Paper Menu >>
Journal Menu >>
 Vol.1, No.3, 127-133 (2009) doi:10.4236/health.2009.13021 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Effects of structural modification of anti-inflammatory steroidal antedrug on pro-inflammatory mediators and inhibitory cytokines in human alveolar epithelial cells Gui-Fang Wang1, Soonjo Kwon2*, Rakesh Sharma1, Hemang Patel2, Henry J. Lee3 1Chemical and Biomedical Engineering, Florida State University, Tallahassee, USA; soonjo.kwon@usu.edu 2Biological Engineering, Utah State University, Logan, USA 3Center for Anti-Inflammatory Research, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, USA Received 23 September 2009; revised 19 October 2009; accepted 20 October 2009. ABSTRACT The anti-inflammatory effects of the new ster- oidal antedrug, 21-acetyloxy-9α-fluoro-11β-hy- droxyl-3, 20-dioxo-1, 4-pregnadieno-[16α, 17α-d] isoxazoline (FP-ISO-21AC), on nitric oxide (NO) and interleukin 8 (IL-8) production, were inves- tigated together with its parent steroid predni- solone (PRED). PRED is one of the anti-in- flammatory steroids but has systemic side ef- fects which limit the use of it. PRED was modi- fied with ‘antedrug concept’ to create safer drugs that attack problems such as inflamma- tion, then quickly become inactive before they can cause systemic side effect. We had a test about the effect of the modified anti-inflamma- tory steroidal antedrug on anti-inflammatory activity. The present study evaluated their ability to inhibit cytokine-induced NO and IL-8 produc- tion in human alveolar epithelial cells. We also investigated their ability to enhance the expres- sion of inhibitory cytokine receptor, interleukin 22 receptor (IL-22R) in human alveolar epithelial cells. Our results showed that FP-ISO-21AC sh- owed higher ability to inhibit the cytokine - in- duced production of NO than PRED. Exogenous IL-22 was added to the media of both human alveolar epithelial cells (A549) and human lung fibroblast (HLF-1). In the presence of the ex- ogenous inhibitory cytokine IL-22, further re- duction of NO production was observed in A549 cells, which express IL-22R, but not in HLF1, which does not express IL-22R. These data suggested that the steroidal antedrugs en- hanced the expression of IL-22R. FP-ISO- 21AC showed higher potency than PRED to restore the expression of IL-22R. FP-ISO-21AC further reduced NO production to 27% and PRED further reduced NO production to 39%. In con- clusion, a synthesized steroidal antedrug FP- ISO-21AC showed higher anti-inflammatory ef- fects than PRED by inhibiting the expression of pro-inflammatory mediator NO and stimulating the expression of IL-22R. Keywords: Steroidal Antedrug; NO Production; IL-8 Production; Anti-Inflammatory Cytokine Receptor; IL-22 Receptor 1. INTRODUCTION Glucocorticoid is one of the most commonly and effec- tively used drugs to relieve inflammation [1-4]. However, these drugs display a number of serious systemic side effects such as suppression on pituitary-adrenal axis and on the immune system, aggravation of diabetes, hyper- tension, retardation of growth in children and osteoporo- sis. These systemic side effects limit the clinical use of corticosteroids [5-9]. Therefore, the main focus of present research is to modify the structure of corticosteroids by reducing the systemic side effects without losing their anti-inflammatory function. A novel strategy was em- ployed by incorporating of metabolically labile functional group onto the steroid chain structure based on the ‘ante- drug concept’. As Lee et al. described in previous papers, antedrug is defined as a compound which acts locally on the target tissue and it is rapidly metabolized to an inac- tive metabolite through enzymatic reaction upon entry into the systemic circulation [10-13]. The new anti-inflammatory steroidal antedrug, 21-Ac- etyloxy-9α-fluoro-11β-hydroxy-3, 20-dioxo-1, 4-pregna- dieno-[16α,17α-d] isoxazoline (FP-ISO-21AC) with C-16, 17-isoxazoline ring systems was synthesized and its pharmaceutical activities were evaluated [14]. Their study also showed that FP-ISO-21AC has high binding  G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 128 affinities to the glucocorticoid receptor prepared from liver cytosol and exhibited its enhanced inhibitory effects on LPS- induced nitric oxide (NO) production in murine macrophage cells (RAW 264.7)[14]. Although this new steroidal antedrug showed more potential anti-inflamma- tory effects, few main mechanisms by which multiple pro-inflammatory pathways are switched off and anti- inflammatory pathways are switched on in asthma fol- lowing pretreatment of steroidal antedrugs have been identified. In present time, multiple pro-inflammatory mediators have been implicated in asthma. However, the inhibitory mechanisms of the inflammatory process still remain to be investigated. There is increasing evidence that certain cytokines have anti-inflammatory or immu- nomodulatory effects and that their secretion might be defective in asthmatic patients. As a result, anti- inflam- matory cytokines (inhibitory cytokines) can block the inflammatory process or suppress the intensity of the inflammatory cascade. The severity and the persistence of asthma depend on the “balance” between the pro- inflammatory cytokines and the anti-inflammatory cyto- kines [15]. The expression of anti-inflammatory cyto- kines can be restored by the treatment with steroids or theophylline [16]. In this study, we had a test on the effect of the modi- fied anti-inflammatory steroid (FP-ISO-21AC, isoxa- zoline derivative, anti-inflammatory steroidal antedrugs) on anti-inflammatory activities. The present study ad- dresses the following fundamental questions: 1) Does structural modification of anti-inflammatory steroidal antedrugs increase the therapeutic index of potent corti- costeroids with reducing their systemic side effects? 2) Do these anti-inflammatory steroidal antedrugs enhance or restore the expression of anti-inflammatory proteins (e.g. inhibitory cytokines)? More specifically, the effect of FP-ISO-21AC, an active synthetic derivative of anti- inflammatory steroidal antedrug, was evaluated on re- duced production of pro-inflammatory mediators (IL-8 and NO). Simultaneously, FP-ISO-21AC was evaluated on enhanced expression of anti-inflammatory cytokine receptor IL-22R in human alveolar epithelial cells. FP- ISO-21AC showed higher potency to restore the expres- sion of IL-22R and further reduced NO production, com- pared with PRED. A synthesized steroidal antedrug FP- ISO-21AC showed higher anti-inflammatory effects than PRED by inhibiting the expression of pro-inflammatory mediator, NO and stimulating the expression of the in- hibitory cytokine receptor, IL-22R. The study has enor- mous diagnostic implications in asthmatic patients and designing novel synthetic anti-inflammatory steroids with less systemic side effects. 2. MATERIALS AND METHODS 2.1. Chemicals and Reagents Recombinant human interleukin-1 (IL-1), recombi- Figure 1. Structure of antedrug FP-ISO-21AC. nant human tumor necrosis factor-a (TNF-), recombi- nant human interferon- (IFN-) and recombinant human interleukin-22 (IL-22) were obtained from R&D systems, Inc (Minneapolis, USA). New steroidal antedrug, 21- Acetyloxy-9α-fluoro-11β-hydroxy-3, 20-dioxo-1, 4-pre- gnadieno-[16α, 17α-d] isoxazoline (FP-ISO-21AC) was synthesized as described previously [17-19]. The struc- ture of FP-ISO-21AC is shown in Figure 1. 2.2. Cell Culture Human alveolar epithelial cells, A549 (ATCC, CCL- 185) and human lung fibroblast, HFL1 (ATCC, CCL- 183) were obtained from ATCC (Manassas, VA, USA). The cells were grown on Ham's F12K medium with 2 mM of L-glutamine adjusted to contain 1.5g/L sodium bicar- bonate, 90% (ATCC, Manassas, VA, USA); and supplied with 10% fetal bovine serum (ATCC, Manassas, VA, USA). The cells were plated onto 75 cm2 flasks and in- cubated at 37 °C with 95% air and 5% CO2. Once the cells reached confluence, cells were typsinized with a solution containing 0.25% (w/v) trypsin and 0.038% (w/v) EDTA-4Na (Invitrogen, Carlsbad, California, USA). Cells were then transferred to 24-well plates at the seeding density of 1 X 104 per well. When the cells reached confluence (approximately 7 days), they were washed twice with PBS (Phosphate Buffered Saline). 2.3. Stimulation with Cytokines and/or Steroidal Antedrugs Once confluent, cells were incubated in serum-free me- dia for 24 h before exposure to cytokine(s) and/or ster- oidal antedrugs. Cells were then pre-incubated for 1 h with PRED and FP-ISO-AC at 1M in cell culture me- dium without serum. After washing cell layers with PBS twice, cells were stimulated with Cytomix (IL-1, TNF- and IFN-) at 50 ng/ml each for 23 h at 37°C in cell culture medium in combination with anti-inflammatory cytokine (IL-22) at 20 ng/ml. Following cytokine expo- sure, serum was returned to the culture media. NO and IL-8 was analyzed at 24 h following stimulation. 2.4. Measurement of Nitric Oxide (NO) NO production was measured by NO Analyzer (inNO, 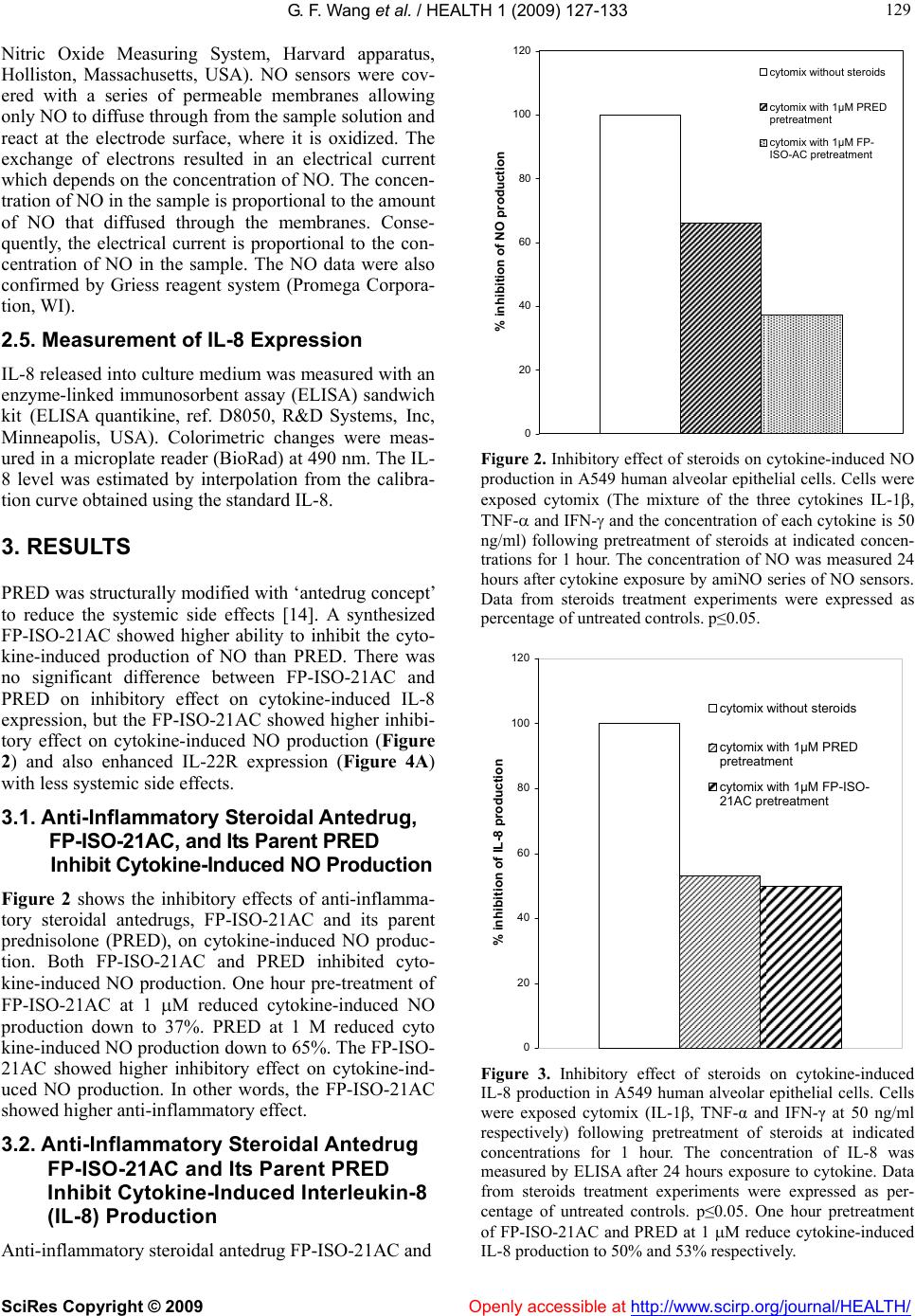 G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 129 129 Nitric Oxide Measuring System, Harvard apparatus, Holliston, Massachusetts, USA). NO sensors were cov- ered with a series of permeable membranes allowing only NO to diffuse through from the sample solution and react at the electrode surface, where it is oxidized. The exchange of electrons resulted in an electrical current which depends on the concentration of NO. The concen- tration of NO in the sample is proportional to the amount of NO that diffused through the membranes. Conse- quently, the electrical current is proportional to the con- centration of NO in the sample. The NO data were also confirmed by Griess reagent system (Promega Corpora- tion, WI). 2.5. Measurement of IL-8 Expression IL-8 released into culture medium was measured with an enzyme-linked immunosorbent assay (ELISA) sandwich kit (ELISA quantikine, ref. D8050, R&D Systems, Inc, Minneapolis, USA). Colorimetric changes were meas- ured in a microplate reader (BioRad) at 490 nm. The IL- 8 level was estimated by interpolation from the calibra- tion curve obtained using the standard IL-8. 3. RESULTS PRED was structurally modified with ‘antedrug concept’ to reduce the systemic side effects [14]. A synthesized FP-ISO-21AC showed higher ability to inhibit the cyto- kine-induced production of NO than PRED. There was no significant difference between FP-ISO-21AC and PRED on inhibitory effect on cytokine-induced IL-8 expression, but the FP-ISO-21AC showed higher inhibi- tory effect on cytokine-induced NO production (Figure 2) and also enhanced IL-22R expression (Figure 4A) with less systemic side effects. 3.1. Anti-Inflammatory Steroidal Antedrug, FP-ISO-21AC, and Its Parent PRED Inhibit Cytokine-Induced NO Production Figure 2 shows the inhibitory effects of anti-inflamma- tory steroidal antedrugs, FP-ISO-21AC and its parent prednisolone (PRED), on cytokine-induced NO produc- tion. Both FP-ISO-21AC and PRED inhibited cyto- kine-induced NO production. One hour pre-treatment of FP-ISO-21AC at 1 M reduced cytokine-induced NO production down to 37%. PRED at 1 M reduced cyto kine-induced NO production down to 65%. The FP-ISO- 21AC showed higher inhibitory effect on cytokine-ind- uced NO production. In other words, the FP-ISO-21AC showed higher anti-inflammatory effect. 3.2. Anti-Inflammatory Steroidal Antedrug FP-ISO-21AC and Its Parent PRED Inhibit Cytokine-Induced Interleukin-8 (IL-8) Production Anti-inflammatory steroidal antedrug FP-ISO-21AC and 0 20 40 60 80 100 120 % inhibition of NO production cytomix without steroids cytomix with 1μM PRED pretreatment cytomix with 1μM FP- ISO-AC pretreatment Figure 2. Inhibitory effect of steroids on cytokine-induced NO production in A549 human alveolar epithelial cells. Cells were exposed cytomix (The mixture of the three cytokines IL-1, TNF- and IFN- and the concentration of each cytokine is 50 ng/ml) following pretreatment of steroids at indicated concen- trations for 1 hour. The concentration of NO was measured 24 hours after cytokine exposure by amiNO series of NO sensors. Data from steroids treatment experiments were expressed as percentage of untreated controls. p≤0.05. 0 20 40 60 80 100 120 % inhibition of IL-8 production cytomix without steroids cytomix with 1µM PRED pretreatment cytomix with 1µM FP-ISO- 21AC pretreatment Figure 3. Inhibitory effect of steroids on cytokine-induced IL-8 production in A549 human alveolar epithelial cells. Cells were exposed cytomix (IL-1β, TNF-α and IFN-γ at 50 ng/ml respectively) following pretreatment of steroids at indicated concentrations for 1 hour. The concentration of IL-8 was measured by ELISA after 24 hours exposure to cytokine. Data from steroids treatment experiments were expressed as per- centage of untreated controls. p≤0.05. One hour pretreatment of FP-ISO-21AC and PRED at 1 M reduce cytokine-induced IL-8 production to 50% and 53% respectively. 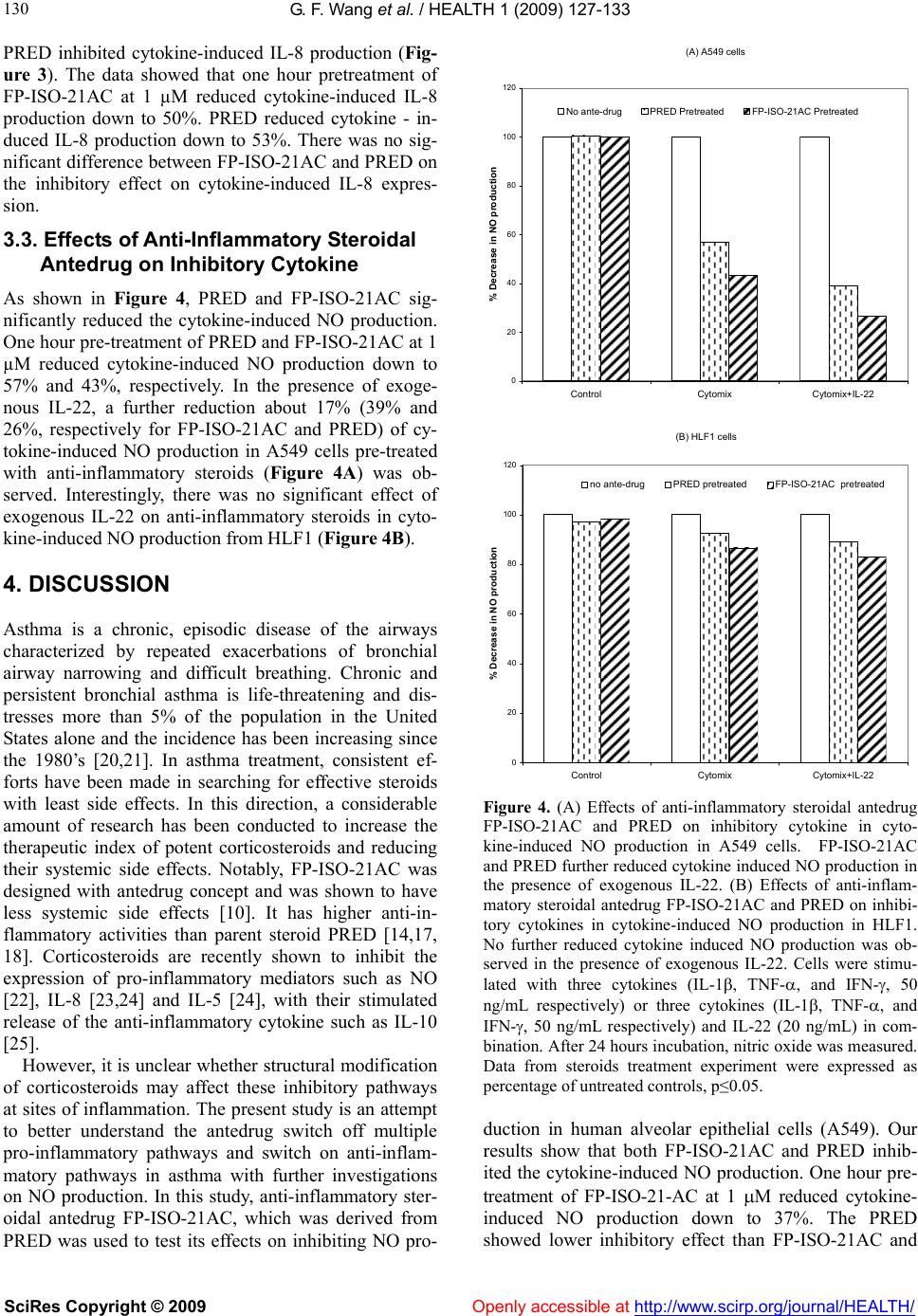 G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 130 PRED inhibited cytokine-induced IL-8 production (Fig- ure 3). The data showed that one hour pretreatment of FP-ISO-21AC at 1 µM reduced cytokine-induced IL-8 production down to 50%. PRED reduced cytokine - in- duced IL-8 production down to 53%. There was no sig- nificant difference between FP-ISO-21AC and PRED on the inhibitory effect on cytokine-induced IL-8 expres- sion. 3.3. Effects of Anti-Inflammatory Steroidal Antedrug on Inhibitory Cytokine As shown in Figure 4, PRED and FP-ISO-21AC sig- nificantly reduced the cytokine-induced NO production. One hour pre-treatment of PRED and FP-ISO-21AC at 1 µM reduced cytokine-induced NO production down to 57% and 43%, respectively. In the presence of exoge- nous IL-22, a further reduction about 17% (39% and 26%, respectively for FP-ISO-21AC and PRED) of cy- tokine-induced NO production in A549 cells pre-treated with anti-inflammatory steroids (Figure 4A) was ob- served. Interestingly, there was no significant effect of exogenous IL-22 on anti-inflammatory steroids in cyto- kine-induced NO production from HLF1 (Figure 4B). 4. DISCUSSION Asthma is a chronic, episodic disease of the airways characterized by repeated exacerbations of bronchial airway narrowing and difficult breathing. Chronic and persistent bronchial asthma is life-threatening and dis- tresses more than 5% of the population in the United States alone and the incidence has been increasing since the 1980’s [20,21]. In asthma treatment, consistent ef- forts have been made in searching for effective steroids with least side effects. In this direction, a considerable amount of research has been conducted to increase the therapeutic index of potent corticosteroids and reducing their systemic side effects. Notably, FP-ISO-21AC was designed with antedrug concept and was shown to have less systemic side effects [10]. It has higher anti-in- flammatory activities than parent steroid PRED [14,17, 18]. Corticosteroids are recently shown to inhibit the expression of pro-inflammatory mediators such as NO [22], IL-8 [23,24] and IL-5 [24], with their stimulated release of the anti-inflammatory cytokine such as IL-10 [25]. However, it is unclear whether structural modification of corticosteroids may affect these inhibitory pathways at sites of inflammation. The present study is an attempt to better understand the antedrug switch off multiple pro-inflammatory pathways and switch on anti-inflam- matory pathways in asthma with further investigations on NO production. In this study, anti-inflammatory ster- oidal antedrug FP-ISO-21AC, which was derived from PRED was used to test its effects on inhibiting NO pro- (A) A549 cells 0 20 40 60 80 100 120 ControlCytomix Cytomix+IL-22 % Decrease in NO production No ante-drugPRED PretreatedFP-ISO-21AC Pretreated (B) HLF1 cells 0 20 40 60 80 100 120 ControlCytomix Cytomix+IL-22 % Decrease in NO production no ante-drugPRED pretreatedFP-ISO-21AC pretreated Figure 4. (A) Effects of anti-inflammatory steroidal antedrug FP-ISO-21AC and PRED on inhibitory cytokine in cyto- kine-induced NO production in A549 cells. FP-ISO-21AC and PRED further reduced cytokine induced NO production in the presence of exogenous IL-22. (B) Effects of anti-inflam- matory steroidal antedrug FP-ISO-21AC and PRED on inhibi- tory cytokines in cytokine-induced NO production in HLF1. No further reduced cytokine induced NO production was ob- served in the presence of exogenous IL-22. Cells were stimu- lated with three cytokines (IL-1, TNF-, and IFN-, 50 ng/mL respectively) or three cytokines (IL-1, TNF-, and IFN-, 50 ng/mL respectively) and IL-22 (20 ng/mL) in com- bination. After 24 hours incubation, nitric oxide was measured. Data from steroids treatment experiment were expressed as percentage of untreated controls, p≤0.05. duction in human alveolar epithelial cells (A549). Our results show that both FP-ISO-21AC and PRED inhib- ited the cytokine-induced NO production. One hour pre- treatment of FP-ISO-21-AC at 1 M reduced cytokine- induced NO production down to 37%. The PRED showed lower inhibitory effect than FP-ISO-21AC and  G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 131 131 reduced cytokine-induced NO production down to 65% (Figure 2). There was no significant difference between FP-ISO-21AC and PRED on inhibitory effect on cyto- kine-induced IL-8 expression, but the FP-ISO-21AC showed higher inhibitory effect on cytokine-induced NO production (Figure 2). To confirm the observed decrease in NO production related to enhanced expression of inhibitory cytokines, exogenous IL-22 was added to the media of A549 in the presence or absence of anti-inflammatory steroids. IL-22 is a member of the human type I IFN family, which in- cludes IL-10. Our data corroborates with earlier report on IL-22 effect in the presence of inflammatory steroids [26]. Other studies support our observations on lung epithelial cells (A549) which express IL-22R1 (inter- leukin 22 receptor) subunit in response to IL-22 [15]. As the results indicated in Figure 4A and 4B, both PRED and FP-ISO-21AC may enhance the expression of IL-22R1, and increase the responsiveness to exogenous IL-22 in A549 cells which do express IL-22R1. An important factor is the balance between the effects of pro-inflammatory and anti-inflammatory chemokines or cytokines in determining the severity of the inflam- matory disease. Corticosteroids have effects on expres- sion of cytokines receptors [27,28]. Corticosteroids are understood to upregulate cytokine receptor expression that correlates with enhanced cytokine effects on target cells [27]. Other earlier studies also suggest that recep- tors for IL-1 [29,30], IL-2 [31], IL-4 [32], IL-6 [33,34], IFN- [35] and GM-CSF [36] are all up-regulated by glucocorticoids. Inflammatory stimuli increase the expression of many inflammatory genes, resulting in the inflammatory re- sponses. The same stimuli may also induce inhibitory cytokines, which can inhibit the expression of these in- flammatory proteins, thus terminating this inflammatory response. There has been increasing evidence that a de- fective expression of inhibitory cytokines is observed in patients with asthma [37]. The reduced expression of inhibitory cytokines leads to increased and more pro- longed inflammation. Subsequent treatment by anti-in- flammatory steroids can restore the secretion of inhibi- tory cytokines [37]. Structural modification of corticos- teroids may restore this defective expression of inhibi- tory cytokines. The structural modification can be an important direction forwards the design of novel anti-in- flammatory steroidal antedrugs for the treatment of asthma. There has been increasing evidences that the role of inhibitory cytokines is crucial on the treatment of asthma [38]. IL-22 is one of the most important inhibitory cyto- kines involved in the inhibition of the inflammation. It can regulate the expression of proinflammatory cyto- kines [39]. However, the effect of steroid on IL-22R is not known. In order to test if steroids can enhance the expression of IL-22R, the effects of IL-22 on the inhibi- tion of NO production induced by the pre-treatment of anti-inflammatory steroids was tested. Our data demon- strated that in the presence of the exogenous inhibitory cytokine IL-22, further reduction of NO production was observed in A549 cells (Figure 4A) as earlier demon- strated to express IL-22R [39] but not in HLF1 (Figure 4B) which do not express IL-22R. These data suggested that the steroidal ante-drugs may enhance the expression the IL-22 receptor. FP-ISO-21AC showed higher po- tency than PRED to restore the expression of IL-22R. FP-ISO-21AC further reduced NO production to 27% and PRED further reduced NO production to 39%. In conclusion, a synthesized antedrug FP-ISO-21AC showed higher anti-inflammatory effects than PRED by inhibiting the expression of pro-inflammatory mediator NO. There was no significant difference between FP-ISO-21AC and PRED on inhibitory effect on cyto- kine-induced IL-8 expression, but the FP-ISO-21AC showed higher inhibitory effect on cytokine-induced NO production. FP-ISO-21AC also showed higher potency than its parent PRED in stimulating the possible expres- sion of IL-22R. This study showed that structural modi- fication of anti-inflammatory steroids with ‘antedrug concept’ (with the direction of reducing the systemic side effects) could enhance their anti-inflammatory ac- tivities with less systemic side effects. REFERENCES [1] D. T. Boumpas, G. P. Chrousos, R. L. Wilder, T. R. Cupps, J. E. Balow, (1993) Glucocorticoid therapy for immune- mediated diseases - basic and clinical correlates, Annals of Internal Medicine, 119, 1198-1208. [2] J. W. Funder, (1997) Glucocorticoid and mineralocorti- coid receptors: biology and clinical relevance, Annual Review of Medicine, 48, 231-240. [3] H. M. Reichardt, G. Schutz, (1998) Glucocorticoid sig- nalling - multiple variations of a common theme, Mo- lecular and Cellular Endocrinology, 146, 1-6. [4] L.I. McKay, JA. Cidlowski, (1999) Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways, Endocrine Reviews, 20, 435-459. [5] L. A. Wolfraim, (2006) Treating autoimmune diseases through restoration of antigen-specific immune tolerance, Archivum Immunologiae Et Therapiae Experimentalis, 54, 1-13. [6] H. Schacke, W. D. Docke, K. Asadullah, (2002) Mecha- nisms involved in the side effects of glucocorticoids, Pharmacology & Therapeutics, 96, 23-43. [7] E. Garbe, J. LeLorier, J. F. Boivin, S. Suissa, (1997) Risk of ocular hypertension or open-angle glaucoma in elderly patients on oral glucocorticoids, Lancet, 350, 979-982. [8] P. B. Jacobson, T. W. von Geldern, L. Ohman, M. Oster- land, J. H. Wang, B. Zinker, D. Wilcox, PT. Nguyen, A. Mika, S. Fung, T. Fey, A. Goos-Nilsson, M. Grynfarb, T.  G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/HEALTH/ 132 Barkhem, K. Marsh, D. W. A. Beno, B. Nga-Nguyen, P. R. Kym, J. T. Link, N. Tu, DS. Edgerton, A. Cherrington, S. Efendic, B. C. Lane, T. J. Opgenorth, (2005) Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes, Journal of Pharmacology and Experimental Therapeutics, 314, 191- 200. [9] J. Nishimura, S. Ikuyama, (2000) Glucocorticoid-Induced osteoporosis: pathogenesis and management, Journal of Bone and Mineral Metabolism, 18, 350-352. [10] H. J. Lee, MRI. Soliman, (1982) Anti-Inflammatory ster- oids without pituitary-adrenal suppression, Science, 215, 989-991. [11] H. J. Lee, M. A. Khalil, J. W. Lee, (1984) Antedrug - a conceptual basis for safer anti-inflammatory steroids, Drugs under Experimental and Clinical Research, 10, 835-844. [12] H. J. Lee, R. Trottier, (1980) Antiinflammatory activity of two novel derivatives of prednisolone, Res Commun Chem Pathol Pharmacol, 27, 611-4. [13] H. Ueno, A. Maruyama, M. Miyake, E. Nakao, K. Nakao, K. Umezu, I. Nitta, (1991) Synthesis and evaluation of antiinflammatory activities of a series of corticosteroid 17-Alpha-Esters containing a Functional-Group, Journal of Medicinal Chemistry, 34, 2468-2473. [14] K. K. Park, D. H. Ko, Z. You, M. O. F. Khan, H. J. Lee, (2006) In vitro anti-inflammatory activities of new ster- oidal antedrugs: [16 alpha, 17 alpha-d] isoxazoline and [16 alpha, 17 alpha-d]-3'-hydroxy-iminoformyl isoxazo- line derivatives of prednisolone and 9 alpha - fluoropred- nisolone, Steroids, 71, 183-188. [15] H. A. Whittington, L. Armstrong, K. M. Uppington, A.B. Millar, (2004Interleukin-22 - A potential immunomodu- latory molecule in the lung, American Journal of Respi- ratory Cell and Molecular Biology, 31, 220-226. [16] B. M. Necela, J. A.Cidlowski, (2003) Crystallization of the human glucocorticoid receptor ligand binding domain: a step towards selective glucocorticoids, Trends in Phar- macological Sciences, 24, 58-61. [17] M. A. Khalil, M. F. Maponya, D. H. Ko, Z. Q. You, E. T. Oriaku, H. J. Lee, (1996) New anti-inflammatory steroids: [16 alpha, 17 alpha-d] isoxazoline derivatives of predni- solone and 9 alpha-fluoroprednisolone, Medicinal Chem- istry Research, 6, 52-60. [18] D. H. Ko, M. F. Maponya, M. A. Khalil, E. T. Oriaku, Z. Q. You, H. J. Lee, (1997) New anti-inflammatory ster- oids: [16 alpha, 17 alpha-d] -3'-hydroxyiminoformyl isoxazoline derivatives of prednisolone and 9 apha- flu ro- prednisolone. Medicinal Chemistry Research, 7, 313-323. [19] T. Kwon, A. S. Heiman, E. T. Oriaku, K. Yoon, H. J. Lee (1995) New steroidal antiinflammatory antedrugs - ster- oidal [16- Alpha,17-Alpha-D]-3'-Carbethoxyisoxazolines. Journal of Medicinal Chemistry, 38, 1048-1051. [20] JA. Elias, Z. Zhu, G. Chupp, RJ. Homer, (1999) Airway remodeling in asthma, Journal of Clinical Investigation; 104, 1001-1006. [21] J. Elias, (1999) Inspirations on asthma, Journal of Clini- cal Investigation, 104, 827-827. [22] N. K. Worrall, T. P. Misko, P. M. Sullivan, J. J. Hui, C. P. Rodi, T. B. Ferguson, (1996) Corticosteroids inhibit ex- pression of inducible nitric oxide synthase during acute cardiac allograft rejection, Transplantation, 61, 324-328. [23] K. Nyhlen, M. Linden, R. Andersson, S. Uppugunduri, (2000) Corticosteroids and interferons inhibit cyto- kine-induced production of IL-8 by human endothelial cells, Cytokine, 12, 355-360. [24] B. Wallwork, W. Coman, F. Feron, A. Mackay-Sim, A. Cervin, (2002) Clarithromycin and prednisolone inhibit cytokine production in chronic rhinosinusitis, Laryngo- scope, 112, 1827-1830. [25] M. John, S. Lim, J. Seybold, P. Jose, A. Robichaud, B. O'Connor, P. J. Barnes, K. F. Chung, (1998) Inhaled cor- ticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1 alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma, American Journal of Respiratory and Critical Care Medicine, 157, 256-262. [26] L. Dumoutier, J. Louahed, J. C. Renauld, Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF): a novel cytokine structurally related to IL-10 and inducible by IL-9, Journal of Immunology l2000, 164, 1814-1819. [27] W. Y. Almawi, H. N. Beyhum, A. A. Rahme, M. J. Rieder, (1996) Regulation of cytokine and cytokine receptor ex- pression by glucocorticoids, Journal of Leukocyte Biol- ogy,60, 563-572. [28] G. J. Wiegers, J. M. H. M. Reul, (1998) Induction of cytokine receptors by glucocorticoids: functional and pathological significance, Trends in Pharmacological Sciences, 19, 317-321. [29] T. Akahoshi, J. J. Oppenheim, K. Matsushima, (1988) Induction of high-affinity Interleukin-1 receptor on hu- man peripheral-blood lymphocytes by glucocorticoid hormones, Journal of Experimental Medicine, 167, 924- 936. [30] P. E. Gottschall, K. Koves, K. Mizuno, I. Tatsuno, A. Arimura, (1991) Glucocorticoid up-regulation of Inter- leukin-1 receptor expression in a glioblastoma Cell-Line,. American Journal of Physiology, 261, E362-E368. [31] G. J. Wiegers, M. S. Labeur, I. E. M. Stec, W. E. F. Klinkert, F. Holsboer, J. M. H. M. Reul, (1995) Gluco- corticoids accelerate anti-T cell receptor-induced T-cell growth, Journal of Immunology, 155, 1893-1902. [32] R. L. K. Paterson, R. Or, J. M. Domenico, G. Delespesse, E. W. Gelfand, (1994) Regulation of Cd23 expression by Il-4 and corticosteroid in human B-lymphocytes - altered response after Ebv infection, Journal of Immunology, 152, 2139-2147. [33] L. Snyers, L. Dewit, J. Content, (1990) Glucocorticoid up-regulation of high-affinity Interleukin-6 receptors on human Epithelial-Cells, Proceedings of the National Academy of Sciences of the United States of America, 87, 2838-2842. [34] S. P. Campos, Y. P. Wang, A. Koj, H. Baumann, (1993) Divergent transforming Growth-Factor-Beta effects on Il-6 regulation of Acute-Phase Plasma-Proteins in Rat Hepatoma-Cells, Journal of Immunology, 151, 7128-7137. [35] R. W. Strickland, L. M. Wahl, D. S. Finbloom, (1986) Corticosteroids enhance the binding of recombinant in- terferon-gamma to cultured human-monocytes, Journal of Immunology, 137, 1577-1580. [36] C. M. Hawrylowicz, L. Guida, E. Paleolog, (1994) Dex- amethasone up-regulates granulocyte-macrophage colony  G. F. Wang et al. / HEALTH 1 (2009) 127-133 SciRes Copyright © 2009 http://www.scirp.org/journal/HEALTH/Openly accessible at 133 133 - stimulating ractor-receptor expression on human mono- cytes, Immunology, 83, 274-280. [37] P. J. Barnes, S. Lim, (1998) Inhibitory cytokines in asthma, Molecular Medicine Today, 4, 452-458. [38] D. Kunz, G. Walker, W. Eberhardt, J. Pfeilschifter, (1996) Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta- stimulated mesangial cells: evidence for the involvement of transcriptional and posttranscriptional regulation, Proceedings of the National Academy of Sciences of the United States of America, 93, 255-259. [39] D. A. Geller, A. K. Nussler, M. Disilvio, C. J. Lowenstein, R. A. Shapiro, S. C. Wang, R. L. Simmons, T. R. Billiar, (1993) Cytokines, endotoxin, and glucocorticoids regu- late the expression of inducible Nitric-Oxide synthase in hepatocytes, Proceedings of the National Academy of Sciences of the United States of America, 90, 522-526. |

