 International Journal of Organic Chemistry, 2011, 1, 143-147 doi:10.4236/ijoc.2011.14021 Published Online December 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC 143 Solvent Free Synthesis of α, ά-Bis(Substituted-benzylidene)cycloalkanones Using Covalently Anchored Sulfonic Acid on Silica Gel (SiO2-R-SO3H) as an Efficient and Reusable Heterogeneous Catalyst Azizollah Habibi1, Enayatollah Sheikhhosseini1*, Mohammadali Bigdeli1, Saeed Balalaie2, Elinaz Farrokhi1 1Faculty of Chemistry, Tarbiat Moallem University, Tehran, Iran 2Peptide Chemistry Research Center, K. N. Toosi University of Technology, Tehran, Iran E-mail: *sheikhhosseiny@gmail.com Received August 2, 2011; revised September 8, 2011; accepted September 16, 2011 Abstract We wish to report a mild and efficient Crossed-Aldol reaction for the synthesis of α, ά-bis(substituted-ben- zylidene)cycloalkanones in the presence of catalytic amounts of covalently anchored sulfonic acid onto sil- ica gel under heterogeneous and solvent-free conditions. The present methodology offers several advantages such as excellent yields, simple procedure and work up steps, short reaction times and easy recovery of the catalyst. We have also demonstrated that the catalyst can be reused successfully. Keywords: Crossed-Aldol Condensation, Heterogeneous Catalysis, α, ά-Bis(Substituted-benzylidene)cycloalkanones, SiO2-R-SO3H, Solvent-Free 1. Introduction Heterogeneous catalysis has emerged as a process in whi- ch catalysts can be recovered and reused to achieve high turn-over numbers [1,2]. Incorporation of an active site onto a large surface solid carrier is one strategy to gener- ate a heterogeneous catalyst. Silica is one such support which displays exceptional chemical and thermal quail- ties [3,4]. Recently silica functionalized sulfonic acid has been used for a variety of reactions [5-8]. Claisen-Schmidt condensation reaction followed by de- hydration affords α-alkylidene or α-arylidene compounds. Such an introduction of alkylidene or arylidene moieties at the α-position of carbonyl compounds has been a use- ful synthetic tool in natural product chemistry [9]. α, ά- Bis(substituted-benzylidene)cycloalkanones exhibit nu- merous biological activities [10-13]. They are also suit- able as nonlinear optical materials [14], in preparation of pyridine derivatives [15], 2,7-disubstituted tropones [16] and in the total synthesis of natural products [17]. Such condensation processes are carried out with the aid of strong acids or bases. The yields, however, are ge- nerally low and cumbersome separation steps are requir- ed [18,19]. It is reported that various complexes of metal ions, such as Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn (II) may be used as catalysts in condensation reactions [20]. The reported yields, however, are less than 38%. In other cases, amino-functionalized ionic liquid [21] and copper (II) trifluoroacetate [22] have also been used to catalyze the reaction. High yield of products, however, can only be obtained at high temperatures and the purify- cation operations are always complicated. Such condensation reactions are also carried out in sol- vent-free conditions [23]. Silica sulfonic acid, nanopor- ous silica-based sulfonic acid and polymer supported sul- fonic acid type catalysts are also employed for such con- densations. Reaction times are reported to be in 3 - 12 hour range [24-26]. In the present work, we have used covalently anchored sulfonic acid on silica gel (Catalyst 1, Scheme 1), which its applications have been reported in a few number of organic transformations [6,8,27,28] under solvent-free conditions to prepare α, ά-bis(substituted- benzylidene) cycloalkanones (Scheme 2) in excellent yields, short reaction times and clean work up procedures. 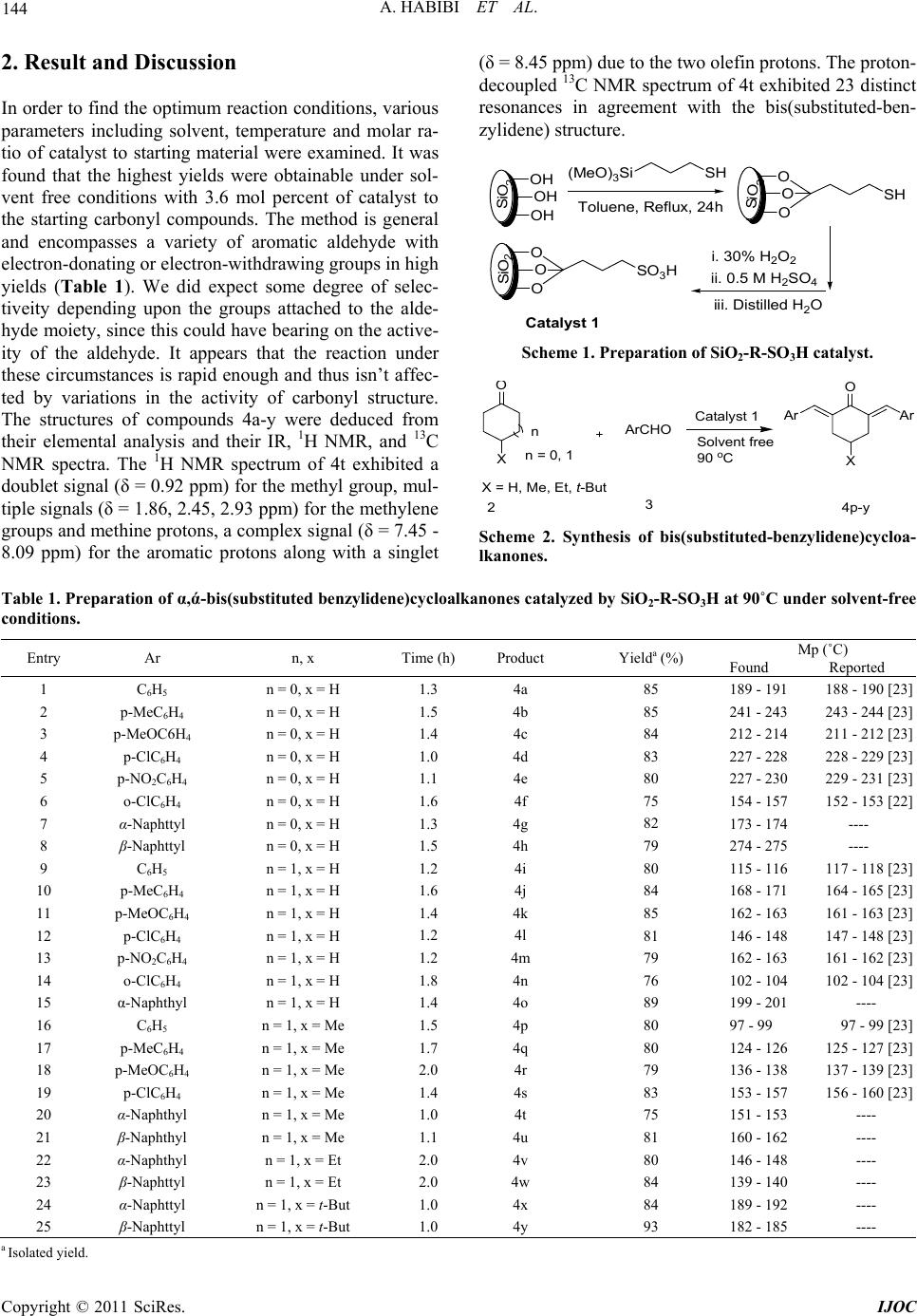 A. HABIBI ET AL. 144 2. Result and Discussion In order to find the optimum reaction conditions, various parameters including solvent, temperature and molar ra- tio of catalyst to starting material were examined. It was found that the highest yields were obtainable under sol- vent free conditions with 3.6 mol percent of catalyst to the starting carbonyl compounds. The method is general and encompasses a variety of aromatic aldehyde with electron-donating or electron-withdrawing groups in high yields (Table 1). We did expect some degree of selec- tiveity depending upon the groups attached to the alde- hyde moiety, since this could have bearing on the active- ity of the aldehyde. It appears that the reaction under these circumstances is rapid enough and thus isn’t affec- ted by variations in the activity of carbonyl structure. The structures of compounds 4a-y were deduced from their elemental analysis and their IR, 1H NMR, and 13C NMR spectra. The 1H NMR spectrum of 4t exhibited a doublet signal (δ = 0.92 ppm) for the methyl group, mul- tiple signals (δ = 1.86, 2.45, 2.93 ppm) for the methylene groups and methine protons, a complex signal (δ = 7.45 - 8.09 ppm) for the aromatic protons along with a singlet (δ = 8.45 ppm) due to the two olefin protons. The proton- decoupled 13C NMR spectrum of 4t exhibited 23 distinct resonances in agreement with the bis(substituted-ben- zylidene) structure. SiO 2 OH OH OH (MeO)3Si SH Toluene, Reflux, 24h SiO 2 O O OSH i. 30% H2O2 ii. 0.5 M H2SO4 iii. Distilled H2O Si O2 O O OSO3H atal t1 Scheme 1. Preparation of SiO2-R-SO3H catalyst. n = 0, 1 n X X = H, Me, Et, t-But ArCHO Catalyst 1 Solvent free 90 o C 3 O X ArAr 24 Scheme 2. Synthesis of bis(substituted-benzylidene)cycloa- lkanones. Table 1. Preparation of α,ά-bis(substituted benzylidene)cycloalkanones catalyzed by SiO2-R-SO3H at 90˚C under solvent-free conditions. Entry Ar n, x Time (h) Product Yielda (%) Mp (˚C) Found Reported 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 C6H5 p-MeC6H4 p-MeOC6H4 p-ClC6H4 p-NO2C6H4 o-ClC6H4 α-Naphttyl β-Naphttyl C6H5 p-MeC6H4 p-MeOC6H4 p-ClC6H4 p-NO2C6H4 o-ClC6H4 α-Naphthyl C6H5 p-MeC6H4 p-MeOC6H4 p-ClC6H4 α-Naphthyl β-Naphthyl α-Naphthyl β-Naphttyl α-Naphttyl β-Naphttyl n = 0, x = H n = 0, x = H n = 0, x = H n = 0, x = H n = 0, x = H n = 0, x = H n = 0, x = H n = 0, x = H n = 1, x = H n = 1, x = H n = 1, x = H n = 1, x = H n = 1, x = H n = 1, x = H n = 1, x = H n = 1, x = Me n = 1, x = Me n = 1, x = Me n = 1, x = Me n = 1, x = Me n = 1, x = Me n = 1, x = Et n = 1, x = Et n = 1, x = t-But n = 1, x = t-But 1.3 1.5 1.4 1.0 1.1 1.6 1.3 1.5 1.2 1.6 1.4 1.2 1.2 1.8 1.4 1.5 1.7 2.0 1.4 1.0 1.1 2.0 2.0 1.0 1.0 4a 4b 4c 4d 4e 4f 4g 4h 4i 4j 4k 4l 4m 4n 4o 4p 4q 4r 4s 4t 4u 4v 4w 4x 4y 85 85 84 83 80 75 82 79 80 84 85 81 79 76 89 80 80 79 83 75 81 80 84 84 93 189 - 191 188 - 190 [23] 241 - 243 243 - 244 [23] 212 - 214 211 - 212 [23] 227 - 228 228 - 229 [23] 227 - 230 229 - 231 [23] 154 - 157 152 - 153 [22] 173 - 174 ---- 274 - 275 ---- 115 - 116 117 - 118 [23] 168 - 171 164 - 165 [23] 162 - 163 161 - 163 [23] 146 - 148 147 - 148 [23] 162 - 163 161 - 162 [23] 102 - 104 102 - 104 [23] 199 - 201 ---- 97 - 99 97 - 99 [23] 124 - 126 125 - 127 [23] 136 - 138 137 - 139 [23] 153 - 157 156 - 160 [23] 151 - 153 ---- 160 - 162 ---- 146 - 148 ---- 139 - 140 ---- 189 - 192 ---- 182 - 185 ---- a Isolated yield. Copyright © 2011 SciRes. IJOC 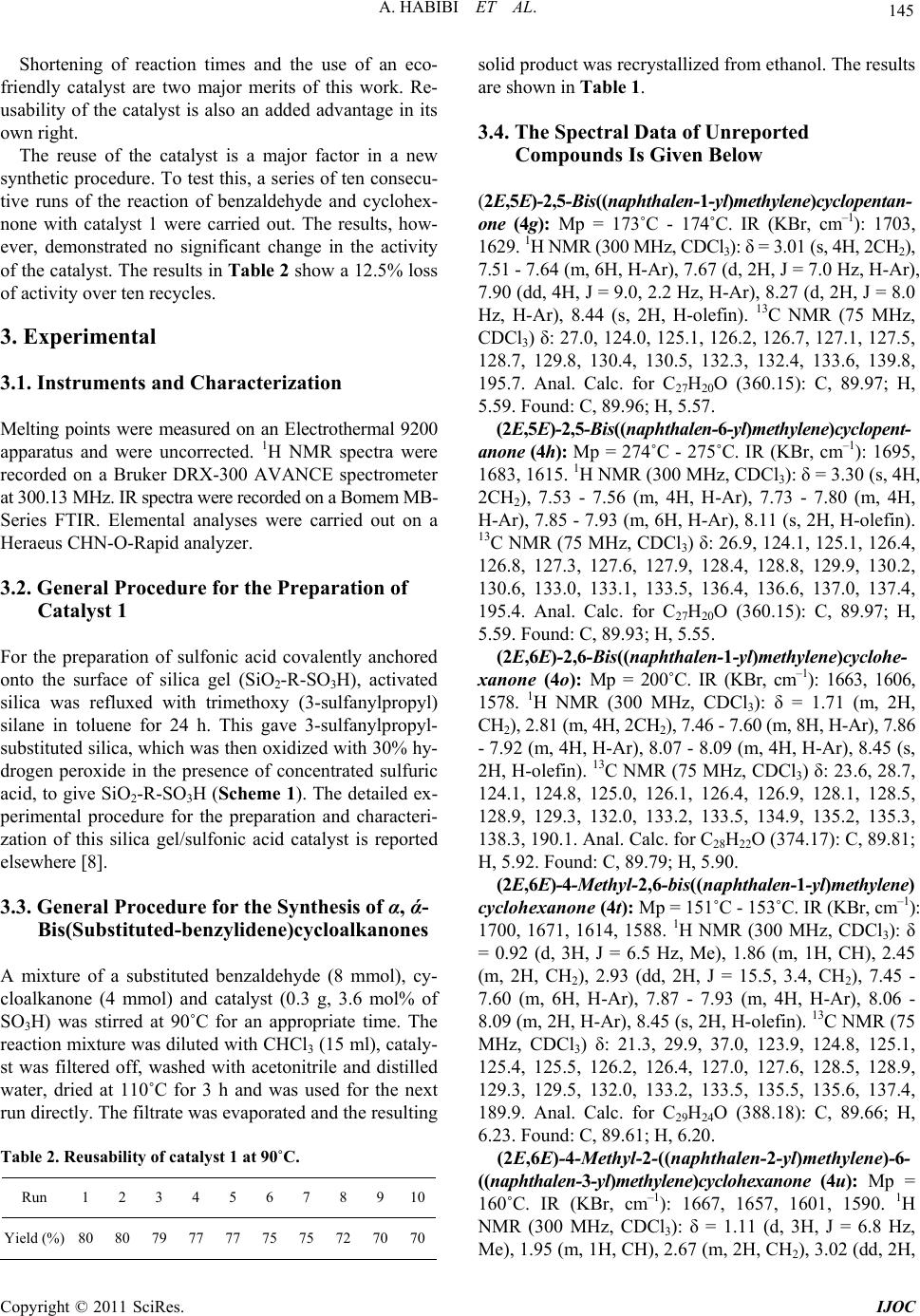 145 A. HABIBI ET AL. Shortening of reaction times and the use of an eco- friendly catalyst are two major merits of this work. Re- usability of the catalyst is also an added advantage in its own right. The reuse of the catalyst is a major factor in a new synthetic procedure. To test this, a series of ten consecu- tive runs of the reaction of benzaldehyde and cyclohex- none with catalyst 1 were carried out. The results, how- ever, demonstrated no significant change in the activity of the catalyst. The results in Table 2 show a 12.5% loss of activity over ten recycles. 3. Experimental 3.1. Instruments and Characterization Melting points were measured on an Electrothermal 9200 apparatus and were uncorrected. 1H NMR spectra were recorded on a Bruker DRX-300 AVANCE spectrometer at 300.13 MHz. IR spectra were recorded on a Bomem MB- Series FTIR. Elemental analyses were carried out on a Heraeus CHN-O-Rapid analyzer. 3.2. General Procedure for the Preparation of Catalyst 1 For the preparation of sulfonic acid covalently anchored onto the surface of silica gel (SiO2-R-SO3H), activated silica was refluxed with trimethoxy (3-sulfanylpropyl) silane in toluene for 24 h. This gave 3-sulfanylpropyl- substituted silica, which was then oxidized with 30% hy- drogen peroxide in the presence of concentrated sulfuric acid, to give SiO2-R-SO3H (Scheme 1). The detailed ex- perimental procedure for the preparation and characteri- zation of this silica gel/sulfonic acid catalyst is reported elsewhere [8]. 3.3. General Procedure for the Synthesis of α, ά- Bis(Substituted-benzylidene)cycloalkanones A mixture of a substituted benzaldehyde (8 mmol), cy- cloalkanone (4 mmol) and catalyst (0.3 g, 3.6 mol% of SO3H) was stirred at 90˚C for an appropriate time. The reaction mixture was diluted with CHCl3 (15 ml), cataly- st was filtered off, washed with acetonitrile and distilled water, dried at 110˚C for 3 h and was used for the next run directly. The filtrate was evaporated and the resulting Table 2. Reusability of catalyst 1 at 90˚C. Run 1 2 3 4 5 6 7 8 910 Yield (%) 80 80 79 77 77 75 75 72 7070 solid product was recrystallized from ethanol. The results are shown in Table 1. 3.4. The Spectral Data of Unreported Compounds Is Given Below (2E,5E)-2,5-Bis((naphthalen-1-yl)methylene)cyclopentan- one (4g): Mp = 173˚C - 174˚C. IR (KBr, cm–1): 1703, 1629. 1H NMR (300 MHz, CDCl3): δ = 3.01 (s, 4H, 2CH2), 7.51 - 7.64 (m, 6H, H-Ar), 7.67 (d, 2H, J = 7.0 Hz, H-Ar), 7.90 (dd, 4H, J = 9.0, 2.2 Hz, H-Ar), 8.27 (d, 2H, J = 8.0 Hz, H-Ar), 8.44 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 27.0, 124.0, 125.1, 126.2, 126.7, 127.1, 127.5, 128.7, 129.8, 130.4, 130.5, 132.3, 132.4, 133.6, 139.8, 195.7. Anal. Calc. for C27H20O (360.15): C, 89.97; H, 5.59. Found: C, 89.96; H, 5.57. (2E,5E)-2,5-Bis((naphthalen-6-yl)methylene)cyclopent- anone (4h): Mp = 274˚C - 275˚C. IR (KBr, cm–1): 1695, 1683, 1615. 1H NMR (300 MHz, CDCl3): δ = 3.30 (s, 4H, 2CH2), 7.53 - 7.56 (m, 4H, H-Ar), 7.73 - 7.80 (m, 4H, H-Ar), 7.85 - 7.93 (m, 6H, H-Ar), 8.11 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 26.9, 124.1, 125.1, 126.4, 126.8, 127.3, 127.6, 127.9, 128.4, 128.8, 129.9, 130.2, 130.6, 133.0, 133.1, 133.5, 136.4, 136.6, 137.0, 137.4, 195.4. Anal. Calc. for C27H20O (360.15): C, 89.97; H, 5.59. Found: C, 89.93; H, 5.55. (2E,6E)-2,6-Bis((naphthalen-1-yl)methylene)cyclohe- xanone (4o): Mp = 200˚C. IR (KBr, cm–1): 1663, 1606, 1578. 1H NMR (300 MHz, CDCl3): δ = 1.71 (m, 2H, CH2), 2.81 (m, 4H, 2CH2), 7.46 - 7.60 (m, 8H, H-Ar), 7.86 - 7.92 (m, 4H, H-Ar), 8.07 - 8.09 (m, 4H, H-Ar), 8.45 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 23.6, 28.7, 124.1, 124.8, 125.0, 126.1, 126.4, 126.9, 128.1, 128.5, 128.9, 129.3, 132.0, 133.2, 133.5, 134.9, 135.2, 135.3, 138.3, 190.1. Anal. Calc. for C28H22O (374.17): C, 89.81; H, 5.92. Found: C, 89.79; H, 5.90. (2E,6E)-4-Methyl- 2,6-bis((naphthalen-1-yl)methylene) cyclohexanone (4t): Mp = 151˚C - 153˚C. IR (KBr, cm–1): 1700, 1671, 1614, 1588. 1H NMR (300 MHz, CDCl3): δ = 0.92 (d, 3H, J = 6.5 Hz, Me), 1.86 (m, 1H, CH), 2.45 (m, 2H, CH2), 2.93 (dd, 2H, J = 15.5, 3.4, CH2), 7.45 - 7.60 (m, 6H, H-Ar), 7.87 - 7.93 (m, 4H, H-Ar), 8.06 - 8.09 (m, 2H, H-Ar), 8.45 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 21.3, 29.9, 37.0, 123.9, 124.8, 125.1, 125.4, 125.5, 126.2, 126.4, 127.0, 127.6, 128.5, 128.9, 129.3, 129.5, 132.0, 133.2, 133.5, 135.5, 135.6, 137.4, 189.9. Anal. Calc. for C29H24O (388.18): C, 89.66; H, 6.23. Found: C, 89.61; H, 6.20. (2E,6E)-4-Methyl-2-((naphthalen-2-yl)methylene)-6- ((naphthalen-3-yl)methylene)cyclohexanone (4u): Mp = 160˚C. IR (KBr, cm–1): 1667, 1657, 1601, 1590. 1H NMR (300 MHz, CDCl3): δ = 1.11 (d, 3H, J = 6.8 Hz, Me), 1.95 (m, 1H, CH), 2.67 (m, 2H, CH2), 3.02 (dd, 2H, Copyright © 2011 SciRes. IJOC  A. HABIBI ET AL. 146 J = 15.5, 3.5, CH2), 7.51 - 7.61 (m, 6H, H-Ar), 7.84 - 7.95 (m, 6H, H-Ar), 8.00 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 21.6, 29.5, 36.6, 126.4, 126.8, 127.6, 127.9, 128.4, 130.2, 133.0, 133.1, 133.5, 135.6, 137.3, 190.1. Anal. Calc. for C29H24O (388.18): C, 89.66; H, 6.23. Found: C, 89.61; H, 6.20. (2E,6E)-4-Ethyl-2,6-bis((naphthalen-1-yl)methylene) cyclohexanone (4 v): Mp = 146˚C - 148˚C. IR (KBr, cm–1): 1669, 1607, 1589. 1H NMR (300 MHz, CDCl3): δ = 0.70 (t, 3H, J = 7.4 Hz, CH3), 1.29 (m, 2H, CH2-Me), 1.62 (m, 1H, CH), 2.46 (m, 2H, CH2), 2.98 (dd, 2H, J = 15.6, 3.3 Hz, CH2), 7.46 - 7.60 (m, 8H, H-Ar), 7.87 - 7.93 (m, 4H, H-Ar), 8.07 - 8.11 (m, 2H, H-Ar), 8.47 (s, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 11.3, 28.1, 34.2, 36.1, 124.8, 125.0, 126.1, 126.4, 126.9, 127.6, 127.9, 128.1, 128.5, 128.9, 132.0, 133.1, 133.5, 135.2, 135.6, 137.3, 190.2. Anal. Calc. for C30H26O (402.2): C, 89.51; H, 6.51. Found: C, 89.46; H, 6.47. (2E,6E)-4-Ethyl-2-((naphthalen-2-yl)methylene)-6-((n aphthalen-3-yl)methylene)cyclohexanone (4w): Mp = 139˚C - 140˚C. IR (KBr, cm–1): 1696, 1663, 1601, 1565. 1H NMR (300 MHz, CDCl3): δ = 0.87 (t, 3H, J = 7.4 Hz, CH3), 1.45 (m, 2H, CH2-Me), 1.71 (m, 1H, CH), 2.67 (m, 2H, CH2), 3.23 (dd, 2H, J = 15.6, 3.3 Hz, CH2), 7.51 - 7.55 (m, 4H, H-Ar and H-olefine), 7.60 (dd, 2H, J = 8.5, 1.3 Hz, H-Ar), 7.84 - 7.90 (m, 6H, H-Ar), 7.95 - 8.0 (m, 4H, H-Ar and H-olefin). 13C NMR (75 MHz, CDCl3) δ: 11.5, 28.5, 34.2, 35.7, 126.4, 126.8, 127.7, 127.9, 128.4, 128.8, 130.2, 133.1, 133.1, 133.6, 135.6, 137.4, 190.4. Anal. Calc. for C30H26O (402.2): C, 89.51; H, 6.51. Found: C, 89.48; H, 6.49. (2E,6E)-4-Tert-butyl-2,6-bis((naphthalen-1-yl)methyle ne)cyclohexanone (4x): Mp = 189˚C - 192˚C IR (KBr, cm–1): 1659, 1595, 1575, 1566. 1H NMR (300 MHz, CDCl3): δ = 0.78 (s, 9H, 3Me), 1.52 (m, 1H, CH), 2.38 (t, 2H, J = 15 Hz, CH2), 3.07 (d, 2H, J = 15.1 Hz, CH2), 7.47 - 7.60 (m, 8H, H-Ar), 7.86 - 7.93 (m, 4H, H-Ar), 8.08 - 8.12 (m, 2H, H-Ar), 8.44 (s br, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 27.0, 27.1, 29.7, 30.0, 32.5, 44.7, 44.9, 124.8, 125.0, 126.1, 126.4, 126.7, 127.5, 127.6, 128.0, 128.5, 128.9, 130.4,132.1, 133.1, 133.5, 135.2, 137.5, 138.1, 190.3. Anal. Calc. for C32H30O (430.23): C, 89.26; H, 7.02. Found: C, 89.21; H, 6.97. (2E,6E)-4-Tert-butyl-2-((naphthalen-2-yl)methylene)- 6-((naphthalen-3-yl)methylene)cyclohexanone (4y): Mp = 182˚C - 185˚C. IR (KBr, cm–1): 1695, 1662, 1600, 1563. 1H NMR (300 MHz, CDCl3): δ = 0.67 (s, 9H, 3Me), 1.51 (m, 1H, CH), 2.57 (t, 2H, J = 15 Hz, CH2), 3.27 (d, 2H, J = 15.1 Hz, CH2), 7.46 - 7.57 (m, 6H, H-Ar), 7.60 - 8.05 (m, 8H, H-Ar), 8.10 (s br, 2H, H-olefin). 13C NMR (75 MHz, CDCl3) δ: 27.3, 29.6, 32.5, 44.5, 126.4, 126.8, 127.5, 127.6, 128.0, 128.4, 130.3, 130.6, 133.0, 133.1, 133.6, 136.4, 136.6, 137.0, 137.4, 190.5. Anal. Calc. for C32H30O (430.23): C, 89.26; H, 7.02. Found: C, 89.23; H, 7.01. 4. Conclusions We are reporting a new method for preparation of α, ά- bis(substituted-benzylidene)cycloalkanones using SiO2- R-SO3H. The catalyst is reusable with only small loss of activity over ten trials. Simple experimental procedure, use of small quantities of catalyst, no toxicity, no corro- siveness, short reaction times, inexpensive solid catalyst, high yields, mild reaction conditions and clean work up steps are significant merits of this work. 5. References [1] D. Choudhary, S. Paul, R. Gupta and J. H. Clark, “Cata- lytic Properties of Several Palladium Complexes Cova- lently Anchored onto Silica for the Aerobic Oxidation of Alcohols,” Green Chemistry, Vol. 8, No. 5, 2006, pp. 479-482. doi:10.1039/b601363e [2] B. Karimi, A. Zamani and J. H. Clark, “A Bipyridyl Pal- ladium Complex Covalently Anchored onto Silica as an Effective and Recoverable Interphase Catalyst for the Aerobic Oxidation of Alcohols,” Organometallics, Vol. 24, No. 19, 2005, pp. 4695-4698. doi:10.1021/om050326m [3] S. Paul and J. H. Clark, “A Highly Active and Reusable Heterogeneous Catalyst for the Suzuki Reaction: Synthe- sis of Biaryls and Polyaryls,” Green Chemistry, Vol. 5, No. 5, 2003, pp. 635-638. doi:10.1039/b306097g [4] M. Lagasi and P. Moggi, “Anchoring of Pd on Silica Func- tionalized with Nitrogen Containing Chelating Groups and Applications in Catalysis,” Journal of Molecular Cataly- sis A: Chemical, Vol. 182-183, 2002, pp. 61-72. doi:10.1016/S1381-1169(01)00478-2 [5] B. Das, K. Venkateswarlu, H. Holla and M. Krishnaiah, “A Remarkably Efficient Heterogeneous Reusable Cata- lyst for α-Monobromination of Carbonyl Compounds Using N-Bromosuccinimide,” Journal of Molecular Ca- talysis A: Chemical, Vol. 253, No. 1-2, 2006, pp. 107- 111. doi:10.1016/j.molcata.2006.03.011 [6] B. Karimi and M. Khalkhali, “Solid Silica-Based Sulfo- nic Acid as an Efficient and Recoverable Interphase Cata- lyst for Selective Tetrahydropyranylation of Alcohols and Phenols,” Journal of Molecular Catalysis A: Chemical, Vol. 232, No. 1-2, 2005, pp. 113-117. doi:10.1016/j.molcata.2005.01.028 [7] P. Gupta, V. Kumar and S. Paul, “Silica Functionalized Sulfonic Acid Catalyzed One-pot Synthesis of 4,5,8a- Triarylhexahydropyrimido-[4,5-d]pyrimidine-2,7(1H,3H) -diones under Liquid Phase Catalysis,” Journal of Bra- zilian Chemical Society, Vol. 21, No. 2, 2010, pp. 249- 252. doi:10.1590/S0103-50532010000200022 [8] R. Gupta, S. Paul and R. Gupta, “Covalently Anchored Sulfonic Acid onto Silica as an Efficient and Recoverable Copyright © 2011 SciRes. IJOC  A. HABIBI ET AL. Copyright © 2011 SciRes. IJOC 147 Interphase Catalyst for the Synthesis of 3,4-Dihydro- pyrimidinones/Thiones,” Journal of Molecular Catalysis A: Chemical, Vol. 266, No. 1-2, 2007, pp. 50-54. doi:10.1016/j.molcata.2006.10.039 [9] A. T. Nielsen and W. J. Houlihan, “The Aldol Condensa- tion,” In: R. Adams, A. H. Blatt, V. Boekelheide, T. L. Cairns, D. J. Cram and H. O. House, Eds., Organic Reac- tions, Wiley, New York, 1968. [10] T. P. Robinson, R. B. Hubbard, T. J. Ehlers, J. L. Arbiser, D. J. Goldsmith and J. P. Bowen, “Synthesis and Bio- logical Evaluation of Aromatic Enones Related to Cur- cumin,” Bioorganic & Medicinal Chemistry, Vol. 13, No. 24, 2005, pp. 4007-4013. doi:10.1016/j.bmc.2005.03.054 [11] A. T. Dinkova-Kostova, C. Abeygunawardana and P. Talalay, “Chemoprotective Properties of Phenylprope- noids, Bis(benzylidene)cycloalkanones, and Related Mi- chael Reaction Acceptors: Correlation of Potencies as Phase 2 Enzyme Inducers and Radical Scave,” Journal of Medicinal Chemistry, Vol. 41, No. 26, 1998, pp. 5287- 5296. doi:10.1021/jm980424s [12] A. Modzelewska, C. Pettit, G. Achanta, N. E. Davidson, P. Huang and S. R. Khan, “Anticancer Activities of Nov- el Chalcone and Bis-Chalcone Derivatives,” Bioorganic & Medicinal Chemistry, Vol. 14, No. 24, 2006, pp. 3491- 3495. doi:10.1016/j.bmc.2006.01.003 [13] C. Piantadosi, I. H. Hall, J. L. Irvine and G. L. Carlson, “Synthesis and Biological Activity of Alpha, Alpha’- dibenzylcycloalkanones,” Journal of Medicinal Chemis- try, Vol. 16, No. 7, 1973, pp. 770-775. doi:10.1021/jm00265a006 [14] J. Kawamata, K. Inoue, T. Inabe, M. Kiguchi, M. Kato and Y. Taniguchi, “Large Second-Harmonic Generation Coefficients of Bis(benzylidine) Cycloalkanones Estim- ated by the Second-Harmonic Wave Generated with the Evanescent Wave Technique,” Chemical Physics Letters, Vol. 249, No. 1-2, 1996, pp. 29-34. doi:10.1016/0009-2614(95)01373-3 [15] J. Deli, T. Lorand, D. Szabo and A. Foldesi, “Potentially Bioactive Pyrimidine Derivatives. 1.2-Amino-4-aryl-8-a- rylidene-3,4, 5, 6,7,8 -he xa hyd roqu inaz ol ine,” Pharmazie, Vol. 39, No. 8, 1984, pp. 539-540. [16] N. J. Leonard, L. A. Miller and J. W. Berry, “The Syn- thesis of 2,7-Disubstituted Tropones via Aromatization,” Journal of the American Chemical Society, Vol. 79, No. 6, 1957, pp. 1482-1485. doi:10.1021/ja01563a056 [17] M. A. Ciufolini and N. E. Byrne. “The Total Synthesis of Cystodytins, ” Journal of the American Chemical Society, Vol. 113, No. 21, 1991, pp. 8016-8024. doi:10.1021/ja00021a031 [18] B. A. Hathaway, “An Aldol Condensation Experiment Using a Number of Aldehydes and Ketones,” Journal of Chemical Education, Vol. 64, No. 4, 1987, p. 367. doi:10.1021/ed064p367 [19] T. Nakano, S. Irifune, S. Umano, A. Inada, Y. Ishii and M. Ogawa, “Cross-Condensation Reactions of Cycloal- kanones with Aldehydes and Primary Alcohols under the Influence of Zirconocene Complexes,” The Journal of Organic Chemistry, Vol. 52, No. 11, 1987, pp. 2239- 2244. doi:10.1021/jo00387a025 [20] K. Irie and K. Watanabe, “Aldol Condensations with Metal(II) Complex Catalysts,” Bulletin of the Chemical Society of Japan, Vol. 53, No. 5, 1980, pp. 1366-1371. doi:10.1246/bcsj.53.1366 [21] L.-Q. Kang, G.-H. Song, et al., “Synthesis of α, ά-Bis (substituted benzylidene)cycloalkanones Catalyzed by Amino-Functionalized Ionic Liquid,” Journal of the Chi- nese Chemical Society, Vol. 55, No. 5, 2008, pp. 1125- 1128. [22] D. Song, Y. Chen, R. Wang, C. Liu, H. Jiang and G. Luo, “Crossed-Aldol Condensation of Cycloalkanones with Aromatic Aldehydes Catalyzed by Copper(II) Trifluo- roacetate,” Preparative Biochemistry & Biotechnology, Vol. 39, No. 2, 2009, pp. 201-207. doi:10.1080/10826060902800874 [23] M. A. Bigdeli, G. H. Mahdavinia and H. Hazarkhani, “Wet 2,4,6-Trichloro[1,3,5]triazine (TCT) an Efficient Catalyst for Synthesis of α, ά-Bis(substituted-benzylidene) cycloalkanones under Solvent-Free Conditions,” Catalysis Communications, Vol. 8, No. 12, 2007, pp. 2228-2230. [24] P. Salehi, M. Dabiri, M. A. Zolfigol and M. A. B. Fard, “Silica Sulfuric Acid as an Efficient and Reusable Re- agent for Crossed-Aldol Condensation of Ketones with Aromatic Aldehydes under Solvent-Free Conditions,” Journal of Brazilian Chemical Society, Vol. 15, No. 5, 2004, pp. 773-777. doi:10.1590/S0103-50532004000500026 [25] M. G. Ziarani, A. Badiei, A. Abbasi and Z. Farahani, “Cross-Aldol Condensation of Cycloalkanones and Aro- matic Aldehydes in the Presence of Nanoporous Silica- Based Sulfonic Acid (SiO2-Pr-SO3H) under Solvent Free Conditions,” Chinese Journal of Chemistry, Vol. 27, No. 8, 2009, pp. 1537-1542. doi:10.1002/cjoc.200990259 [26] L.-T. An, J.-P. Zou and L.-L. Zang, “Polymer-Supported Sulphonic Acid Catalyzed Cross-Aldol Condensation: An Expeditious Synthesis of α, ά-Bis(substituted benzyli- dene)cycloalkanones,” Catalysis Communications, Vol. 9, No. 3, 2008, pp. 349-354. doi:10.1016/j.catcom.2007.06.004 [27] G. H. Mahdavinia, M. A. Bigdeli and S. Y. Hayeniaz, “Covalently Anchored Sulfonic Acid on Silicagel (SiO2- R-SO3H) as an Efficient and Reusable Heterogeneous Catalyst for the One-Pot Synthesis of 1,8-Dioxo-octahy- droxanthenes under Solvent-Free Conditions,” Chinese Chemical Letters, Vol. 20, No. 5, 2009, pp. 539-541. doi:10.1016/j.cclet.2008.12.026 [28] G. A. Ziarani, A. Badiei, M. Hassanzadeh and S. Mousa- vi, “Synthesis of 1,8-Dioxo-decahydroacridine Deriva- tives Using Sulfonic Acid Functionalized Silica (SiO2- Pr-SO3H) under Solvent-Free Conditions,” Arabian Jour- nal of Chemistry, 2011, in press.
|