Paper Menu >>
Journal Menu >>
 Vol.1, No.3, 167-172 (2009) doi:10.4236/health.2009.13027 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Protective role of buffalo pineal proteins on arsenic-induced oxidative stress in blood and kidney of rats Vijay K. Bharti*, R. S. Srivastava Neurophysiology Laboratory, Division of Physiology & Climatology, Indian Veterinary Research Institute, Izatnagar, U.P., India; vijaykbharti@rediffmail.com *DIHAR (FRL), DRDO, C/o-56 APO, Leh-194101, India Received 26 September 2009; revised 12 October 2009; accepted 13 October 2009. ABSTRACT Objective: Exposure to various toxic metals has become an increasingly recognized source of ill- ness in human and animals, worldwide. Arsenic (As) and its compounds cause adverse health effects in animals and humans. Recently, it has been suggested that the pineal gland may also have antioxidants role due to secretary product other than melatonin. With keeping this view, pre- sent investigation tested effect of buffalo (Bubalus bubalis) pineal proteins (PP) on arsenic-induced oxidative stress in RBCs (Red blood cells) and kidney of rats. Methods: Eighteen adult female Wistar rats were grouped into group-I (Control), group-II (Arsenic control), and group-III (Arsenic + Pineal proteins). Experimental rats were given 100 ppm arsenic (p.o.) for 4 weeks alone or along with pineal proteins at a dose of 100 µg/kg body weight (i.p.). Results: Interestingly, arsenic ex- posure led to the stimulation of kidney catalase (CAT) activity, but inhibition of RBCs CAT activ- ity and significantly (P<0.05) increased the RBCs and kidney lipid peroxidation level (LPO). How- ever, arsenic treatment caused depletion of glu- tathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GR) in kidney tissues. In RBCs, only GR and CAT activity were significantly (P<0.05) declined. These changes were significantly (P<0.05) reversed by PP treatment in arsenic exposed animals. Conclusion: Therefore, present study indicated the significant protecting effect of buf- falo (Bubalus bubalis) PP against arsenic in- duced-oxidative stress through antioxidant de- fense systems in rats. Keywords: Arsenic Toxicity; Blood; Buffalo Pineal Proteins; Kidney; Oxidative Stress; Free-Radicals; Rat 1. INTRODUCTION Recent studies by Flora, 1999 [1], Bhadauria and Flora, 2007 [2] and Modi et al., 2007 [3] have suggested that arsenic toxicity is associated with the induction of oxida- tive stress in vital organs through overproduction of re- active free radicals (ROS) and inhibition of antioxidant enzymes activity. Research performed by Halliwell and Gutteridge, 1992 [4], shows the importance of certain enzymes in oxidative defense systems as they metabolize either free radicals or reactive oxygen intermediates to no-radical products. These enzymes play important role in free radical scavenging and amelioration of oxidative stress (OS). Study done by Halliwell and Gutteridge, 1992 [4] and Halliwell, 2006 [5], further revealed that antioxidant defense mechanisms in our vital organs are not sufficient to prevent toxicity-related increase in oxi- dative damage and therefore exogenous intake of anti- oxidants might have protecting role for preserving their physiological functions. A growing number of studies have been designed to test the antioxidant effect of some agents to prevent toxicity-related degenerative changes in vital organs. Studies done by Varner et al., 1998 [6] have shown the vital role of kidney in xenobiotics metabolism. Since, kidney and blood are among the most vulnerable biological system during oxidative stress and any xeno- biotics metabolic disturbances. Therefore, kidney damage due to OS further aggravates the kidney dysfunction. This may result in additional toxic metabolites load to renal system and may cause more insult to kidney due to high OS. Hence, in present study these organs are sampled for study. As per studies of Reiter, 1991 [7] and Halliwell, 2006 [5], supplementations of antioxidants are needed to enhance these antioxidative enzymes level for preventing OS. Studies of Reiter et al., 1995 [8] illustrated the neu- roendocrine functions of the pineal affect on wide variety of physiological role in our body. Few studies of Si- monneaux and Ribelayga, 2003 [9] and Tandon et al., 2006 [10] revealed that beside melatonin (MEL), the  V. K. Bharti et al. / HEALTH 1 (2009) 167-172 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 168 Table 1. Distribution of experimental rats to different treatments. Groups Body weight (g)Treatments Dose Route of administration I Control (28 D) 141.67 a ±3.57 Drinking water + Normal saline Adlibitum Oral Intra peritoneal II As 218.33 b ±11.53 Arsenic + Normal saline 100 ppm As Oral with drinking water Intra peritoneal III As+PP 145.00 a ±6.58 Arsenic + Pineal Proteins 100 ppm As 100 µg/kg BW Oral with drinking water Intra peritoneal Values (n=6); Means ±S.E) in the same column bearing no superscript (a, b, c) common vary significantly (P<0.05). As: arsenic; PP: Buffalo pineal proteins pineal gland secretes and expresses certain proteins es- sential for various physiological functions. The study of Bharti and Srivastava, 2009a [11] has suggested that the pineal gland may also have antioxidants role due to sec- retory product other than MEL. Literature regarding the physiological role of PP and peptides in arsenic-induced OS is completely lacking. However, earlier laboratory findings revealed that, these buffalo PP and peptides have certain physiological functions viz. immunopotentiation by Ramasamy, 2006 [12], heat stress by Sejian, 2006 [13], amelioration of fluoride-induced OS and apoptosis by Bharti, 2008 [14] and Bharti and Srivastava, 2009b [15]. These findings suggest the test for protecting role of PP against As-induced OS in kidney and blood. Therefore, the present study will investigate the physiological role of buffalo pineal proteins in amelioration of arsenic-induced oxidative stress through modulation of certain antioxidant defense systems. The present study will further substan- tiate the physiological role of PP on antioxidant defense systems, and findings may have significant implications in elucidating the therapeutic use of pineal proteins as antioxidants drugs and management of toxicity. 2. MATERIALS AND METHODS All the procedures conducted on the experimental ani- mals were duly approved by the Institutional Animal Ethics Committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). 2.1. Chemicals The chemical, glass and plastic were procured as per requirement from different suppliers. All these metal salts are soluble in water. All other chemicals used in the study were of analytical grade from HiMedia, Loba Chemie (Mumbai, India); Sigma Chemical Co., St. Louis; USA; Genei, Bangalore; SRL Chemicals, India. 2.2. Experimental Animals Experimental adult female Wistar rats (123-142 g BW) were procured from the Laboratory Animal Resource (LAR) Section, Indian Veterinary Research Institute, Izatnagar-243122, U.P., India. Rats on arrival were ex- amined for any abnormality or overt ill health. After an acclimatization period of one week, they were weighed and randomly assigned to various groups so as to give approximately equal initial group mean body weights. Rats were housed in polypropylene cages and rice husk was used as the nesting material. Animal room tempera- ture and relative humidity were set at 21±2 oC and 50±10%, respectively and lighting was controlled to give 12 h light and 12 h darkness. All the animals had free access to standard laboratory animal diet and clean water. The animals were checked daily for the health and hus- bandry conditions. 2.3. Experimental Design Eighteen adult female Wistar rats were grouped into control (Group I), arsenic control (Group II), and arsenic and buffalo pineal proteins (PP) (Group III) and given different treatment (see Table 1). Before the start of ex- periment, appropriate dose of arsenic and pineal proteins were optimized in pilot trial. Arsenic (As) level in drinking water was calculated and thereafter-required concentration of As (100 ppm) was made by additional sodium arsenite daily for 28 days. We used buffalo PP as our agent since we have previously published this reagent. Dose of pineal proteins was calculated based on animal body weight and thereafter, they were dissolved in vehicle (normal saline) and administered (daily for 28 day) in- traperitoneally (i.p.). Solutions for administration in ex- perimental animals were prepared daily to minimize possible instability of the chemicals in the mixture. 2.4. Sample Collection Daily observations were taken for the behavioral changes, clinical signs of toxicity and mortality, if any, throughout the experimental period. The rats were euthanized by using ether at the end of experiments (28 day), then blood and kidney were collected. The heparinised blood samples were centrifuged at 2000 rpm for 15 min, and RBCs (Red blood cells) were separated. The resulting erythrocyte (RBCs) pellet was washed thrice with phosphate buffer saline (PBS, pH 7.4) and dilution (33%) was made in PBS (pH 7.4). This 33% packed RBCs was used for the estimation of lipid per- oxidation (LPO), reduced glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx) and hae-  V. K. Bharti et al. / HEALTH 1 (2009) 167-172 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 169 169 moglobin (Hb). Another 1:10 dilution of packed RBCs in PBS was used for the estimation of catalase (CAT) and superoxide dismutase (SOD). Immediately, kidney was cleaned, rinsed in chilled sa- line, blotted and weighed. Thereafter, 200 mg of sample was weighed and taken in 2 ml of ice-cold saline. Another 200 mg of sample was weighed separately and taken in 2 ml of 0.02 M EDTA for GSH estimation. Kidney ho- mogenates were prepared using IKA homogenizer (Ger- many), under ice-cold condition. Homogenate were col- lected and centrifuged for 10 min at 3000 rpm at 4˚C. Thereafter, cell free supernatant were collected and trans- ferred to pre-cooled microfuge tube in duplicate and stored at below -20 oC. These supernatants were used for estima- tion of total proteins, LPO, CAT, SOD, GPx, GR activity, and GSH concentration. 2.5. Analytical Procedures Enzymatic (CAT, SOD, GR, GPx) and non-enzymatic (GSH) antioxidant defense systems are essential to combat OS in the body. These parameters are up regulated by antioxidants supplementation. Therefore, to test anti- oxidant properties of PP, we examined these parameters. Absorbance of all the tissue biochemical estimations was read, using Double Beam UV-VIS Spectrophotometer (UV 5704 SS, ECIL, India). 2.5.1. Lipid Peroxidation (LPO) Membrane peroxidative damage in RBCs and kidney tissues due to free radicals was determined in terms of malondialdehyde (MDA) production by the method of Rehman, 1984 [16]. Lipid peroxidation in blood was expressed as n M of MDA per ml of RBCs homogenates and in kidney tissues as nM of MDA formed per g of wet tissue. 2.5.2. Reduced Glutathione (GSH) The concentration of GSH in kidney homogenates was estimated by evaluating free-SH groups, using DTNB method described by Sedlak and Lindsay, 1968 [17] and the results have been expressed as µM/g of GSH per g of wet tissue. However, in blood, GSH was estimated by the 5, 5’dithiobis (2-nitrobenzoic acid; DTNB) method of Prins and Loos, 1969 [18] and expressed as mM GSH per ml of packed RBCs. 2.5.3. Catalase (CAT) Activities of catalase enzymes in blood and kidney tissues were estimated by spectrophotometric method as describ- ed by Bergmeyer, 1983 [19] and were expressed as nM H2O2 utilized /min /mg protein and in blood, mM H2O2 utilized /min /mg Hb 2.5.4. Superoxide Dismutase (SOD) Superoxide dismutase activities in blood and kidney homogenates were estimated as per the method described by Madesh and Balasubramanian, 1998 [20]. It involves generation of superoxide by Pyrogallol autoxidation and the inhibition of superoxide-dependent reduction of the tetrazolium dye MTT [3-(4-5 dimethyl thiazol 2-xl) 2, 5 diphenyl tetrazolium bromide] to its formazan, measured at 570 nm. Superoxide dismutase activity in blood was expressed as U/g of Hb and as U/g of protein in kidney tissues. 2.5.5. Glutathione Peroxidase (GPx) Glutathione peroxidase (GPx) activities in blood and kidney were determined by the method of Paglia and Valantine, 1967 [21]. The enzyme activity in kidney has been expressed as U/mg of protein, and one unit of en- zyme activity is defined as 1 nM of substrate (NADPH) utilized/min/mg protein and in blood as µmole NADPH oxidized to NADP/ g of Hb/min. 2.5.6. Glutathione Reductase (GR) The GR activities in kidney and blood were assayed by the method of Goldberg and Spooner, 1983 [22]. The GR activity in kidney tissues has been expressed as nM NADPH oxidized to NADP/ g of protein/min and in blood as nM NADPH oxidized to NADP/ g of Hb/min. 2.5.7. Protein Assay Protein contents in kidney homogenates were determined and calculated by the method of Lowry et al., 1951 [23] and result has been expressed as mg/g of tissue. 2.5.8. Hb Estimation Haemoglobin concentrations in packed RBCs were esti- mated by spectrophotometric method using Drabkin’s solution (Span India Kit). 2.5.9. Statistical Analysis Differences between groups were statistically analyzed by one-way ANOVA, and the differences between the means of groups were separated by least significant dif- ference (LSD) test. All data were presented as mean±standard error. Values of p< 0.05 were regarded as significant. A computer program (SPSS 10.01, SPSS Inc. Chicago, IL, USA) was used for statistical analysis. 3. RESULTS We evaluated different enzymatic and non-enzymatic antioxidant defense system to assess the As-induced OS and its amelioration by PP. All the comparisons were made between vehicle and PP with control animals (28 days). We did not find any abnormal behavior in animals during the entire period of investigation and none of the animals died in any group. We did not observe any gross abnor- malities in the organs of female rats in different treat- ments. Experimental data obtained in present study were presented in tabular form (Means ±S.E) (see Tables 2, 3). A significant (P<0.05) increase in lipid peroxides level along with a concomitant decrease in the activities of CAT and GR antioxidants enzymes were observed in RBC (Red blood cells) of arsenic administered (100 ppm As/day in 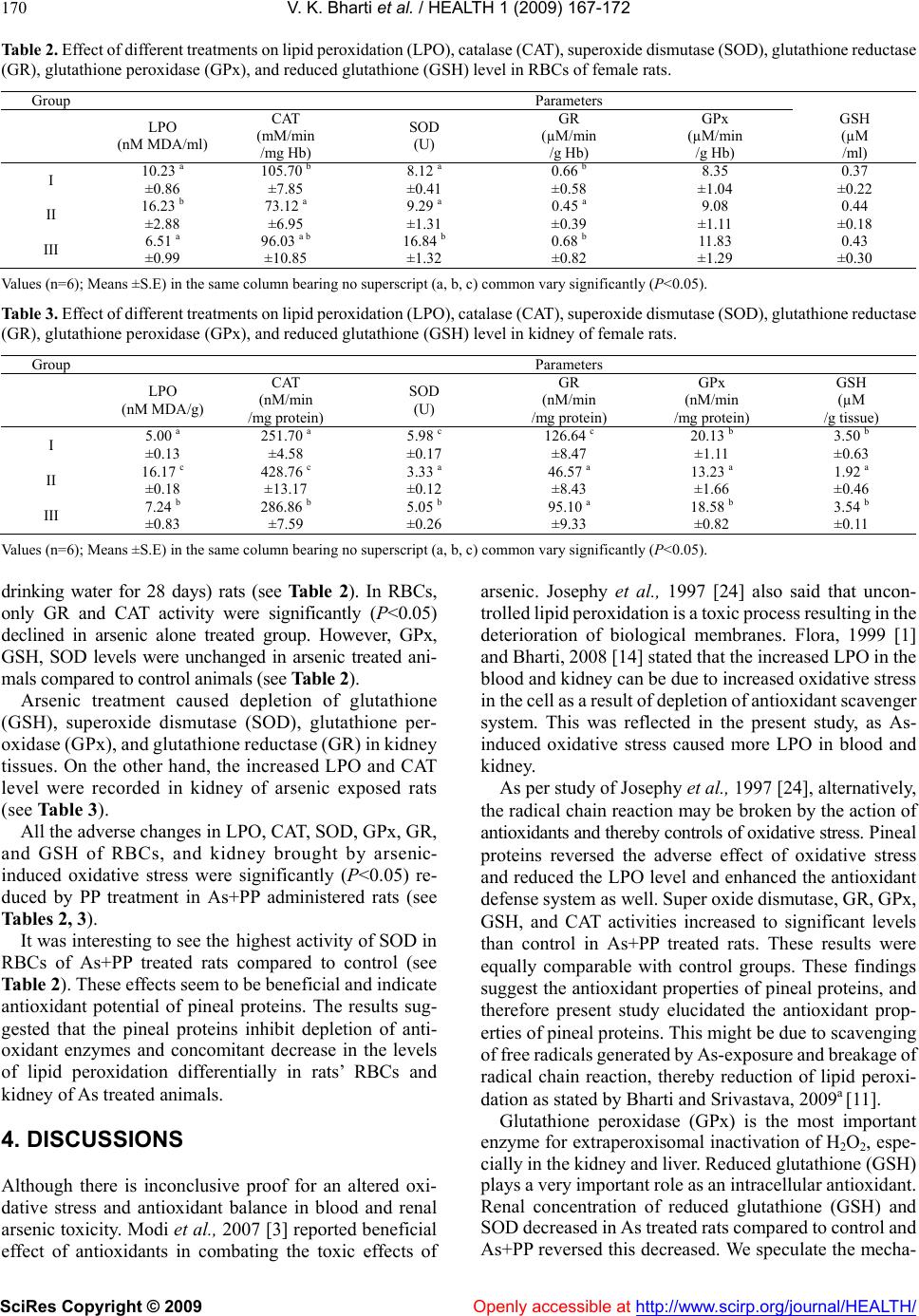 V. K. Bharti et al. / HEALTH 1 (2009) 167-172 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 170 Table 2. Effect of different treatments on lipid peroxidation (LPO), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx), and reduced glutathione (GSH) level in RBCs of female rats. Group Parameters LPO (nM MDA/ml) CAT (mM/min /mg Hb) SOD (U) GR (µM/min /g Hb) GPx (µM/min /g Hb) GSH (µM /ml) I 10.23 a ±0.86 105.70 b ±7.85 8.12 a ±0.41 0.66 b ±0.58 8.35 ±1.04 0.37 ±0.22 II 16.23 b ±2.88 73.12 a ±6.95 9.29 a ±1.31 0.45 a ±0.39 9.08 ±1.11 0.44 ±0.18 III 6.51 a ±0.99 96.03 a b ±10.85 16.84 b ±1.32 0.68 b ±0.82 11.83 ±1.29 0.43 ±0.30 Values (n=6); Means ±S.E) in the same column bearing no superscript (a, b, c) common vary significantly (P<0.05). Table 3. Effect of different treatments on lipid peroxidation (LPO), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx), and reduced glutathione (GSH) level in kidney of female rats. Group Parameters LPO (nM MDA/g) CAT (nM/min /mg protein) SOD (U) GR (nM/min /mg protein) GPx (nM/min /mg protein) GSH (µM /g tissue) I 5.00 a ±0.13 251.70 a ±4.58 5.98 c ±0.17 126.64 c ±8.47 20.13 b ±1.11 3.50 b ±0.63 II 16.17 c ±0.18 428.76 c ±13.17 3.33 a ±0.12 46.57 a ±8.43 13.23 a ±1.66 1.92 a ±0.46 III 7.24 b ±0.83 286.86 b ±7.59 5.05 b ±0.26 95.10 a ±9.33 18.58 b ±0.82 3.54 b ±0.11 Values (n=6); Means ±S.E) in the same column bearing no superscript (a, b, c) common vary significantly (P<0.05). drinking water for 28 days) rats (see Table 2). In RBCs, only GR and CAT activity were significantly (P<0.05) declined in arsenic alone treated group. However, GPx, GSH, SOD levels were unchanged in arsenic treated ani- mals compared to control animals (see Table 2). Arsenic treatment caused depletion of glutathione (GSH), superoxide dismutase (SOD), glutathione per- oxidase (GPx), and glutathione reductase (GR) in kidney tissues. On the other hand, the increased LPO and CAT level were recorded in kidney of arsenic exposed rats (see Table 3). All the adverse changes in LPO, CAT, SOD, GPx, GR, and GSH of RBCs, and kidney brought by arsenic- induced oxidative stress were significantly (P<0.05) re- duced by PP treatment in As+PP administered rats (see Tables 2, 3). It was interesting to see the highest activity of SOD in RBCs of As+PP treated rats compared to control (see Table 2). These effects seem to be beneficial and indicate antioxidant potential of pineal proteins. The results sug- gested that the pineal proteins inhibit depletion of anti- oxidant enzymes and concomitant decrease in the levels of lipid peroxidation differentially in rats’ RBCs and kidney of As treated animals. 4. DISCUSSIONS Although there is inconclusive proof for an altered oxi- dative stress and antioxidant balance in blood and renal arsenic toxicity. Modi et al., 2007 [3] reported beneficial effect of antioxidants in combating the toxic effects of arsenic. Josephy et al., 1997 [24] also said that uncon- trolled lipid peroxidation is a toxic process resulting in the deterioration of biological membranes. Flora, 1999 [1] and Bharti, 2008 [14] stated that the increased LPO in the blood and kidney can be due to increased oxidative stress in the cell as a result of depletion of antioxidant scavenger system. This was reflected in the present study, as As- induced oxidative stress caused more LPO in blood and kidney. As per study of Josephy et al., 1997 [24], alternatively, the radical chain reaction may be broken by the action of antioxidants and thereby controls of oxidative stress. Pineal proteins reversed the adverse effect of oxidative stress and reduced the LPO level and enhanced the antioxidant defense system as well. Super oxide dismutase, GR, GPx, GSH, and CAT activities increased to significant levels than control in As+PP treated rats. These results were equally comparable with control groups. These findings suggest the antioxidant properties of pineal proteins, and therefore present study elucidated the antioxidant prop- erties of pineal proteins. This might be due to scavenging of free radicals generated by As-exposure and breakage of radical chain reaction, thereby reduction of lipid peroxi- dation as stated by Bharti and Srivastava, 2009a [11]. Glutathione peroxidase (GPx) is the most important enzyme for extraperoxisomal inactivation of H2O2, espe- cially in the kidney and liver. Reduced glutathione (GSH) plays a very important role as an intracellular antioxidant. Renal concentration of reduced glutathione (GSH) and SOD decreased in As treated rats compared to control and As+PP reversed this decreased. We speculate the mecha-  V. K. Bharti et al. / HEALTH 1 (2009) 167-172 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 171 171 nism of protective effect of PP against As-induced oxi- dative stress through scavenging of free radicals gener- ated by As-exposure and breakage of radical chain reac- tion, thereby reduction of lipid peroxidation. As per ex- perimental study of Bharti and Srivastava, 2009a [11], this physiological role of PP may be due to presence of certain enzymes influencing it or via melatonin secretion on body antioxidant defense systems. Therefore, administration of pineal proteins is proved to be beneficial on vital organs antioxidant defense system during As-induced oxidative stress in rats. Therefore, these findings support the hypothesis of protecting role of buffalo pineal proteins against arse- nic-induced oxidative stress through its antioxidant properties and also find agreement with earlier findings of Bharti and Srivastava, 2009b [15] about free radical scav- enging ability of pineal proteins. 5. CONCLUSIONS Pineal proteins reduced the arsenic-induced OS level in kidney and blood as reflected by low LPO and higher activities of catalase, GPx, GR, SOD, and GSH level. Hence, these findings indicate the protecting role of PP against As-induced OS in kidney and blood through modulation of certain antioxidant defense systems. The present study further substantiate the physiological role of PP on antioxidant defense systems and findings have significant implications in elucidating the therapeutic use of pineal proteins as antioxidants drugs and management of toxicity. 6. ACKNOWLEDGEMENTS Project grant and facilities provided by Indian Veterinary Research Institute for conducting this study is duly ac- knowledged. I also acknowledge the tireless efforts of our lab and animal shed assistants. REFERENCES [1] S. J. S. Flora, (1999) Arsenic induced oxidative stress and its reversibility following combined administration of N- acetyl cysteine and meso 2, 3-dimercaptosuccinic acid in rats. Clinical and Experimental Pharmacology Physiology, 26, 865-869. [2] S. Bhadauria and S. J. S. Flora, (2007) Response of arse- nic-induced oxidative stress, DNA damage, and metal imbal- ance to combined administration of DMSA and monoisoa- myl-DMSA during chronic arsenic poisoning in rats. Cell Biology and Toxicology, 23, 91-104. [3] M. Modi, M. Mittal, and S. J. S. Flora, (2007) Combined administration of selenium and meso-2, 3-dimercapto- succinic acid on arsenic mobilization and tissue oxidative stress in chronic arsenic-exposed male rats. Indian Journal of Pharmacology, 39, 107-114. [4] B. Halliwell and J. M. Gutteridge, (1992) Free radicals, antioxidants, and human disease: where are we now. Journal of Laboratory and Clinical Medicine, 119, 598-620. [5] B. Halliwell, (2006) Oxidative stress and neurodegenera- tion: where are we known. Journal of Neurochemistry, 97, 1634-1658. [6] J. A. Varner, K. F. Jenson, W. Horvath, and R. L. Isaacson, (1998) Chronic administration of aluminum fluoride or sodium fluoride to rats in drinking water: alteration in neuronal and cerebrovascular integrity. Brain Research, 784, 284-298. [7] R. J. Reiter, (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocri- nology Review, 12, 151-180. [8] R. J. Reiter, D. Melchiorri, E. Sewerynek, and B. Poeggler, (1995) A review of the evidence supporting melatonin’s role as an antioxidant. Journal of Pineal Research, 23, 43-50. [9] V. Simonneaux and C. Ribelayga, (2003) Generation of the melatonin endocrine message in mammals: A Review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacological Review, 55, 325-395. [10] M. Tandon, R. S. Srivastava, S. K. Meur, and M. Saini, (2006) Proteins and peptides present in pineal gland and other brain structures of buffaloes. Indian Journal of Animal Science, 76(5), 383-394. [11] V . K. Bharti and R. S. Srivastava, (2009a) Pineal proteins up-regulate specific antioxidant defense systems in the brain. Oxidative Medicine and Cellular Longevity, 2, 88-92. [12] M. Ramasamy, (2006) Studies on bubaline pineal pro- teins/peptides below 20 kDa and their immunopotentia- tion in guinea pigs. Ph.D. Thesis. Indian Veterinary Re- search Institute, Izatnagar, India. [13] V. Sejian, (2006) Studies on pineal-adrenal relationship in goats (Capra hircus) under thermal stress. Ph.D.Thesis. Indian Veterinary Research Institute, Izatnagar, India. [14] V. K. Bharti, (2008) Studies on buffalo (Bubalus bubalis) pineal proteins on fluoride-induced oxidative stress and apoptosis in rats. Ph.D. Thesis. Indian Veterinary Re- search Institute, Izatnagar, India. [15] V. K. Bharti and R. S. Srivastava, (2009b) Fluoride-in duced oxidative stress in rat's brain and its amelioration by buffalo (Bubalus bubalis) pineal proteins and melatonin. Biological Trace Element Research, 130, 131-140. [16] S. Rehman, (1984) Lead-induced regional lipid peroxida- tion in brain. Toxicology Letter, 21 (3), 333-337. [17] J. Sedlak and R. H. Lindsay, (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry, 25(1), 192-205. [18] H. K. Prins and J. A. Loos, (1969) In Glutathione, Bio- chemical methods in red cell genetics, edited by J. J. Yunis. Academic Press, New York, 127-129. [19] H. U. Bergmayer, (1983) UV method of catalase assay. In Methods of Enzymatic Analysis, 3, Weinheim Deer field Beach, Florida, Bansal, 273. [20] M. Madesh and K. A. Balasubramanian, (1998) Microtitre plate assay for superoxide dismutase using MTT reduction by superoxide. Indian Journal of Biochemistry and Bio- physics, 35, 184-188.  V. K. Bharti et al. / HEALTH 1 (2009) 167-172 SciRes Copyright © 2009 http://www.scirp.org/journal/HEALTH/Openly accessible at 172 [21] D. E. Paglia and W. N. Valentine, (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158-169. [22] D. M. Goldberg and R. J. Spooner, (1983) Glutathione Reductase, J. Bergmeyer, M. Grassi, eds, Methods in En- zymatic Analysis, VCH Weinheim, Germany, 258-265. [23] O. H. Lowry, N. J. Rosebrough, A. I. Farr, and R. J. Randall, (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265-275. [24] P. D. Josephy, B. Mannervik, and P. O. Montellano, (1997) Oxidative stress in the erythrocyte. Molecular Toxicology, First Edition, Oxford University Press, New York, USA. |

