 Chinese Medicine, 2011, 2, 130-137 doi:10.4236/cm.2011.24022 Published Online December 2011 (http://www.SciRP.org/journal/cm) Copyright © 2011 SciRes. CM Effects of Bofutsushosan and Gardeniae Fructus on Diabetic Serum Parameters in Stre ptozotocin-Induced Diabetic Mice Qing Yu1, Mai Yasuda1, Tatsuo Taka h a sh i 1, Masaaki Nomura2, Nobuyoshi Hagino3, Shinjiro Kobayashi1* 1Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Hokuriku University, Kanazawa, Japan 2Department of Education Center of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Hokuriku University, Kanazawa, Japan 3Laboratory of Integrative Medicine, US-Japan Cooperative Biomedical Research Laboratories, Tulane University Herbert Research Center and Department of Medicine, Tulane University Health Science Center, School of Medicine, Belle Chasse, USA E-mail: *s-kobayashi@hokuriku-u.ac.jp Received July 9, 2011; revised August 14, 2011; accepted August 30, 2011 Abstract Streptozotocin (STZ)-induced diabetic mice increased levels of serum glucose, triglyceride and cholesterol, and decreased level of serum insulin. Effects of Bofutsushosan (BOF: Pulvis ledebouriellae compositae: 防風通聖 散) and its composed crude drug, gardeniae fructus (GF: 山梔子) were investigated on levels of these diabetic parameters (serum glucose, insulin, triglyceride and cholesterol) in STZ-diabetic mice. BOF and GF were ex- tracted in 10 volumes of distilled water with an automatic extractor “Torobi”. STZ-induced diabetic mice with serum glucose level of over 600 mg/dl at 3 - 4 weeks after intravenous injection of 150 mg/kg STZ were used for experiments. BOF extract, GF extract, geniposide (a main constituent of GF), and glibenclamide were ad- ministered intraperitoneally into 3-hour-fasted STZ-diabetic mice. At 6 hours after administration, BOF extract (100 - 300 mg/kg) decreased high levels of serum glucose, triglyceride and cholesterol, and also increased low level of serum insulin in STZ-diabetic mice in a dose-dependent manner, respectively. Anti-diabetic drug glibenclamide (0.3 - 1 mg/kg) as positive control significantly decreased serum glucose and cholesterol levels, and increased serum insulin level in the diabetic mice. GF extract (30 - 300 mg/kg) decreased serum glucose, triglyceride and cholesterol levels but did not affect serum insulin level in the diabetic mice. Geniposide (10 - 100 mg/kg), decreased serum glucose level but did not affect serum insulin and triglyceride levels in the dia- betic mice. These results demonstrated that intraperitoneally administrated BOF extract improved abnormal levels of serum glucose, insulin, triglyceride and cholesterol in the STZ-diabetic mice as being similar to glibenclamide. GF extract has an important role in a part of improving actions of BOF in the diabetic mice. The action of GF extract on serum glucose was parallel with the action of geniposide in the diabetic mice, support- ing roles of geniposide in anti-hyperglycemic action of GF. Keywords: Bofutsushosan (Pulvis Ledebouriellae Compositae: 防風通聖散), Gardeniae Fructus (山梔子), Geniposide, Streptozotocin-Induced Diabetic Mice, Anti-Hyperglycemic Action, Anti-Hyperlipidemic Action 1. Introduction Diabetes mellitus is a chronic heterogeneous disease cha- racterized by hyperglycemia resulting from both insulin resistance and insulin deficiency secondary to pancreatic cell failure. Diabetes mellitus through hyperglycemia is widely known to be a major factor that leads to micro- vascular and neural complication [1]. Hyperglycemia-  131 Q. YU ET AL. induced end organ damage in diabetes mellitus is associ- ated with 1) accumulation of advanced glycation end products [2,3]; 2) increased oxidative stress [4,5]; 3) pol- yol metabolic pathway [6,7]; and 4) activation of protein kinase C pathway [8,9]. Insulin deficiency in diabetes mellitus stimulates lipolysis in the adipose tissue, and gives rise to hyperlipidemia and fatty liver [10]. Althou- gh diabetes mellitus is characterized as a disease of car- bohydrate metabolism, abnormalities of lipid and lipo- protein metabolism are commonly observed. Hyperlipi- demia [11] and atherosclerosis [12] are frequently asso- ciated with diabetes mellitus, indicating alterations of cholesterol metabolism in this diabetes [13]. In addition, there is triglyceride enrichment of both high-density lipoprotein (HDL) and low-density lipoprotein (LDL). Therefore, the lipid profile in type 2 diabetic subjects generally consists of elevated triglycerides and LDL cho- lesterol, and reduced HDL cholesterol [14,15]. Diabetes- related dyslipidaemia has also been shown to be an im- portant contributor to vascular dysfunction. Atheroscle- rosis is, however, associated with macrovascular disease and thus the role of dyslipidaemia versus hyperglycemia in the pathogenesis of microvascular disease remains unclear and continues to be debated [16]. Streptozotocin (STZ) is N-nitroso derivative of gluco- samine and has been commonly used to induce not only animal models of insulin-dependent diabetes mellitus (IDDM, type 1) but also non-insulin-dependent diabetes mellitus (NIDDM, type 2) with hypoinsulinemia by STZ administration to neonates (1 or 2 day old mice) [17-19]. It has been reported that STZ is capable of producing mild to severe types of diabetes according to the dosages used when it is given to animals by either single intrave- nous or intraperitoneal (i.p.) injection [20]. Using this experimental model, we sought to investi- gate the efficacy of traditional Chinese medicine, bofu- tsushosan (BOF: Pulvis ledebouriellae compositae) on the prevention of abnormal levels of diabetic parameters, serum glucose, insulin, triglyceride and cholesterol. BOF is consisted of 18 crude drugs as described in Table 1 [21]. BOF is indicated for the relief of the following sy- mptoms of those patients with hypertension, insulin re- sistance and thick subcutaneous fat in the abdomen and tendency to constipation [21]. BOF has been successfully used in patients with hyper functional constitution who exhibit risk factors for cerebrovascular events. Gardeniae fructus (GF) has traditionally classified as an antipyretic agent in BOF. Chemical isolated from GF has been sug- gested to increase glucose-stimulated insulin release fr- om pancreatic cells of type 2 diabetes mellitus model [22]. In the present study, effects of extracts of BOF and GF were compared on levels of the diabetic parameters in STZ-diabetic mice to study a role of GF in action of BOF in the STZ-diabetic mice. 2. Materials and Methods 2.1. Preparation of Streptozotocin-Diabetic Mice Fed male mice (ddY strain; 4 weeks of age; 16 - 20 g; Japan SLC, Shizuoka, Japan) were injected with a single dose (150 mg/kg) of STZ (Sigma, St. Louis, MO, U.S.A.) in saline into the tail vein. STZ-induced diabetic mice (7- 8 weeks of age; blood glucose over 600 mg/dl) were used for experiments at 3 - 4 weeks after the injection of STZ. Age-matched normal male mice (ddY strain; 7 - 8 weeks of age) were used in the control experiments. These mice were given CRF-1 (Oriental Yeast Co., To- kyo, Japan) and water ad libitum and kept at 25˚C - 26˚C with lights on from 7 a.m. to 7 p.m. Drugs were adminis- tered intraperitoneally to mice that had been fasted for 3 hours. The Ethics Review Committee for Animal Expe- rimentation of Hokuriku University approved the expe- rimental protocol. 2.2. Preparation and Administration of Drugs BOF consists of Ephedrae Herba, Saposhnikoviae Radix, Zingiberis Rhizoma, Schizonepetae Spica, Rhei Rhizo- ma, Natrium Sulfuricum, Glycyrrhizae Radix, Forsythiae Table 1. Crude drugs composed in bofutsushosan. Crude drugs content (g)a 1 Ephedrae Herba 1.2 2 Saposhnikoviae Radix 1.2 3 Zingiberis Rhizoma 0.4 4 Schizonepetae Spica 1.2 5 Rhei Rhizoma 1.5 6 Natrium Sulfuricum 0.6 7 Glycyrrhizae Radix 2.0 8 Forsythiae Fructus 1.2 9 Platycodi Radix 2.0 10 Cnidii Rhizoma 1.2 11 Scutellariae Radix 2.0 12 Gardeniae Fructus 1.2 13 Gypsum Fibrosum 2.0 14 Talcum 3.0 15 Angelicae Radix 1.2 16 Paeoniae Radix 1.2 17 Atractylodis Lanceae Rhizoma 2.0 18 Menthae Herba 1.2 aEach value (g) was represented as dry weight. Copyright © 2011 SciRes. CM  Q. YU ET AL. Copyright © 2011 SciRes. CM 132 Fructus, Platycodi Radix, Cnidii Rhizoma, Scutellariae Radix, GF, Gypsum Fibrosum, Talcum, Angelicae Radix, Paeoniae Radix, Atractylodis Lanceae Rhizoma, and Men- thae Herba. BOF and GF were purchased from Tsumura Co. (Tokyo) and extracted in 10 volumes of distilled wa- ter with an automatic extractor “Torobi” (Tochimoto, Osaka, Japan) for 60 min. A water extract of the drug was filtered through a mesh (No. 42, Sanpo, Tokyo), ly- ophilized with a freeze-drier (DF-03G, ULVAC, Tokyo), and stored at 4˚C [23,24]. The dry weight yields of BOF and GF extracts were 27.5% and 67.7% (w/w), respecti- vely. BOF extract, GF extract, geniposide (Wako, Osaka), a main constituent of GF, and glibenclamide (Wako) we- re administered intraperitoneally (0.1 ml/10 g body wei- ght) into 3-hour-fasted STZ-diabetic mice. 2.3. Measurement of Glucose, Insulin, Triglyceride and Cholesterol Levels in Serum Blood was collected from the neck vein plexus of diabe- tic mice at 6 hours after the administration of drugs, and centrifuged at 8000 rpm at 25˚C for 5 min. Serum gluco- se level of the supernatant was measured by the glucose oxidase method with a serum glucose monitor set (MED- ISAFE MINI, Terumo, Tokyo). Serum glucose levels were measured in fasted mice before, and 6 hours after the administration of drugs or saline, respectively. The fall % of serum glucose (SG) was calculated as [SG (be- fore drug treatment) – SG (after drug treatment)]/[SG (before drug treatment) – 85] × 100. The average of SG of 3-hour-fasted normal mice is 85 mg/dl [23,24]. Serum insulin levels of 3-hour-fasted mice were measured with a mouse ELISA kit for insulin (Morinaga, Yokohama, Japan) 6 hours after the administration of drugs or saline. Serum total triglyceride and cholesterol levels were measured with ELISA kits for triglyceride and choles- terol (Wako), respectively. 2.4. Statistical Analyses All values were expressed as means ± S.E.M.. Differen- ces between group data were evaluated by unpaired t-test at p = 0.05 or 0.01. A value of p < 0.05 was considered statistically significant. 3. Results 3.1. STZ-Induced Changes of Levels of Body Weight, Serum Glucose, Insulin, Triglyceride and Cholesterol in ddY Mice Levels of body weight, serum glucose, insulin, triglyc- eride and cholesterol in ddY mice during 3 weeks after injection of STZ were compared with those in age- matched normal mice in Table 2. The body weights of STZ-treated mice were significantly decreased to 93% of those of age-matched normal mice. Levels of diabetic parameters in sera of 3-hour-fasted ddY mice with STZ treatment were compared with those of 3-hour-fasted normal mice. Serum glucose levels of STZ-treated mice were 996.8 mg/dl and significantly 5.4-fold greater than those of normal mice. Serum insulin levels of STZ- treated mice were 183.7 pg/ml and lowered by 82% of normal insulin level. Levels of serum triglyceride and serum cholesterol of STZ-treated mice were 187.3 and 183.2 mg/dl, respectively and increased to 87% and 35% of normal levels. Therefore, we used STZ-treated mice as the diabetic model. 3.2. Effects of Bofutsushosan (BOF) and Glibenclamide on Levels of Serum Glucose and Insulin in STZ-Diabetic Mice Effects of BOF extract (30 - 300 mg/kg) on level of blood glucose were compared with that of anti-diabetic drug glibenclamide as positive control in i.p. treatment during 6 hours. After the drug injection, BOF extract (30 - 300 mg/kg) decreased high level of serum glucose of STZ-diabetic mice in a dose-dependent manner (Figure 1). Glibenclamide (Glc; 0.3 - 1 mg/kg) also significantly decreased high serum glucose level of STZ-diabetic mice. Glibenclamide showed anti-hyperglycemic action by its i.p. administration as well as BOF extract did. BOF ex- tract (30 - 100 mg/kg) was significantly elevated serum insulin level in a dose-dependent manner. Glibenclamide (0.3 - 1 mg/kg) also significantly increased serum insulin level (Figure 2 ) in the STZ-diabetic mice. Effects of BOF extract and glibenclamide on elevation of serum insulin levels were parallel with those anti-hyperglycemic ef- fects in STZ-diabetic mice. Table 2. Body weight, serum glucose, insulin, triglyceride (TG) and cholesterol (CH) of STZ-diabetic mice. Body weight (g) Glucose (mg/dl) Insulin (pg/ml) TG (mg/dl) CH (mg/dl) Normal mice 37.5 ± 0.3 183.3 ± 3.7 1024 ± 64.2 100.2 ± 5.2 135.4 ± 3.7 STZ mice 34.9 ± 0.3** 996.8 ± 43.3** 183.7 ± 25.0** 187.3 ± 10.3** 183.2 ± 4.8** Values represent means ± S.E.M. **p < 0.01: Significantly different from normal mice. 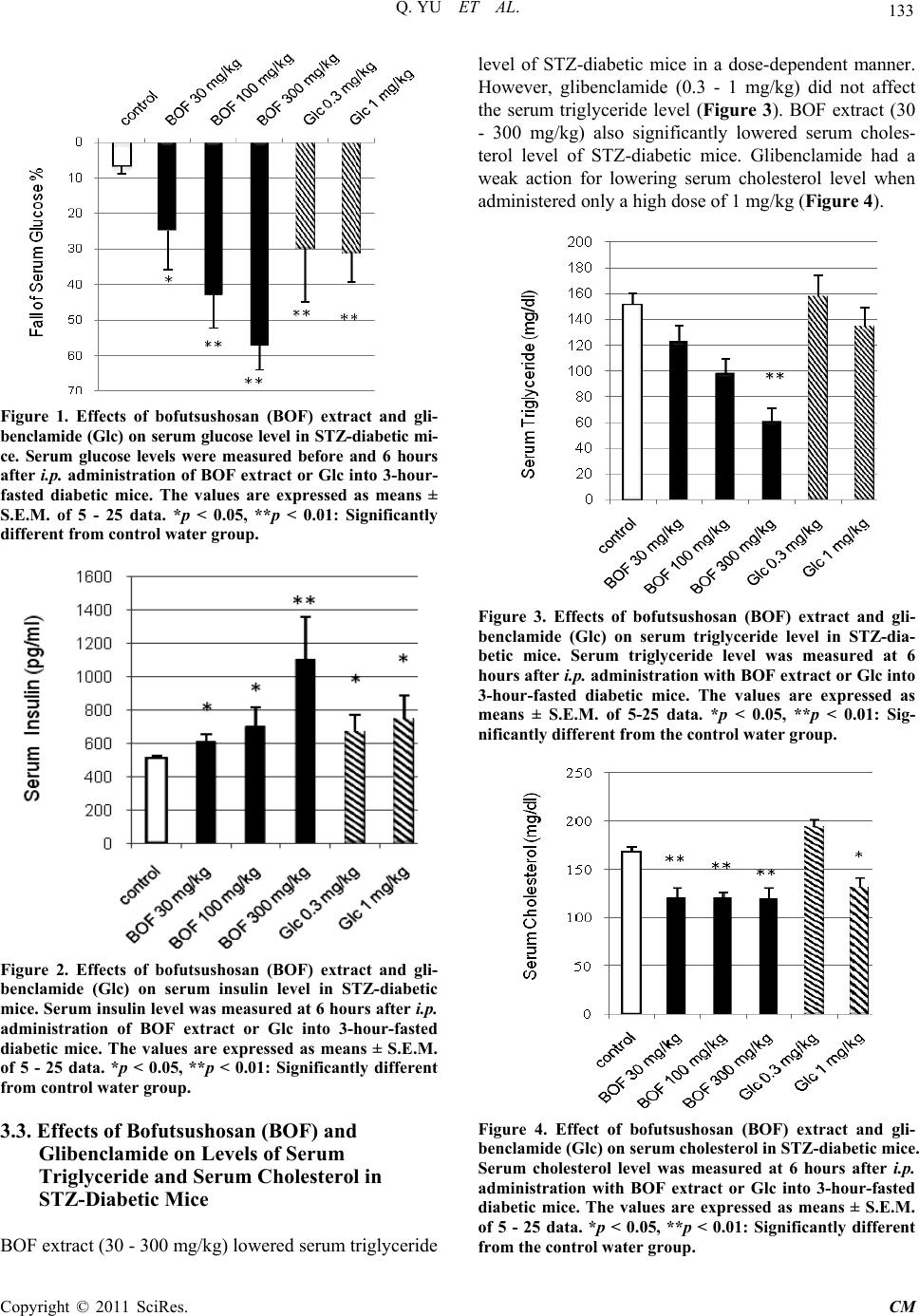 133 Q. YU ET AL. Figure 1. Effects of bofutsushosan (BOF) extract and gli- benclamide (Glc) on serum glucose level in STZ-diabetic mi- ce. Serum glucose levels were measured before and 6 hours after i.p. administration of BOF extract or Glc into 3-hour- fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 25 data. *p < 0.05, **p < 0.01: Significantly different from contr ol water group. Figure 2. Effects of bofutsushosan (BOF) extract and gli- benclamide (Glc) on serum insulin level in STZ-diabetic mice. Serum insulin level was measured at 6 hours after i.p. administration of BOF extract or Glc into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 25 data. *p < 0.05, **p < 0.01: Significantly different from control water group. 3.3. Effects of Bofutsushosan (BOF) and Glibenclamide on Levels of Serum Triglyceride and Serum Cholesterol in STZ-Diabetic Mice BOF extract (30 - 300 mg/kg) lowered serum triglyceride level of STZ-diabetic mice in a dose-dependent manner. However, glibenclamide (0.3 - 1 mg/kg) did not affect the serum triglyceride level (Figure 3). BOF extract (30 - 300 mg/kg) also significantly lowered serum choles- terol level of STZ-diabetic mice. Glibenclamide had a weak action for lowering serum cholesterol level when administered only a high dose of 1 mg/kg (Figure 4 ). Figure 3. Effects of bofutsushosan (BOF) extract and gli- benclamide (Glc) on serum triglyceride level in STZ-dia- betic mice. Serum triglyceride level was measured at 6 hours after i.p. administration with BOF extract or Glc into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5-25 data. *p < 0.05, **p < 0.01: Sig- nificantly different from the control water group. Figure 4. Effect of bofutsushosan (BOF) extract and gli- benclamide (Glc) on serum cholesterol in STZ-diabetic mice. Serum cholesterol level was measured at 6 hours after i.p. administration with BOF extract or Glc into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 25 data. *p < 0.05, **p < 0.01: Significantly different from the control water group. Copyright © 2011 SciRes. CM 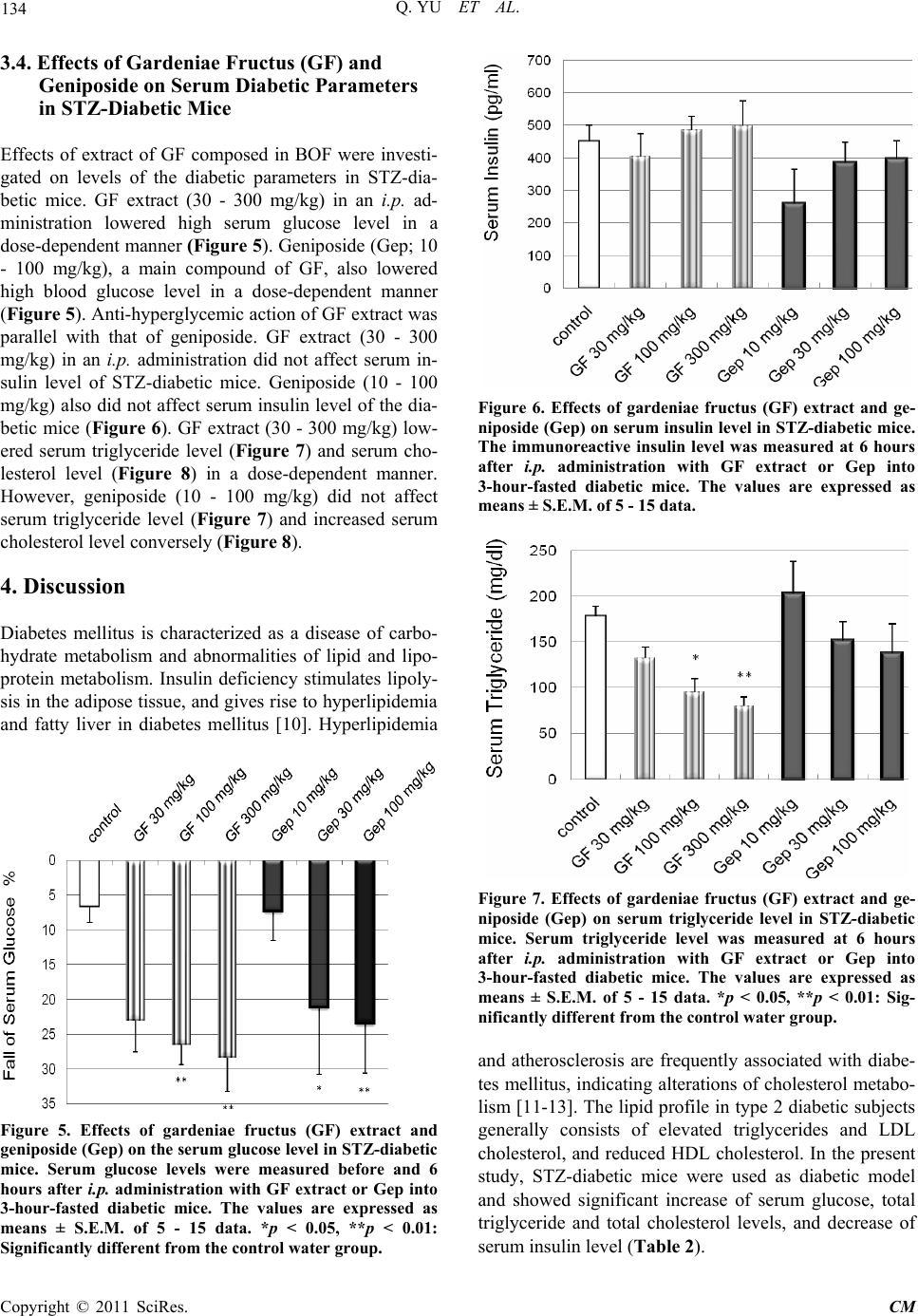 Q. YU ET AL. 134 3.4. Effects of Gardeniae Fructus (GF) and Geniposide on Serum Diabetic Parameters in STZ-Diabetic Mice Effects of extract of GF composed in BOF were investi- gated on levels of the diabetic parameters in STZ-dia- betic mice. GF extract (30 - 300 mg/kg) in an i.p. ad- ministration lowered high serum glucose level in a dose-dependent manner (Figure 5). Geniposide (Gep; 10 - 100 mg/kg), a main compound of GF, also lowered high blood glucose level in a dose-dependent manner (Figure 5). Anti-hyperglycemic action of GF extract was parallel with that of geniposide. GF extract (30 - 300 mg/kg) in an i.p. administration did not affect serum in- sulin level of STZ-diabetic mice. Geniposide (10 - 100 mg/kg) also did not affect serum insulin level of the dia- betic mice (Figure 6). GF extract (30 - 300 mg/kg) low- ered serum triglyceride level (Figure 7) and serum cho- lesterol level (Figure 8) in a dose-dependent manner. However, geniposide (10 - 100 mg/kg) did not affect serum triglyceride level (Figure 7) and increased serum cholesterol level conversely (Figure 8). 4. Discussion Diabetes mellitus is characterized as a disease of carbo- hydrate metabolism and abnormalities of lipid and lipo- protein metabolism. Insulin deficiency stimulates lipoly- sis in the adipose tissue, and gives rise to hyperlipidemia and fatty liver in diabetes mellitus [10]. Hyperlipidemia Figure 5. Effects of gardeniae fructus (GF) extract and geniposide (Gep) on the serum glucose level in STZ-diabetic mice. Serum glucose levels were measured before and 6 hours after i.p. administration with GF extract or Gep into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 15 data. *p < 0.05, **p < 0.01: Significan t ly different from the control water group. Figure 6. Effects of gardeniae fructus (GF) extract and ge- niposide (Gep) on serum insulin level in STZ-diabetic mice. The immunoreactive insulin level was measured at 6 hours after i.p. administration with GF extract or Gep into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 15 data. Figure 7. Effects of gardeniae fructus (GF) extract and ge- niposide (Gep) on serum triglyceride level in STZ-diabetic mice. Serum triglyceride level was measured at 6 hours after i.p. administration with GF extract or Gep into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 15 data. *p < 0.05, **p < 0.01: Sig- nificantly different from the control water group. and atherosclerosis are frequently associated with diabe- tes mellitus, indicating alterations of cholesterol metabo- lism [11-13]. The lipid profile in type 2 diabetic subjects generally consists of elevated triglycerides and LDL cholesterol, and reduced HDL cholesterol. In the present study, STZ-diabetic mice were used as diabetic model and showed significant increase of serum glucose, total triglyceride and total cholesterol levels, and decrease of serum insulin level (Table 2). Copyright © 2011 SciRes. CM 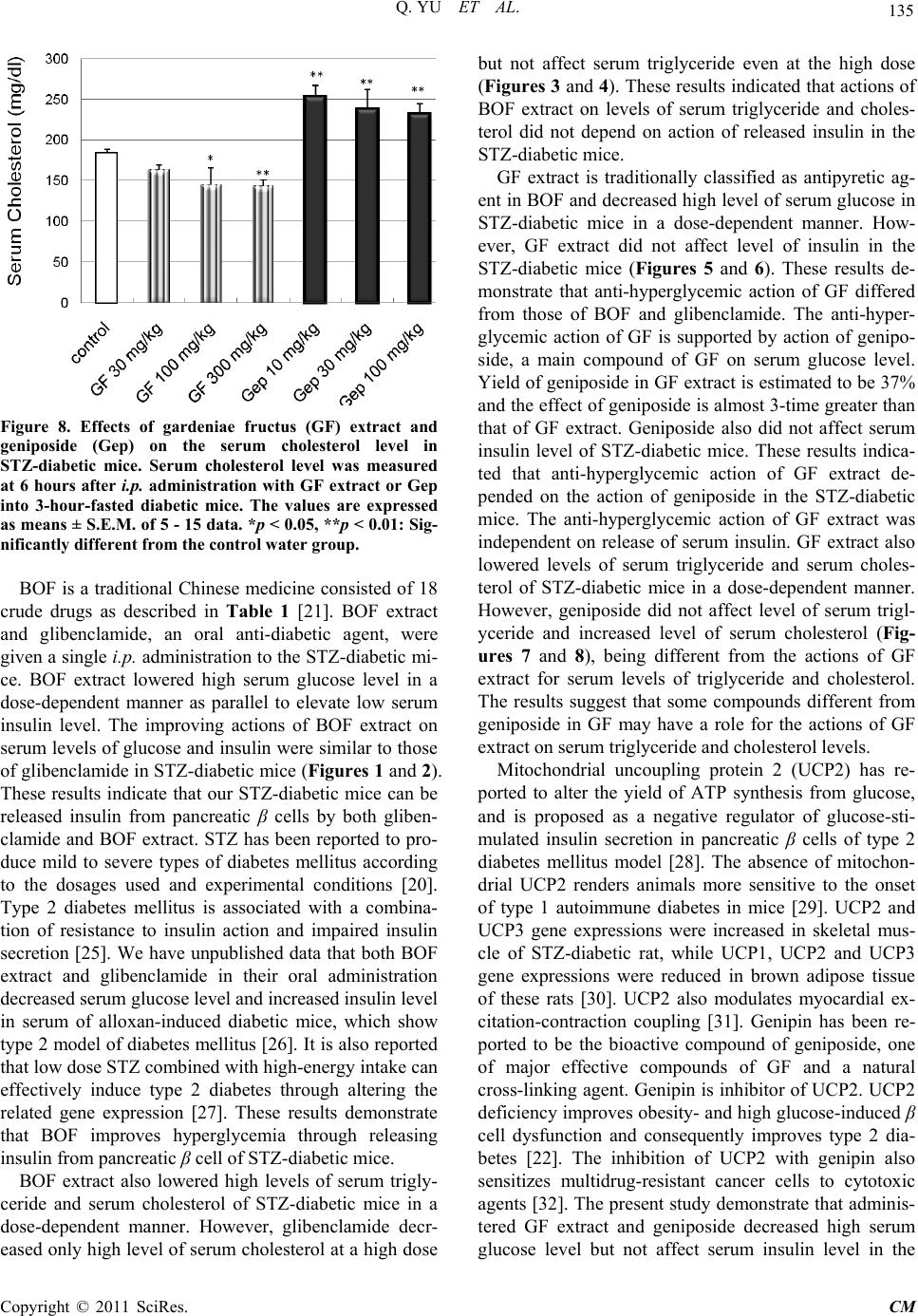 135 Q. YU ET AL. Figure 8. Effects of gardeniae fructus (GF) extract and geniposide (Gep) on the serum cholesterol level in STZ-diabetic mice. Serum cholesterol level was measured at 6 hours after i.p. administration with GF extract or Gep into 3-hour-fasted diabetic mice. The values are expressed as means ± S.E.M. of 5 - 15 data. *p < 0.05, **p < 0.01: Sig- nificantly different from the control water group. BOF is a traditional Chinese medicine consisted of 18 crude drugs as described in Table 1 [21]. BOF extract and glibenclamide, an oral anti-diabetic agent, were given a single i.p. administration to the STZ-diabetic mi- ce. BOF extract lowered high serum glucose level in a dose-dependent manner as parallel to elevate low serum insulin level. The improving actions of BOF extract on serum levels of glucose and insulin were similar to those of glibenclamide in STZ-diabetic mice (Figures 1 and 2). These results indicate that our STZ-diabetic mice can be released insulin from pancreatic β cells by both gliben- clamide and BOF extract. STZ has been reported to pro- duce mild to severe types of diabetes mellitus according to the dosages used and experimental conditions [20]. Type 2 diabetes mellitus is associated with a combina- tion of resistance to insulin action and impaired insulin secretion [25]. We have unpublished data that both BOF extract and glibenclamide in their oral administration decreased serum glucose level and increased insulin level in serum of alloxan-induced diabetic mice, which show type 2 model of diabetes mellitus [26]. It is also reported that low dose STZ combined with high-energy intake can effectively induce type 2 diabetes through altering the related gene expression [27]. These results demonstrate that BOF improves hyperglycemia through releasing insulin from pancreatic β cell of STZ-diabetic mice. BOF extract also lowered high levels of serum trigly- ceride and serum cholesterol of STZ-diabetic mice in a dose-dependent manner. However, glibenclamide decr- eased only high level of serum cholesterol at a high dose but not affect serum triglyceride even at the high dose (Figures 3 and 4). These results indicated that actions of BOF extract on levels of serum triglyceride and choles- terol did not depend on action of released insulin in the STZ-diabetic mice. GF extract is traditionally classified as antipyretic ag- ent in BOF and decreased high level of serum glucose in STZ-diabetic mice in a dose-dependent manner. How- ever, GF extract did not affect level of insulin in the STZ-diabetic mice (Figures 5 and 6). These results de- monstrate that anti-hyperglycemic action of GF differed from those of BOF and glibenclamide. The anti-hyper- glycemic action of GF is supported by action of genipo- side, a main compound of GF on serum glucose level. Yield of geniposide in GF extract is estimated to be 37% and the effect of geniposide is almost 3-time greater than that of GF extract. Geniposide also did not affect serum insulin level of STZ-diabetic mice. These results indica- ted that anti-hyperglycemic action of GF extract de- pended on the action of geniposide in the STZ-diabetic mice. The anti-hyperglycemic action of GF extract was independent on release of serum insulin. GF extract also lowered levels of serum triglyceride and serum choles- terol of STZ-diabetic mice in a dose-dependent manner. However, geniposide did not affect level of serum trigl- yceride and increased level of serum cholesterol (Fig- ures 7 and 8), being different from the actions of GF extract for serum levels of triglyceride and cholesterol. The results suggest that some compounds different from geniposide in GF may have a role for the actions of GF extract on serum triglyceride and cholesterol levels. Mitochondrial uncoupling protein 2 (UCP2) has re- ported to alter the yield of ATP synthesis from glucose, and is proposed as a negative regulator of glucose-sti- mulated insulin secretion in pancreatic β cells of type 2 diabetes mellitus model [28]. The absence of mitochon- drial UCP2 renders animals more sensitive to the onset of type 1 autoimmune diabetes in mice [29]. UCP2 and UCP3 gene expressions were increased in skeletal mus- cle of STZ-diabetic rat, while UCP1, UCP2 and UCP3 gene expressions were reduced in brown adipose tissue of these rats [30]. UCP2 also modulates myocardial ex- citation-contraction coupling [31]. Genipin has been re- ported to be the bioactive compound of geniposide, one of major effective compounds of GF and a natural cross-linking agent. Genipin is inhibitor of UCP2. UCP2 deficiency improves obesity- and high glucose-induced β cell dysfunction and consequently improves type 2 dia- betes [22]. The inhibition of UCP2 with genipin also sensitizes multidrug-resistant cancer cells to cytotoxic agents [32]. The present study demonstrate that adminis- tered GF extract and geniposide decreased high serum glucose level but not affect serum insulin level in the Copyright © 2011 SciRes. CM  Q. YU ET AL. 136 STZ-diabetic mice. Genipin may involve the anti-hyper- glycemic and anti-hyperlipidemic actions of GF extract. We need further experiments for high doses of geni- poside and genipin, and duration of their treatment. 5. Conclusions BOF extract improved abnormal levels of serum glucose, insulin, triglyceride and cholesterol in the STZ-diabetic mice. Extract of GF in BOF also improved levels of se- rum glucose, triglyceride and cholesterol but not level of serum insulin in the diabetic mice. These results indicate that GF extract has an important role in a part of im- proving actions of BOF in the diabetic mice. Anti-hy- perglycemic action of BOF extract may have two, insulin release-dependent and non-insulin release-dependent me- chanisms in the STZ-diabetic mice. 6. Acknowledgements This work was supported in part by a grant (to SK) for the “Academic Frontier” project for Private Universities (2005-2009) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Japan. 7. References [1] M. Brownlee, “Biochemistry and Molecular Cell Biology of Diabetic Complications,” Nature, Vol. 414, 2001, pp. 813-820. doi:10.1038/414813a [2] S. Kobayashi, M. Suzuki, H. Tsuneki, R. Nagai, S. Horiuchi and N. Hagino, “Overproduction of Nε-(Carbox- ymethyl)lysine-Induced Neovascularization in Cultured Choroidal Explant of Streptozotocin-Diabetic Rat,” Bio- logical & Pharmaceutical Bulletin, Vol. 27, No. 10, 2004, pp. 1565-1571. doi:10.1248/bpb.27.1565 [3] Y. Yamamoto, I. Kato, T. Doi, H. Yonekura, S. Ohashi, M. Takeuchi, T. Watanabe, S. I. Yamagishi, S. Sakurai, S. Takasawa, H. Okamoto and H. Yamamoto, “Development and Prevention of Advanced Diabetic Nephropathy in RAGE-Overexpressing Mice,” The Journal of Clinical In- vestigation, Vol. 108, 2001, pp. 261-268. [4] N. E. Cameron and M. A. Cotter, “Effects of Antioxi- dants on Nerve and Vascular Dysfunction in Experimen- tal Diabetes,” Diabetes Research and Clinical Practice, Vol. 45, No. 2, 1999, pp. 137-146. doi:10.1016/S0168-8227(99)00043-1 [5] L. J. Copp. ey, J. S. Gellett, E. P. Davidson, J. A. Dunlap, D. D. Lund and M. A. Yorek, “Effect of Antioxidant Treatment of Streptozotocin-Induced Rats on Endoneurial Blood Flow, Motor Nerve Condition Velocity, and Vas- cular Reactivity of Epineurial Arterioles of the Sciatic Nerve,” Diabetes, Vol. 50, No. 8, 2001, pp. 1927-1937. doi:10.2337/diabetes.50.8.1927 [6] S. Yagihashi, S. I. Yamagishi, R. I. Wada, M. Baba, T. C. Hohman, C. Yabe-Nishimura and Y. Kokai, “Neuropathy in Diabetic Mice Overexpressing Human Aldose Reduc- tase and Effects of Aldose Reductase Inhibitor,” Brain, Vol. 124, No. 12, 2001, pp. 2448-2458. doi:10.1093/brain/124.12.2448 [7] I. G. Obrosova, C. Van Huysen, L. Fathallah, X. Cao, D. A. Greene and M. J. Stevens, “An Aldose Reductase In- hibitors Reverses Early Diabetes-Induced Changes in Pe- ripheral Nerve Function, Metabolism and Antioxidative Defense,” The FASEB Journal, Vol. 16, 2002, pp. 123- 125. [8] P. Xia, T. Inoguchi, S. Kern, R. L. Engerman, P. J. Oates and G. L. King, “Characterization of the Mechanism of the Chronic Activation of Diacylglycerol Protein Kinase C Pathway in Diabetes and Hypergalactosomia,” Diabe- tes, Vol. 43, No. 9, 1994, pp. 1122-1129. doi:10.2337/diabetes.43.9.1122 [9] G. Booth, T. J. Stalker, A. M. Lefer and R. Scalia, “Mechanisms of Amelioration of Glucose-Induced En- dothelial Dysfunction Following Inhibition of Protein Kinase C in vivo,” Diabetes, Vol. 51, No. 5, 2002, pp. 1556-1564. doi:10.2337/diabetes.51.5.1556 [10] G. M. Reaven, “Banting Lecture 1988. Role of Insulin Resistance in Human Disease,” Diabetes, Vol. 37, No. 12, 1988, pp. 1595-1607. doi:10.2337/diabetes.37.12.1595 [11] W. V. Brown, “Lipoprotein Disorders in Diabetes Melli- tus,” Medical Clinics of North America, Vol. 78, 1994, pp. 143-161. [12] I. I. Kessler, “Mortality Experience of Diabetic Patients. A Twenty-Six-Year Follow-up Study,” The American Journal of Medicine, Vol. 51, No. 6, 1971, pp. 715-724. doi:10.1016/0002-9343(71)90299-3 [13] L. J. Bennion, “Effects of Diabetes mellitus on Cholesterol Metabolism in Man,” The New England Journal of Medi- cine, Vol. 296, 1977, pp. 1365-1371. doi:10.1056/NEJM197706162962401 [14] P. Kundsen, J. Ericksson, S. Lahdenpera, J. Kahri, L. Groop and M. R. Taskinen, “Changes of Lipolytic En- zymes Cluster with Insulin Resistance Syndrome. Botnia Study Group,” Diabetologia, Vol. 38, No. 3, 1995, pp. 344-350. doi:10.1007/BF00400640 [15] S. M. Grandy, I. J. Benjamin, G. L. Burke, A. Chait, R. H. Eckel, B. V. Howard, W. Mitch, S. C. Smith Jr. and J. R. Sowers, “Diabetes and Cardiovascular Disease: A State- ment for Healthcare Professionals from the American Heart Association,” Circulation, Vol. 100, 1999, pp. 1134-1146. [16] P. Libby and J. Plutzky, “Diabetic Macrovascular Disease: The Glucose Paradox?” Circulation, Vol. 106, 2002, pp. 2760-2763. doi:10.1161/01.CIR.0000037282.92395.AE [17] A. Sachin, K. O. Shreesh and V. Divya, “Characterization of Streptozotocin Induced Diabetes Mellitus in Swiss Al- bino Mice,” Global Journal of Pharmacology, Vol. 3, 2009, pp. 81-84. [18] K. Srinivasan, B. Viswanad, L. Asrat, C. L. Kaul and P. Ramarao, “Combination of High-Fat Diet-Fed and Low-Dose Streptozotocin-Treated Rat: A Model for Type Copyright © 2011 SciRes. CM  Q. YU ET AL. Copyright © 2011 SciRes. CM 137 2 Diabetes and Pharmacological Screening,” Pharmacol- ogical Research, Vol. 52, No. 4, 2005, pp. 313-320. doi:10.1016/j.phrs.2005.05.004 [19] R. N. Wan, L. Bouwens and G. Kloppel, “Beta Cell Pro- liferation in Normal and Streptozotocin-Treated New Born Rats. Site, Dynamics and Capacity,” Diabetologia, Vol. 37, No. 11, 1994, pp. 1088-1090. doi:10.1007/BF00418372 [20] A. Junod, A. E. Lambert, L. Orci, R. Pictet, A. E. Gonet and A. E. Renold, “Studies of the Diabetogenic Action of Streptozotocin,” Proceedings of the Society for Experi- mental Biology and Medicine, Vol. 126, 1967, pp. 201- 205. [21] C. Hioki, K. Yoshimoto and T. Yoshida, “Efficacy of Bofu-Tsusho-San, an Oriental Herbal Medicine, in Obese Japanese Women with Impaired Glucose Tolerance,” Clinical and Experimental Pharmacology and Physiology, Vol. 31, No. 9, 2004, pp. 614-619. doi:10.1111/j.1440-1681.2004.04056.x [22] C. Y. Zhang, L. E. Parton, C. P. Ye, S. Krauss, R. Shen, C. T., Lin, J. A. Porco Jr. and B. B. Lowell, “Genipin In- hibits UCPs-Mediated Proton Leak and Acutely Reverses Obesity- and Glucose-Induced B Cell Dysfunction in Isolated Pancreatic Islets,” Cell Metabolism, Vol. 3, No. 6, 2006, pp. 417-427. doi:10.1016/j.cmet.2006.04.010 [23] Y. Y. Liu, S. Kobayashi, T. Tsutsumi and H. Kontani, “Combined Effects of Stephania Radix and Astragali Radix in Antihyperglycemic Action of Boi-ogi-to (Fang- ji-huang-qi-tang) in Streptozotocin-Induced Diabetic Mice,” Journal of Traditional Medicines, Vol. 17, 2000, pp. 253-260. [24] T. Tsutsumi, S. Kobayashi, Y. Y. Liu and H. Kontani, “Anti-Hyperglycemic Effect of Fangchinoline Isolated from Stephania Tetrandra Radix in Streptozotocin-Di- abetic Mice,” Biological & Pharmaceutical Bulletin, Vol. 26, No. 3313, 2003, pp. 313-317. doi:10.1248/bpb.26.313 [25] S. I. Taylor, “Deconstructing Type 2 Diabetes,” Cell, Vol. 97, 1999, pp. 9-12. doi:10.1016/S0092-8674(00)80709-6 [26] I. Kimura, N. Nakashima, Y. Sugihara, F. J. Chen and M. Kimura, “The Antihyperglycaemic Blend Effect of Tra- ditional Chinese Medicine Byakko-Kaninjin-to on Al- loxan and Diabetic KK-CAy Mice,” Phytotherapy Re- search, Vol. 13, No. 6, 1999, pp. 484-488. doi:10.1002/(SICI)1099-1573(199909)13:6<484::AID-P TR485>3.0.CO;2-X [27] H. J. Wang, Y. X. Jin, W. Shen, J. Neng, T. Wu, Y. J. Li and Z. W. Fu, “Low Dose Streptozotocin (STZ) Com- bined with High Energy Intake Can Effectively Induce Type 2 Diabetes through Altering the Related Gene Ex- pression,” Asia Pacific Journal of Clinical Nutrition, Vol. 16, 2007, pp. 412-417. [28] C. Y. Zhang, G. Baffy, P. Perret, S. Krauss, O. Peroni, D. Grujic, T. Hagen, A. J. Vidal-Puig, O. Boss and Y. B. Kim, “Uncoupling Protein-2 Negatively Regulates Insu- lin Secretion and Is a Major Link between Obesity, Beta Cell Dysfunction, and Type 2 Diabetes,” Cell, Vol. 105, No. 6, 2001, pp. 745-755. doi:10.1016/S0092-8674(01)00378-6 [29] Y. Emre, C. Hurtaud, M. Karaca, T. Nubel, F. Zavala and D. Ricquier, “Role of Uncoupling Protein UCP2 in Cell-Mediated Immunity: How Macrophage-Mediated Insulitis Is Accelerated in a Model of Autoimmune Dia- betes,” The Proceedings of the National Academy of Sci- ences U.S.A., Vol. 27, 2007, pp. 19085-19090. [30] H. Kageyama, A. Suga, M. Kashiba, J. Oka, T. Osaka, T. Kashiwa, T. Hirano, K. Nemoto, Y. Namba, D. Ricquier, J.-P. Giacobino and S. Inoue, “Increased Uncoupling Protein-2 and -3 Gene Expressions in Skeletal Muscle of STZ-Induced Diabetic Rats,” The Federation of Euro- pean Biochemical Societies Letters, Vol. 440, No. 3, 1998, pp. 450-453. doi:10.1016/S0014-5793(98)01506-3 [31] J. D. Turner, L. D. Gaspers, G. Wang and A. P. Thomas, “Uncoupling Protein-2 Modulates Myocardial Excitation- Contraction Coupling,” Circulation Research, Vol. 106, 2010, pp. 730-738. doi:10.1161/CIRCRESAHA.109.206631 [32] R. J. Mailloux, C. N.-K. Adjeitey and M.-E. Harper, “Genipin-Induced Inhibition of Uncoupling Protein-2 Sensitizes Drug-Resistant Cancer Cells to Toxic Agents,” PLoS ONE, Vol. 5, 2010, pp. 1-10. doi:10.1371/journal.pone.0013289
|