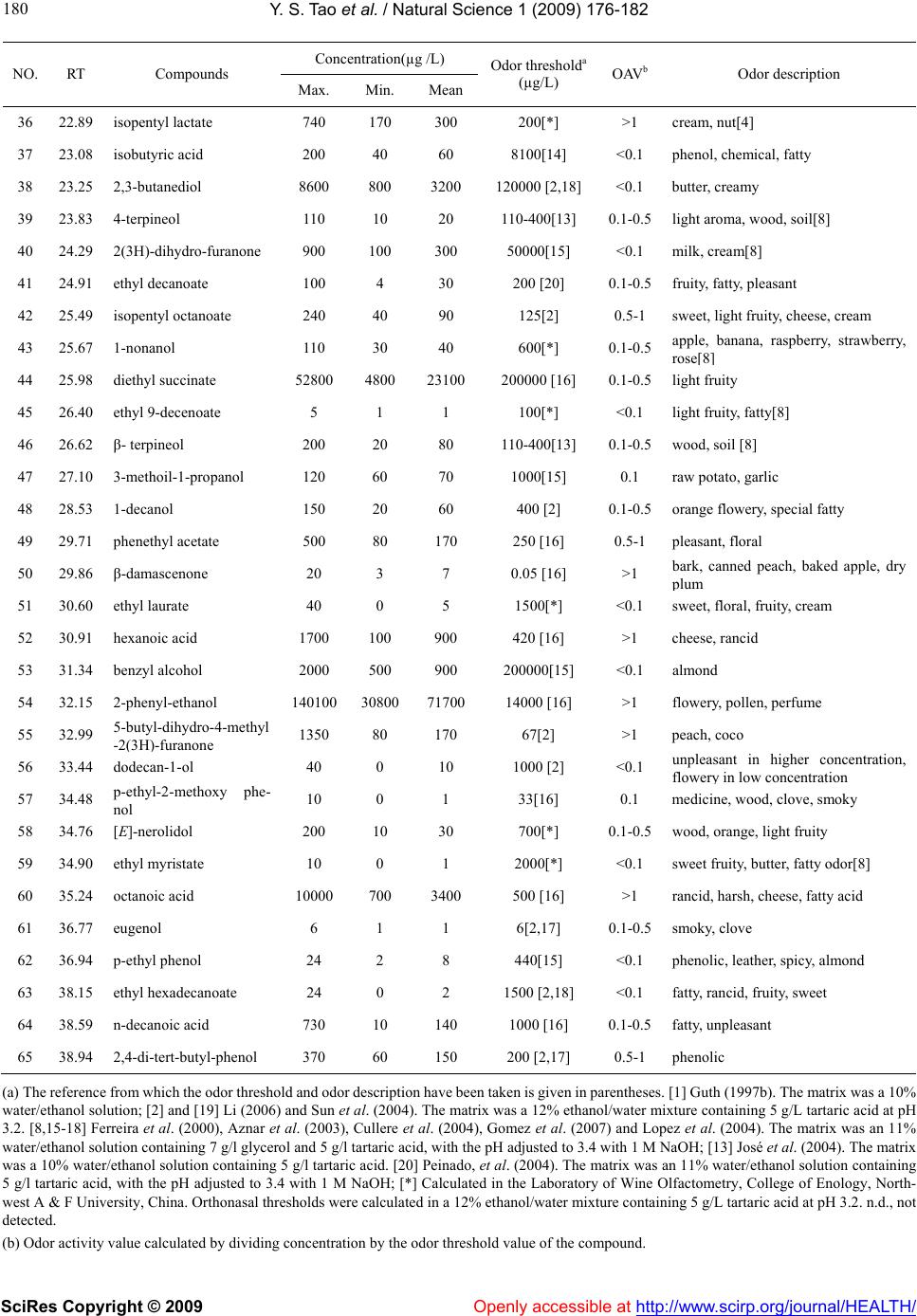 Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 http://www.scirp.org/journal/HEALTH/Openly accessible at 180 Concentration(µg /L) NO. RT Compounds Max. Min. Mean Odor thresholda (µg/L) OAVbOdor description 36 22.89 isopentyl lactate 740 170 300 200[*] >1 cream, nut[4] 37 23.08 isobutyric acid 200 40 60 8100[14] <0.1 phenol, chemical, fatty 38 23.25 2,3-butanediol 8600 800 3200 120000 [2,18] <0.1 butter, creamy 39 23.83 4-terpineol 110 10 20 110-400[13] 0.1-0.5light aroma, wood, soil[8] 40 24.29 2(3H)-dihydro-furanone 900 100 300 50000[15] <0.1 milk, cream[8] 41 24.91 ethyl decanoate 100 4 30 200 [20] 0.1-0.5fruity, fatty, pleasant 42 25.49 isopentyl octanoate 240 40 90 125[2] 0.5-1 sweet, light fruity, cheese, cream 43 25.67 1-nonanol 110 30 40 600[*] 0.1-0.5apple, banana, raspberry, strawberry, rose 8 44 25.98 diethyl succinate 52800 4800 23100200000 [16] 0.1-0.5light fruity 45 26.40 ethyl 9-decenoate 5 1 1 100[*] <0.1 light fruity, fatty[8] 46 26.62 β- terpineol 200 20 80 110-400[13] 0.1-0.5wood, soil [8] 47 27.10 3-methoil-1-propanol 120 60 70 1000[15] 0.1 raw potato, garlic 48 28.53 1-decanol 150 20 60 400 [2] 0.1-0.5orange flowery, special fatty 49 29.71 phenethyl acetate 500 80 170 250 [16] 0.5-1 pleasant, floral 50 29.86 β-damascenone 20 3 7 0.05 [16] >1 bark, canned peach, baked apple, dry lu 51 30.60 ethyl laurate 40 0 5 1500[*] <0.1 sweet, floral, fruity, cream 52 30.91 hexanoic acid 1700 100 900 420 [16] >1 cheese, rancid 53 31.34 benzyl alcohol 2000 500 900 200000[15] <0.1 almond 54 32.15 2-phenyl-ethanol 140100 308007170014000 [16] >1 flowery, pollen, perfume 55 32.99 5-butyl-dihydro-4-methyl -2 3H -furanone 1350 80 170 67[2] >1 peach, coco 56 33.44 dodecan-1-ol 40 0 10 1000 [2] <0.1 unpleasant in higher concentration, flower in low concentration 57 34.48 p-ethyl-2-methoxy phe- nol 10 0 1 33[16] 0.1 medicine, wood, clove, smoky 58 34.76 [E]-nerolidol 200 10 30 700[*] 0.1-0.5wood, orange, light fruity 59 34.90 ethyl myristate 10 0 1 2000[*] <0.1 sweet fruity, butter, fatty odor[8] 60 35.24 octanoic acid 10000 700 3400 500 [16] >1 rancid, harsh, cheese, fatty acid 61 36.77 eugenol 6 1 1 6[2,17] 0.1-0.5smoky, clove 62 36.94 p-ethyl phenol 24 2 8 440[15] <0.1 phenolic, leather, spicy, almond 63 38.15 ethyl hexadecanoate 24 0 2 1500 [2,18] <0.1 fatty, rancid, fruity, sweet 64 38.59 n-decanoic acid 730 10 140 1000 [16] 0.1-0.5fatty, unpleasant 65 38.94 2,4-di-tert-butyl-phenol 370 60 150 200 [2,17] 0.5-1 phenolic (a) The reference from which the odor threshold and odor description have been taken is given in parentheses. [1] Guth (1997b). The matrix was a 10% water/ethanol solution; [2] and [19] Li (2006) and Sun et al. (2004). The matrix was a 12% ethanol/water mixture containing 5 g/L tartaric acid at pH 3.2. [8,15-18] Ferreira et al. (2000), Aznar et al. (2003), Cullere et al. (2004), Gomez et al. (2007) and Lopez et al. (2004). The matrix was an 11% water/ethanol solution containing 7 g/l glycerol and 5 g/l tartaric acid, with the pH adjusted to 3.4 with 1 M NaOH; [13] José et al. (2004). The matrix was a 10% water/ethanol solution containing 5 g/l tartaric acid. [20] Peinado, et al. (2004). The matrix was an 11% water/ethanol solution containing 5 g/l tartaric acid, with the pH adjusted to 3.4 with 1 M NaOH; [*] Calculated in the Laboratory of Wine Olfactometry, College of Enology, North- west A & F University, China. Orthonasal thresholds were calculated in a 12% ethanol/water mixture containing 5 g/L tartaric acid at pH 3.2. n.d., not detected. (b) Odor activity value calculated by dividing concentration by the odor threshold value of the compound.  Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 181 they made up of about 75% of the total aromatic com- pounds. Aromatic compounds with OAVs higher than one were isobutyl alcohol, isopentyl alcohol, 3-methyl-1- pentyl alcohol, 1-hexanol, (E,Z)-3-hexen-1-ol and 2- phenyl-ethanol. Isobutyl and isopentyl alcohols have fusel characters and may give a bitter or harsh sensory odor when in high concentrations. 3-methyl-1-pentyl al- cohol has soil and mushroom nuances. 1-hexanol and hexen-1-ol contribute green grass, herb odor. 2-phenyl- ethanol gives flowery, pollen, and perfume nuances. 3.3. Fatty Acids Four fatty acids were detected in the sample wines. Their concentrations ranged from 0.816 to 12.63 mg/L and accounted for 0.26-0.98% of the total volatile compounds. The OAVs of hexanoic and octanoic acids were higher than one. They contributed cheese and cream flavors in lower concentrations, while giving a rancid and harsh odor in higher concentration. Although the presence of C6–C10 fatty acids is usually related to the occurrence of negative odors, they are very important for aromatic equilibrium in wines because they oppose the hydrolysis of the corresponding esters [22]. 3.4. Terpenols Numerous studies have reported that the terpenoid com- pounds could be used analytically for varietal charac- terization. Terpene compounds belong to the secondary plant constituents, of which biosynthesis begins with acetyl-coenzyme A (CoA). Terpenes are not changed by yeast metabolism during fermentation [20]. Five terpenes were detected in sample wines: linalool, linalool oxide, 4-terpineol, β-terpineol and [E]-nerolidol. Only linalool had an OAV greater than one and contributed muscat, flowery and fruity odors. Because they have overlapping effects, terpenols may play an important role in contrib- uting to the overall aroma. 3.5. Norisoprenoids In this chemical group, α-ionol, β-ionol, and β-damas- cenone, three norisoprenoids often reported, were all detected in sample wines, and all had odor activity. The ionols are responsible for the raspberry, violet and sweet fruity nuances, while β-damascenone contributes odors of bark, canned peach, baked apple, and dry plum. 3.6. Volatile Phenols Some volatile phenols, such as eugenol and guaiacol, may be the major differences between Cabernet Sauvignon and other red variety wines [8]. Four phenols were iden- tified in our study, but all seemed to have no odor con- tribution. Eugenol and 2,4-di-tert-butyl phenol have OAVs between 0.5 to 1. Eugenol has smoky and clove odors. 2,4-di-tert-butyl phenol gives phenols’ chemical character. 3.7. Others An AEDA study regarding the odorants of Bordeaux Cabernet Sauvignon red wines showed that 3-methiol- 1-propanol, furaneol and homofuraneol had high flavor dilution (FD) factors [6]. In our work, two furans and one sulfur compound were detected in the sample wines: 2(3H)-dihydrofuran, 5-butyl-dihydro-4-methyl-2(3H)- furan and 3-methiol-1-propanol. Only 5-butyl-dihydro- 4-methyl-2(3H)-furan had odor activity and smelled of peach and cocoa. 4. CONCLUSIONS Aroma compounds in Cabernet Sauvignon dry red wine from Changli County were evaluated by SPME-GC-MS; 65 volatile compounds were identified and quantified. Their concentrations ranged from 0.5 μg/L to 2.23 g/L. Twenty-one volatile compounds were considered to be the powerful impact odorants of this wine because their OAVs were more than one. These active volatile com- pounds include eight esters, seven higher alcohols, two fatty acids, one terpenols (linalool), α/β-ionol and β-dam- ascenone, one compound of furan. These compounds have different sensory characters and give the wine very complicated aroma, which included not only pleasant floral and fruity odors, but also cheese, clove flavors, and grassy and smoky aromas. Taste or olfactory experiments could be designed to confirm the sensory characteristics of the wine. 5. ACKNOWLEDGEMENTS This project was supported by the China National Science Fund (30571281). The authors are grateful to the Huaxia Winemaking Com- pany and the Yueqiannian Winemaking Company (Changli County) for supplying the samples used in this study. REFERENCES [1] H. Guth, (1997) Quantiation and sensory studies of char- acter impact odorants of different white wine varieties. Journal of Agriculture and Food Chemistry, 45, 3027-3032. [2] H. Li, (2006) Wine Tasting. Beijing, China: China Science Press 29-106. [3] R. Perestrelo, A. Fernandes, F. F. Albuquerque, J. C. Marques, and J. S. Camara, (2006) Analytical characteriza- tion of the aroma of Tinta Negra Mole red wine: Identifica- tion of the main odorants compounds. Analytica Chimica Acta, 563, 154-164. [4] H. Li, Y. S.Tao, W. H. Kang, and C. L. Yin, (2006) Wine aroma analytical investigation progress on GC (Review). Journal of Food Science and Biotechnology (China), 25, 99-104. [5] M. Vilanova, and C. Martinez, (2007) First study of deter- mination of aromatic compounds of red wine from Vitis vinifera CV, Castanal grown in Galicia (NW Spain). Euro-  Y. S. Tao et al. / Natural Science 1 (2009) 176-182 SciRes Copyright © 2009 http://www.scirp.org/journal/HEALTH/Openly accessible at 182 pean Food Research and Technology, 224, 431-436. [6] Y. Kotseridis and R. Baumes, (2000) Identification of impact odorants in Bordeaux red grape juice, in the com- mercial yeast used for its fermentation, and in the pro- duced wine. Journal of Agriculture and Food Chemistry, 48, 400-406. [7] R. Lopez, V. Ferreira, P. Hernamdez, and J. F. Cacho, (1999) Identification of impact odorants of young red wines made with Merlot, Cabernet Sauvignon and Gren- ache grape varieties: A comparative study. Journal of the Science of Food and Agriculture, 79, 1461-1467. [8] V. Ferreira, R. Lopez, and J. F. Cacho, (2000) Quantitative determination of the odorants of young red wines from different grape varieties. Journal of the Science of Food and Agriculture, 80, 1659-1667. [9] M. Zhang, Q. Xu, C. Duan, and Y. Wu, (2007) Compara- tive study of aromatic compounds in young red wines from Cabernet Sauvignon, Cabernet Franc, and Cabernet Gernischet varieties in China. Journal of Food Science, 72(5), c248-c252. [10] Y. S. Tao, H. Li, and H. Wang, (2007) Optimization of wine aroma analysis by solid-phase microextraction. Journal of Northwest A & F University: Natural Science Edition, 35(12), 181-185. [11] O. I. V. (1990) Recueil des methods internationals d’ana- lyse des vins et des mouts. Paris: Office International de la Vigne et du Vin. [12] V. Ferreira, R. Lopez, A. Escudero, and J. F Cacho, (1998) Quantitative-determination of trace and ultra-trace flavor ac- tive compounds in red wines through Gas-Chromatographic Ion-Trap Mass-Spectrometric analysis of micro-extracts, Journal of Chromatography A, 806, 349-354. [13] M. O. José, M. A. Isabel, M. P. Óscar, S. M. José, and J. A. António, (2004) Characterization and differentiation of five “Vinhos Verdes” grape varieties on the basis of monoterpenic compounds. Analytica Chimica Acta, 513(1), 269-275. [14] H. Li , Y. S. Tao, H. Wang, and L. Zhang, (2008) Impact odorants of Chardonnay dry white wine from Changli County (China). European Food Research and Technology, 227(1), 287-292. [15] M. Aznar, R. Lopez, J. Cacho, and V. Ferreira, (2003) Prediction of aged red wine aroma properties from aroma chemical composition, Partial least squares regression models. Journal of Agriculture and Food Chemistry, 51, 2700-2707. [16] L. Cullere, A. Escudero, J. Cacho, and V. Ferreira, (2004) Gas chromatograpgy-Olfactory and chemical qualitative study of the aroma of six premium quality Spanish aged red wines. Journal of Agriculture and Food Chemistry, 52, 1653-1660. [17] M. J. Gomez, J. F. Cacho, V. Ferreira, I. M. Vicario, and F. J. Heredia, (2007) Volatile components of Zalema white wines. Food Chemistry, 100, 1464-1473. [18] R. Lopez, E. Ezpeleta, I. Sanchez, J. Cacho, and V. Ferreira, (2004) Analysis of the aroma intensities of vola- tile compounds released from mild acid hydrolysates of odorless precursors extracted from Tempranillo and Grenache grapes using gas chromatography-olfactometry. Food Chemistry, 88, 95-103. [19] B. G. Sun and Y. P. Liu, (2004) Food Spice and Flavor Handbook, China Petroleum Press. [20] R. A. Peinado, J. Moreno, M. Medina, and J. C. Mauricio, (2004) Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnology Letters, 26(9), 757-762. [21] M. Gil, J. M. Cabellos, T. Arroyo, and M. Prodanov, (2006) Characterization of the volatile fraction of young wines from the Denomination of Origin “Vinos de Madrid” (Spain). Analytica Chimica Acta, 563, 145–153. [22] A. Bertrand, (1981) Formation des substances volatiles au cours de la fermentation alcoolique, Incidence sur la qualit´e du vin. Colloque Soc. Fr. Microbiol., Reims, 252–267.
|