 Open Journal of Stomatology, 2011, 1, 126-139 doi:10.4236/ojst.2011.14020 Published Online December 2011 (http://www.SciRP.org/journal/ojst/ OJST ). Published Online December 2011 in SciRes. http://www.scirp.org/journal/OJST Which treatment protocol, among classical methods and/or various laser applications is the most effective in root canal disinfection, in vitro? A systematic review Joanna Theodosopoulou1, Alexandra Tsigarida2, Konstantinos Chochlidakis3 1Harvard School of Dental Medicine (HSDM), The Forsyth Institute, Private Practice, Athens, Greece; 2Department of Periodontology, The Ohio State University, Columbus, USA; 3Department of Prosthodontics, Eastman Institute for Oral Health, Rochester, USA. Email: joanna_theodosopoulou@yahoo.gr Received 20 July 2011; revised 11 October 2011; accepted 26 October 2011. ABSTRACT Purpose: The aim of this systematic review was to answer the question “Which treatment protocol, am- ong classical methods and/or various laser applica- tions is the most effective in root canal disinfection, in vitro”. Materials and Methods: A MEDLINE, a Co- chrane and an Embase search (three specified search- es) were conducted to identify randomized controlled trials (RCT) until June 2010, conducted on human teeth and published in English, German or French language, examining the root canal disinfection after the use of lasers with or without mechanical instru- mentation. Additionally, hand search was conducted and contact with authors, when needed. Results: The MEDLINE, the Cochrane and the EMBASE search identified 240, 28, and 35 published articles, respec- tively. Ten articles from the MEDLINE and 5 articles from the Cochrane search (that were also identified in the MEDLINE search) met the inclusion and va- lidity assessment criteria. In E. faecalis elimination, instrumentation of the root canal and diode laser/665 nanometer/1 Watt (diode laser/665 nm/1 W) irradia- tion with the combined effect of Methylene Blue (MB) as photosensitizing agent (logCFU/ml = 1.636) seemed to be the best method. In P. aeruginosa and in A. naeslundii elimination, instrumentation of the root canal followed by irrigation with 5.5% NaOCl (log- CFU/ml = 0) seemed to be the best method. In gen- eral, instrumentation of the root canal followed by irrigation with 5.25% NaOCl (logCFU/ml = 0) and instrumentation of the root canal and Er: YAG laser/ 2940 nm/0.8 W irradi ation (logCFU/ml = 1.924) seemed to be the best (polymicrobial studies). Conclusions: There are treatment protocols with the assistance or not of laser irradiation that can eliminate E. faecalis, E. coli and S. aureus inside the root canal. However, there is a serious number of S. anginosus, F. nuclea- tum, A. naeslundii and P. aeru ginosa that remain in- side the root canal even after laser irradiation. New research is needed in order to set a treatment proto- col effective in the root canal disinfection from all bac- teria that are related to endodontic origin pathology. Keywords: Lasers; Classical Methods; Endodontic; Root Canal Therapy; Dentin; Bacteria; Disinfection 1. INTRODUCTION One of the most crucial and fundamental stages of endo- dontic therapy is the root canal disinfection in its three- dimensional network of dentinal tubules. Nevertheless, persistence of infection in the root canal is responsible for long-term failures and need for endodontic therapy retreatments. It is generally accepted that microorganisms tend to remain in the root canal even after proper preparation and are responsible for flare-ups, after the completion of the endodontic therapy. The most common of these mi- croorganisms are: Fusobacterium nucleatum, Enterococ- cus faecalis, Prevotella intermedia, Streptococcus angi- nosa, Treponima denticolla, Porphyromonas gingivalis [1-4]. During the last years, laser irradiation has been addi- tionally introduced in root canal preparation, trying to gain acceptance for its disinfection ability in comparison with the common mechanical instrumentation and irriga- tion procedures. Many studies examine the effectiveness of Nd:YAG, diode, Er,Cr:YSGG and Er:YAG laser, when used in dif- ferent wavelengths, solely, or in addition with various so- lutions in the bacterial elimination inside the root canals. The purpose of this systematic review was to answer the question “Which treatment protocol, among classical me- thods and/or various laser applications is the most ef- fective in root canal disinfection, in vitro”. 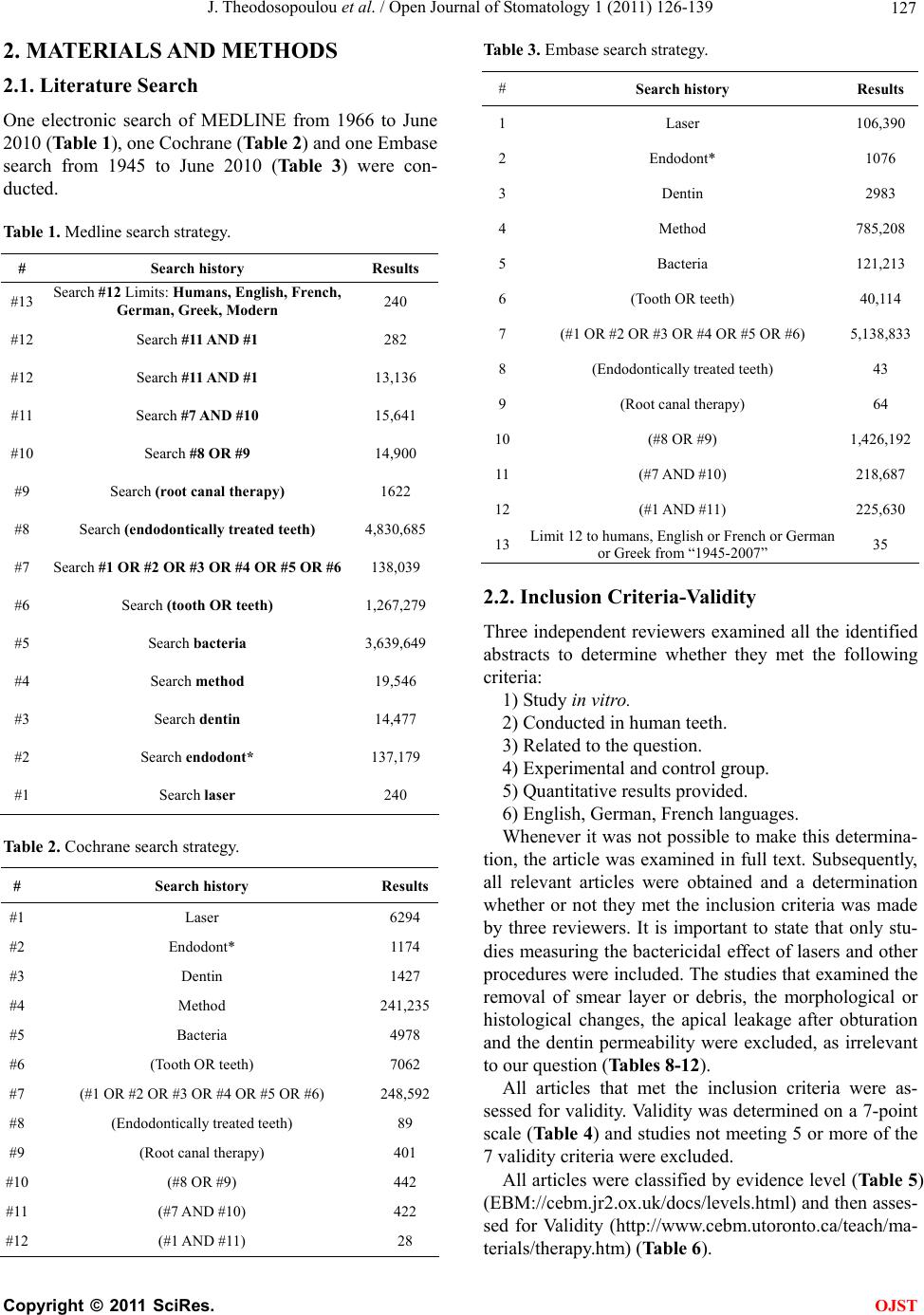 J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 127 2. MATERIALS AND METHODS 2.1. Literature Search One electronic search of MEDLINE from 1966 to June 2010 (Table 1), one Cochrane (Table 2) and one Embase search from 1945 to June 2010 (Table 3) were con- ducted. Table 1. Medline search strategy. # Search history Results #13 Search #12 Limits: Humans, English, French, German, Greek, Modern 240 #12 Search #1 1 AND #1 282 #12 Search #11 AND #1 13,136 #11 Search #7 AND #10 15,641 #10 Search #8 OR #9 14,900 #9 Search (root canal therapy) 1622 #8 Search (endodontically treated teeth) 4,830,685 #7 Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 138,039 #6 Search (tooth OR teeth) 1,267,279 #5 Search bacteria 3,639,649 #4 Search method 19,546 #3 Search dentin 14,477 #2 Search endodont* 137,179 #1 Search laser 240 Table 2. Cochrane search strategy. # Search history Results #1 Laser 6294 #2 Endodont* 1174 #3 Dentin 1427 #4 Method 241,235 #5 Bacteria 4978 #6 (Tooth OR teeth) 7062 #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) 248,592 #8 (Endodontically treated teeth) 89 #9 (Root canal therapy) 401 #10 (#8 OR #9) 442 #11 (#7 AND #10) 422 #12 (#1 AND #11) 28 Table 3. Embase search strategy. # Search history Results 1 Laser 106,390 2 Endodont* 1076 3 Dentin 2983 4 Method 785,208 5 Bacteria 121,213 6 (Tooth OR teeth) 40,114 7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) 5,138,833 8 (Endodontically treated teeth) 43 9 (Root canal therapy) 64 10 (#8 OR #9) 1,426,192 11 (#7 AND #10) 218,687 12 (#1 AND #11) 225,630 13 Limit 12 to humans, English or French or German or Greek from “1945-2007” 35 2.2. Inclusion Criteria-Validity Three independent reviewers examined all the identified abstracts to determine whether they met the following criteria: 1) Study in vitro. 2) Conducted in human teeth. 3) Related to the question. 4) Experimental and control group. 5) Quantitative results provided. 6) English, German, French languages. Whenever it was not possible to make this determina- tion, the article was examined in full text. Subsequently, all relevant articles were obtained and a determination whether or not they met the inclusion criteria was made by three reviewers. It is important to state that only stu- dies measuring the bactericidal effect of lasers and other procedures were included. The studies that examined the removal of smear layer or debris, the morphological or histological changes, the apical leakage after obturation and the dentin permeability were excluded, as irrelevant to our question (Tables 8-12). All articles that met the inclusion criteria were as- sessed for validity. Validity was determined on a 7-point scale (Table 4) and studies not meeting 5 or more of the 7 validity criteria were excluded. All articles were classified by evidence level (Table 5) (EBM://cebm.jr2.ox.uk /docs/levels.html) and then asses- sed for Validity (http://www.cebm.utoronto.ca/teach/ma- terials/therapy.htm) (Table 6). C opyright © 2011 SciRes. OJST 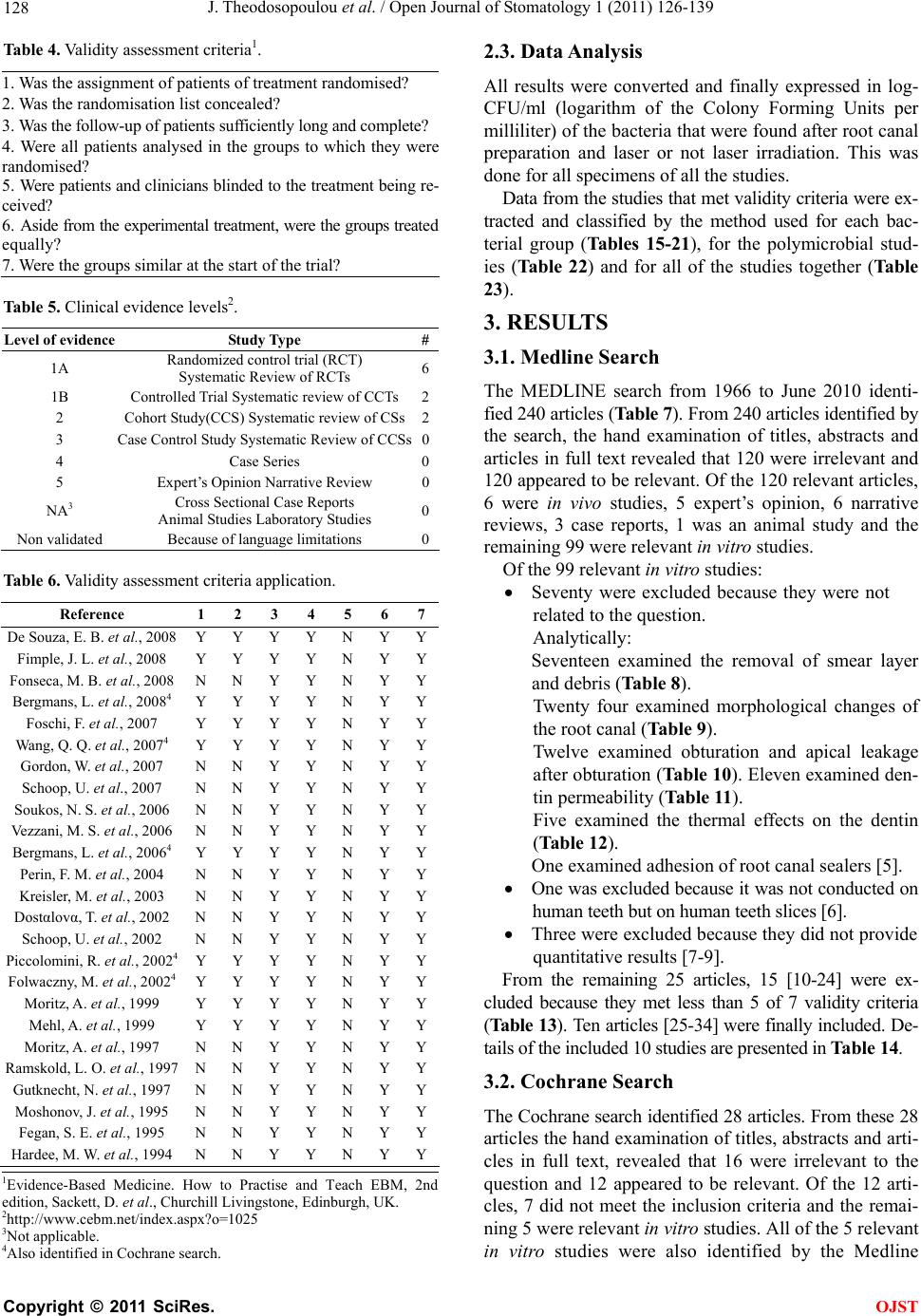 J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 128 Table 4. Validity assessment criteria1. 1. Was the assignment of patients of treatment randomised? 2. Was the randomisation list concealed? 3. Was the follow-up of patients sufficiently long and complete? 4. Were all patients analysed in the groups to which they were randomised? 5. Were patients and clinicians blinded to the treatment being re- ceived? 6. Aside from the experimental treatment, were the groups treated equally? 7. Were the groups similar at the start of the trial? Table 5. Clinical evidence levels2. Level of evidence Study Type # 1A Randomized control trial (RCT) Systematic Review of RCTs 6 1B Controlled Trial Systematic review of CCTs2 2 Cohort Study(CCS) Systematic review of CSs2 3 Case Control Study Systematic Review of CCSs0 4 Case Series 0 5 Expert’s Opinion Narrative Review 0 NA3 Cross Sectional Case Reports Animal Studies Laboratory Studies 0 Non validated Because of language limitations 0 Table 6. Validity assessment criteria application. Reference 1 2 3 4 5 6 7 De Souza, E. B. et al., 2008 Y Y Y Y N Y Y Fimple, J. L. et al., 2008 Y Y Y Y N Y Y Fonseca, M. B. et al., 2008 N N Y Y N Y Y Bergmans, L. et al., 20084 Y Y Y Y N Y Y Foschi, F. et al., 2007 Y Y Y Y N Y Y Wang, Q. Q. et al., 20074 Y Y Y Y N Y Y Gordon, W. et al., 2007 N N Y Y N Y Y Schoop, U. et al., 2007 N N Y Y N Y Y Soukos, N. S. et al., 2006 N N Y Y N Y Y Vezzani, M. S. et al., 2006 N N Y Y N Y Y Bergmans, L. et al., 20064 Y Y Y Y N Y Y Perin, F. M. et al., 2004 N N Y Y N Y Y Kreisler, M. et al., 2003 N N Y Y N Y Y Dostαlovα, T. et al., 2002 N N Y Y N Y Y Schoop, U. et al., 2002 N N Y Y N Y Y Piccolomini, R. et al., 20024 Y Y Y Y N Y Y Folwaczny, M. et al., 20024 Y Y Y Y N Y Y Moritz, A. et al., 1999 Y Y Y Y N Y Y Mehl, A. et al., 1999 Y Y Y Y N Y Y Moritz, A. et al., 1997 N N Y Y N Y Y Ramskold, L. O. et al., 1997 N N Y Y N Y Y Gutknecht, N. et al., 1997 N N Y Y N Y Y Moshonov, J. et al., 1995 N N Y Y N Y Y Fegan, S. E. et al., 1995 N N Y Y N Y Y Hardee, M. W. et al., 1994 N N Y Y N Y Y 2.3. Data Analysis All results were converted and finally expressed in log- CFU/ml (logarithm of the Colony Forming Units per milliliter) of the bacteria that were found after root canal preparation and laser or not laser irradiation. This was done for all specimens of all the studies. Data from the studies that met validity criteria were ex- tracted and classified by the method used for each bac- terial group (Tables 15-21), for the polymicrobial stud- ies (Ta b l e 22 ) and for all of the studies together (Table 23). 3. RESULTS 3.1. Medline Search The MEDLINE search from 1966 to June 2010 identi- fied 240 articles (Table 7). From 240 articles identified by the search, the hand examination of titles, abstracts and articles in full text revealed that 120 were irrelevant and 120 appeared to be relevant. Of the 120 relevant articles, 6 were in vivo studies, 5 expert’s opinion, 6 narrative reviews, 3 case reports, 1 was an animal study and the remaining 99 were relevant in vitro studies. Of the 99 relevant in vitro studies: Seventy were excluded because they were not related to the question. Analytically: Seventeen examined the removal of smear layer and debris (Table 8). Twenty four examined morphological changes of the root canal (Table 9). Twelve examined obturation and apical leakage after obturation (Table 10). Eleven examined den- tin permeability (Table 11). Five examined the thermal effects on the dentin (Table 12). One examined adhesion of root canal sealers [5]. One was excluded because it was not conducted on human teeth but on human teeth slices [6]. Three were excluded because they did not provide quantitative results [7-9]. From the remaining 25 articles, 15 [10-24] were ex- cluded because they met less than 5 of 7 validity criteria (Table 13). Ten articles [25-34] were finally included. De- tails of the included 10 studies are presented in Table 14. 3.2. Cochrane Search The Cochrane search identified 28 articles. From these 28 articles the hand examination of titles, abstracts and arti- cles in full text, revealed that 16 were irrelevant to the question and 12 appeared to be relevant. Of the 12 arti- cles, 7 did not meet the inclusion criteria and the remai- ning 5 were relevant in vitro studies. All of the 5 relevant in vitro studies were also identified by the Medline 1Evidence-Based Medicine. How to Practise and Teach EBM, 2nd edition, Sackett, D. et al., Churchill Livingstone, Edinburgh, UK. 2http://www.cebm.net/index.aspx?o=1025 3Not applicable. 4Also identified in Cochrane search. C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 Copyright © 2011 SciRes. 129 Table 7. Search results. MEDLINE COCHRANE EMBASE (n = 240) (n = 28) (n = 35) Irrelevant (n= 120)(n=16)(n=35) Relevant studies (n = 120) (n = 12) (n = 0) In Vitro studies (n = 99) (n = 13) (n = 0) Meeting the Inclusion and Validity Criteria (n = 10) (n = 5) (n = 0) In Vivo (n = 6)(n = 0) (n = 0) Expert’s opinion (n = 5)(n = 0) (n = 0) Narrative reviews(n = 6)(n = 0) (n = 0) Case reports (n = 3)(n = 0) (n = 0) In animals (n = 1)(n = 0) (n = 0) Not Found (n = 0)(n = 0) (n = 0) Not meeting the Inclusion Criteria (n = 74)(n = 7)(n = 0) Not meeting the Validity Criteria (n = 15)(n = 0)(n = 0) Table 8. Excluded studies: remove smear layer/debris. Reference Detail Soares F. et al., 2008 Remove smear layer Moshonov J. et al., 2004 Remove smear layer Moshonov J. et al., 2003 Remove smear layer Matsuoka E. et al., 1998 Remove smear layer Takeda F. H. et al., 1998 Remove smear layer Arrastia-Jitosho A. M. et al., 1998 Remove smear layer Takeda F. H. et al., 1998 Remove smear layer Radatti D. A. et al., 2006 Remove debris Blum J. Y. et al., 1997 Remove debris Harashima T. et al., 1997 Remove debris Saunders W. P. et al., 1995 Remove debris Machida T. et al. , 1995 Remove debris, Thermal effects Moshonov J. et al., 1995 Remove debris Bahcall J. K. et al., 1993 Remove debris, Morphological Frentzen M. et al., 1991 Remove debris Liesenhoff T. et al., 1989 Remove debris, Morphological Pini R. et al., 1989 Remove debris search, met all the inclusion criteria and 5 or more of the validity assessment criteria. So, they were finally inc- luded (Table 6). 3.3. Embase Search The Embase search from 1945 to June 2010 identified 35 articles. From these 35 articles identified by the sear- ch, the hand examination of titles, abstracts and articles in full text, revealed that all of them were irrelevant. In order to be able to compare the bactericidal ability of the various treatment protocols that were applied on the human teeth in vitro, by each different group of re- searchers, the results were expressed in logCFU/ml. The CFU/ml of the bacteria that were found in the root canals after bacterial contamination and consecutive standard root canal preparation and/or laser irradiation was cal- culated and converted in logCFU/ml. The closer the value of logCFU/ml is to 0, the greater the positive ef- fect the treatment protocol has to the root canal disin- fecttion. LogCFU/ml of all bacteria is plotted in Ta b l e s 1 5 - 2 3 (Clustered bars and 3-D clustered bars). On the X-axis lies the logCFU/ml and on the Y-axis the various treat- ment protocols. OJST 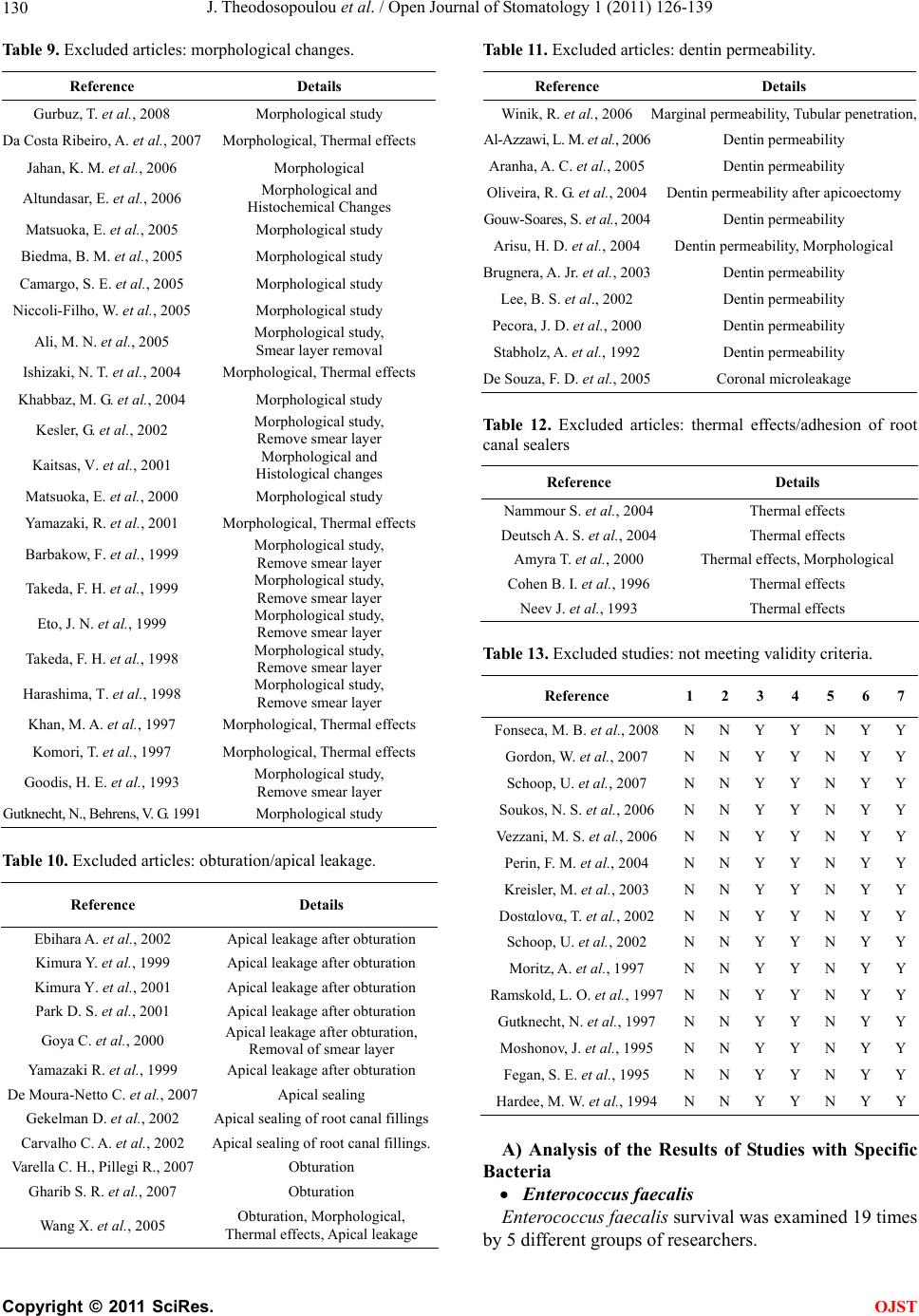 J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 130 Table 9. Excluded articles: morphological changes. Reference Details Gurbuz, T. et al., 2008 Morphological study Da Costa Ribeiro, A. et al., 2007 Morphological, Thermal effects Jahan, K. M. et al., 2006 Morphological Altundasar, E. et al., 2006 Morphological and Histochemical Changes Matsuoka, E. et al., 2005 Morphological study Biedma, B. M. et al., 2005 Morphological study Camargo, S. E. et al., 2005 Morphological study Niccoli-Filho, W. et al., 2005 Morphological study Ali, M. N. et al., 2005 Morphological study, Smear layer removal Ishizaki, N. T. et al., 2004 Morphological, Thermal effects Khabbaz, M. G. et al., 2004 Morphological study Kesler, G. et al., 2002 Morphological study, Remove smear layer Kaitsas, V. et al., 2001 Morphological and Histological changes Matsuoka, E. et al., 2000 Morphological study Yamazaki, R. et al., 2001 Morphological, Thermal effects Barbakow, F. et al., 1999 Morphological study, Remove smear layer Takeda, F. H. et al., 1999 Morphological study, Remove smear layer Eto, J. N. et al., 1999 Morphological study, Remove smear layer Takeda, F. H. et al., 1998 Morphological study, Remove smear layer Harashima, T. et al., 1998 Morphological study, Remove smear layer Khan, M. A. et al., 1997 Morphological, Thermal effects Komori, T. et al., 1997 Morphological, Thermal effects Goodis, H. E. et al., 1993 Morphological study, Remove smear layer Gutknecht, N., Behrens, V. G. 1991 Morphological study Table 10. Excluded articles: obturation/apical leakage. Reference Details Ebihara A. et al., 2002 Apical leakage after obturation Kimura Y. et al., 1999 Apical leakage after obturation Kimura Y. et al., 2001 Apical leakage after obturation Park D. S. et al., 2001 Apical leakage after obturation Goya C. et al., 2000 Apical leakage after obturation, Removal of smear layer Yamazaki R. et al., 1999 Apical leakage after obturation De Moura-Netto C. et al., 2007 Apical sealing Gekelman D. et al., 2002 Apical sealing of root canal fillings Carvalho C. A. et al., 2002 Apical sealing of root canal fillings. Varella C. H., Pillegi R., 2007 Obturation Gharib S. R. et al., 2007 Obturation Wang X. et al., 2005 Obturation, Morphological, Thermal effects, Apical leakage Table 11. Excluded articles: dentin permeability. Reference Details Winik, R. et al., 2006 Marginal permeability, Tubular penetration, Al-Azzawi, L. M. et al., 2006Dentin permeability Aranha, A. C. et al., 2005Dentin permeability Oliveira, R. G. et al., 2004Dentin permeability after apicoectomy Gouw-Soares, S. et al., 2004Dentin permeability Arisu, H. D. et al., 2004Dentin permeability, Morphological Brugnera, A. Jr. et al., 2003Dentin permeability Lee, B. S. et al., 2002 Dentin permeability Pecora, J. D. et al., 2000Dentin permeability Stabholz, A. et al., 1992Dentin permeability De Souza, F. D. et al., 2005Coronal microleakage Table 12. Excluded articles: thermal effects/adhesion of root canal sealers Reference Details Nammour S. et al., 2004 Thermal effects Deutsch A. S. et al., 2004 Thermal effects Amyra T. et al., 2000 Thermal effects, Morphological Cohen B. I. et al., 1996 Thermal effects Neev J. et al., 1993 Thermal effects Table 13. Excluded studies: not meeting validity criteria. Reference 1 2 3 4 567 Fonseca, M. B. et al., 2008N N Y Y N Y Y Gordon, W. et al., 2007 N N Y Y N Y Y Schoop, U. et al., 2007 N N Y Y N Y Y Soukos, N. S. et al., 2006 N N Y Y N Y Y Vezzani, M. S. et al., 2006N N Y Y N Y Y Perin, F. M. et al., 2004 N N Y Y N Y Y Kreisler, M. et al., 2003 N N Y Y N Y Y Dostαlovα, T. et al., 2002 N N Y Y N Y Y Schoop, U. et al., 2002 N N Y Y N Y Y Moritz, A. et al., 1997 N N Y Y N Y Y Ramskold, L. O. et al., 1997N N Y Y N Y Y Gutknecht, N. et al., 1997 N N Y Y N Y Y Moshonov, J. et al., 1995 N N Y Y N Y Y Fegan, S. E. et al., 1995 N N Y Y N Y Y Hardee, M. W. et al., 1994N N Y Y N Y Y A) Analysis of the Results of Studies with Specific Bacteria Enterococcus faec alis Enterococcus faecalis survival was examined 19 times by 5 different groups of researchers. C opyright © 2011 SciRes. OJST 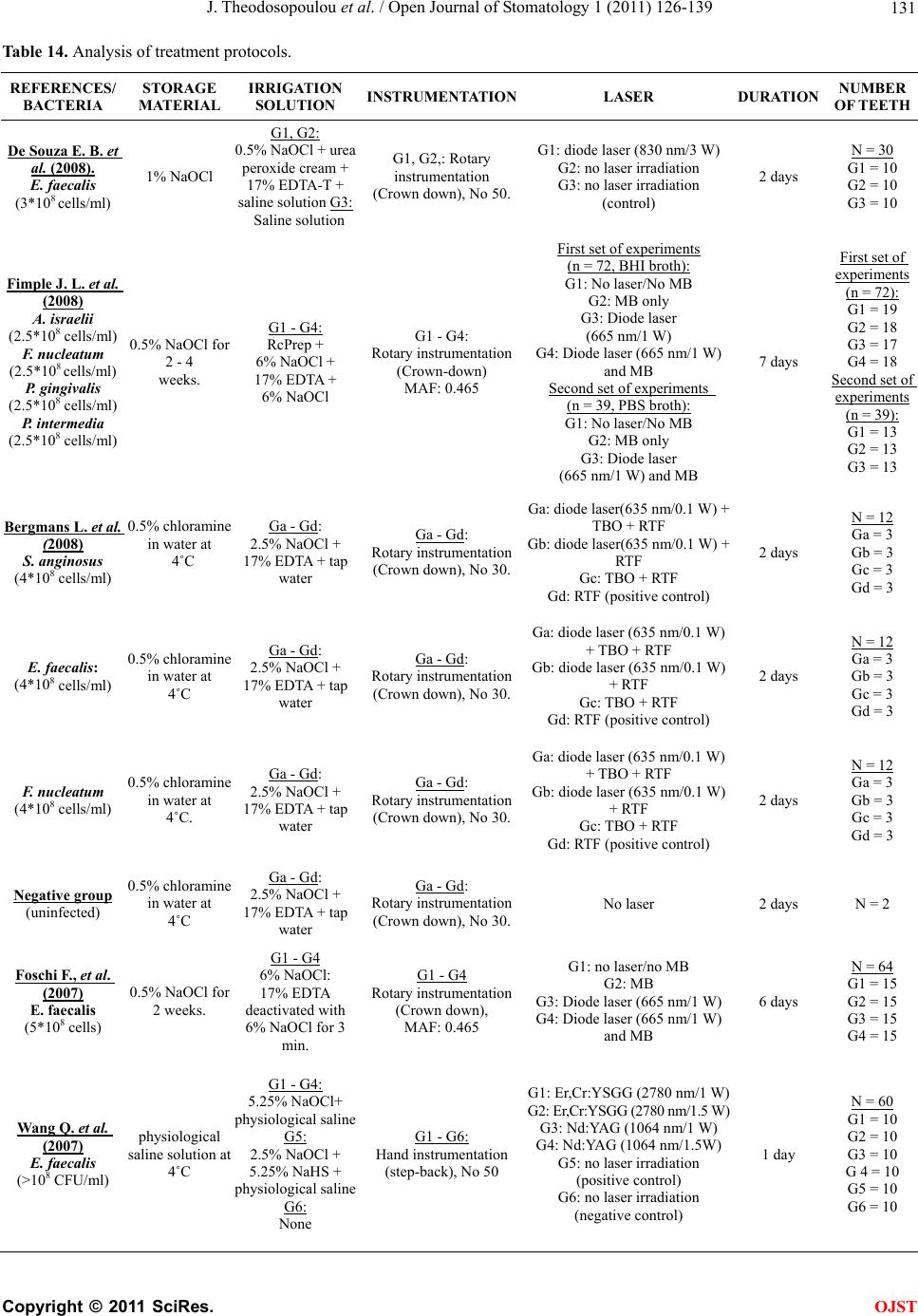 J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 Copyright © 2011 SciRes. 131 OJST Table 14. Analysis of treatment protocols. REFERENCES/ BACTERIA STORAGE MATERIAL IRRIGATION SOLUTION INSTRUMENTATIONLASER DURATION NUMBER OF T EETH De Souza E. B. et al. (2008). E. faecalis (3*108 cells/ml) 1% NaOCl G1, G2: 0.5% NaOCl + urea peroxide cream + 17% EDTA-T + saline solution G3: Saline solution G1, G2,: Rotary instrumentation (Crown down), No 50. G1: diode laser (830 nm/3 W) G2: no laser irradiation G3: no laser irradiation (control) 2 days N = 30 G1 = 10 G2 = 10 G3 = 10 Fimple J. L. et al. (2008) A. israelii (2.5*108 cells/ml) F. nucleatum (2.5*108 cells/ml) P. gingivalis (2.5*108 cells/ml) P. intermedia (2.5*108 cells/ml) 0.5% NaOCl for 2 - 4 weeks. G1 - G4: RcPrep + 6% NaOCl + 17% EDTA + 6% NaOCl G1 - G4: Rotary instrumentation (Crown-down) MAF: 0.465 First set of experiments (n = 72, BHI broth): G1: No laser/No MB G2: MB only G3: Diode laser (665 nm/1 W) G4: Diode laser (665 nm/1 W) and MB Second set of experiments (n = 39, PBS broth): G1: No laser/No MB G2: MB only G3: Diode laser (665 nm/1 W) and MB 7 days First set of experiments (n = 72): G1 = 19 G2 = 18 G3 = 17 G4 = 18 Second set of experiments (n = 39): G1 = 13 G2 = 13 G3 = 13 Bergmans L. et al. (2008) S. anginosus (4*108 cells/ml) 0.5% chloramine in water at 4˚C Ga - Gd: 2.5% NaOCl + 17% EDTA + tap water Ga - Gd: Rotary instrumentation (Crown down), No 30. Ga: diode laser(635 nm/0.1 W) + TBO + RTF Gb: diode laser(635 nm/0.1 W) + RTF Gc: TBO + RTF Gd: RTF (positive control) 2 days N = 12 Ga = 3 Gb = 3 Gc = 3 Gd = 3 E. faecalis: (4*108 cells/ml) 0.5% chloramine in water at 4˚C Ga - Gd: 2.5% NaOCl + 17% EDTA + tap water Ga - Gd: Rotary instrumentation (Crown down), No 30. Ga: diode laser (635 nm/0.1 W) + TBO + RTF Gb: diode laser (635 nm/0.1 W) + RTF Gc: TBO + RTF Gd: RTF (positive control) 2 days N = 12 Ga = 3 Gb = 3 Gc = 3 Gd = 3 F. nucleatum (4*108 cells/ml) 0.5% chloramine in water at 4˚C. Ga - Gd: 2.5% NaOCl + 17% EDTA + tap water Ga - Gd: Rotary instrumentation (Crown down), No 30. Ga: diode laser (635 nm/0.1 W) + TBO + RTF Gb: diode laser (635 nm/0.1 W) + RTF Gc: TBO + RTF Gd: RTF (positive control) 2 days N = 12 Ga = 3 Gb = 3 Gc = 3 Gd = 3 Negative group (uninfected) 0.5% chloramine in water at 4˚C Ga - Gd: 2.5% NaOCl + 17% EDTA + tap water Ga - Gd: Rotary instrumentation (Crown down), No 30. No laser 2 days N = 2 Foschi F., et al. (2007) E. faecalis (5*108 cells) 0.5% NaOCl for 2 weeks. G1 - G4 6% NaOCl: 17% EDTA deactivated with 6% NaOCl for 3 min. G1 - G4 Rotary instrumentation (Crown down), MAF: 0.465 G1: no laser/no MB G2: MB G3: Diode laser (665 nm/1 W) G4: Diode laser (665 nm/1 W) and MB 6 days N = 64 G1 = 15 G2 = 15 G3 = 15 G4 = 15 Wang Q. et al. (2007) E. faecalis (>108 CFU/ml) physiological saline solution at 4˚C G1 - G4: 5.25% NaOCl+ physiological saline G5: 2.5% NaOCl + 5.25% NaHS + physiological saline G6: None G1 - G6: Hand instrumentation (step-back), No 50 G1: Er,Cr:YSGG (2780 nm/1 W) G2: Er,Cr:YSGG (2780 nm/1.5 W) G3: Nd:YAG (1064 nm/1 W) G4: Nd:YAG (1064 nm/1.5W) G5: no laser irradiation (positive control) G6: no laser irradiation (negative control) 1 day N = 60 G1 = 10 G2 = 10 G3 = 10 G 4 = 10 G5 = 10 G6 = 10  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 132 Bergmans L., et al. (2006) E. faecalis (4*10 8 CFU/ml) 0.5% chloramine in water at 4˚C G1, G2: 2.5% NaOCl+ 17% EDTA+ tap water + 0.9% sterile saline G3: 2.5% NaOCl + 17% EDTA+ tap water G1 - G3: Rotary instrumentation (Crown down), No 30 G1: Nd:YAG laser (1064 nm/1.5 W/15 Hz) + RTF G2: RTF, no laser irradiation, Infected (positive control) G3: RTF, no laser irradiation, (uninfected, negative control) 2 days N = 8 G1 = 3 G2 = 3 G3 = 2 Piccolomini R., et al. (2002) A. naeslundii (1.8* 108 CFU/ml) none SubA, SubB1, SubB2 Physiological saline + EDTA SubC: physiological saline + EDTA + 5.25% NaOCl All groups: Hand instrumentation (Crown-down). SubA: no laser irradiation (control) SubB1: Nd:YAG laser (1064 nm/5 Hz) Sub B2: Nd:YAG laser (1064 nm/10 Hz) SubC: no laser irradiation 1 day. N = 60 SubA = 5 SubB1 = 10 SubB2 = 10 SubC = 5 P. aeruginosa (1.8* 108 CFU/ml) none SubA, SubB1, SubB2 Physiological saline + EDTA SubC: physiological saline+ EDTA+ 5.25% NaOCl All groups: Hand instrumentation (Crown-down). SubA: no laser irradiation (control) SubB1:Nd:YAG laser (1064 nm/5 Hz) Sub B2: Nd:YAG laser (1064 nm/10 Hz) SubC: no laser irradiation 1 day. SubA = 5 SubB1 = 10 SubB2 = 10 SubC = 5 Folwaczny M., et al. (2002) Escherichia Coli (8.67*106 CFU/ml) Sterile saline solution G1a-G1d: saline solution G1d; saline solution+ 1% NaOCl G1a - G1d: Hand instrumentation, No 40. G1a:No laser irradiation (positive control) G1b: Nd:YAG laser (1064 nm/0.005 W) G1c: Nd:YAG laser (1064 nm/0.01 W) G1d: No laser irradiation 1 day N = 114 G1a = 13 G1b = 13 G1c = 13 G1d = 13 Folwaczny M., et al. (2002) S. aureus (1.44*106 CFU/ml) Sterile saline solution G2a - G2d: saline solution G2d; saline solution+ 1% NaOCl G2a-G2d: Hand instrumentation, No 40. G2a:No laser irradiation (positive control) G2b: Nd:YAG laser (1064 nm /0.005 W) G2c: Nd:YAG laser (1064 nm/ 0.01 W) G2d: No laser irradiation 1 day G2a = 13 G2b = 13 G2c = 13 G2d = 13 Negative control (uninfected) Sterile saline solution saline solution Hand instrumentation, No 40. G3: no laser irradiation (negative control) 1 day G3 = 10 Moritz A., et al. (1999) Escherichia coli and Enterococcus fae- calis ( 5*105 CFU/mL) Physiological saline solution All groups: EDTA+ physio- logical saline solution All groups: Rotary instrumentation, (Step-back) G1: Positive control (untreated) G2: Nd:YAG laser (0.8 W/1064 nm) G3: Nd:YAG laser (1.5 W/1064 nm) G4: Ho: YAG laser (0.8 W/2130 nm) G5: Ho:YAG laser, (1.5 W/2130 nm) G6: Er:YAG laser, (0.8 W/2940 nm) G7: Er:YAG laser, (1.5 W/2940 nm) 1 day N = 40 G1: 10 G2: 5 G3: 5 G4: 5 G5: 5 G6: 5 G7: 5 Mehl A., et al. (1999) Escherichia coli (1.1*106 CFU/ml) none Ge1-Ge4: sterile saline solution Ge4: 1.25% NaOCl Hand instrumentation, No 40. Gs1: no laser irradiation (positive control) Gs2: Er:YAG (2940 nm/0.003 W/15 sec/50 mJ) Gs3: Er:YAG (2940 nm/0.001 W/60 sec/50 mJ) Gs4: no laser irradiation (1.25% NaOCl ) 1 day N = 90 Ge1: 10 Ge2: 10 Ge3: 10 Ge4: 10 C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 Copyright © 2011 SciRes. 133 S. aureus (1.53*106 CFU/ml) none Gs1-Gs4: sterile saline solution Gs4: 1.25% NaOCl Hand instrumentation, No 40. Gs1:no laser irradiation (positive control) Gs2: Er:YAG (2940 nm/0.003 W/15 sec/50 mJ) Gs3:Er:YAG (2940 nm/0.001 W/60 sec/50 mJ) Gs4: no laser irradiation (1.25% NaOCl ) 1 day Gs1: 10 Gs2: 10 Gs3: 10 Gs4: 10 Negative control (uninfected) none None Hand instrumentation, No 40. Gn: negative (uninfected) 1 day Gn: 10 Table 15. Enterococcus faecalis survival. Table 18. Escheric h ia co l i survival. Table 19. Actinomyces naeslundii survival. Table 16. Streptococcus anginosus survival. Table 20. Staphylococcus aureus survival. Table 17. Fusobacterium nucleatum survival. Table 21. Pseudomonas aeruginosa survival. Piccolomini R. et al(2002) In the first place, they contaminated the root canals with E. faecalis. In the second place they performed chemo-mechanical preparation and/or laser irradiation with or without specific solutions. The logCFU/ml ran- ges from 1.636 to 8.818 (Table 15). The best treatment protocols for root canal disinfect- tion in descending order are as follows: Instrumentation of the root canal and diode laser/665 nanometer/1 Watt irradiation with the combined effect OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 134 Table 22. Polymicrobial survival. Table 23. Mean microbial survival. of Methylene Blue (MB) as photosensitizing agent (log- CFU/ml = 1.636) [33]. Instrumentation of the root canal and diode laser/665 nm/1 W irradiation (logCFU/ml = 2.061) [33]. Instrumentation of the root canal combined with MB as photosensitizing agent (logCFU/ml = 2.190) [33]. Instrumentation followed by irrigation with 2.5% NaOCl (sodium hypochloride) (logCFU/ml = 2.602) [31]. When instrumentation and diode laser/830 nm/3 W were used, no CFU/ml was found. However, as the au- thor explained, this result is due to the fact that the level of sensitivity of the methodology used, was insufficient for detecting viable cells in low concentrations (contact with the author via e-mail) [32]. Streptococc u s an gi nosus Streptococcus anginosus survival was examined 4 ti- mes by 1 group of researchers. In the first place they per- formed chemo-mechanical preparation and in the second place they contaminated the root canals with S. angino- sus. Consequently, they completed root canal preparation and either used or not laser irradiation with specific so- lutions. The logCFU/ml ranges from 5.000 to 6.204 (Ta- ble 16) [30]. These results demonstrate no satisfying reduction of S. anginosus after diode laser/635 nm/0.1 W irradiation combined with RTF (reduced transferred fluid) and/or TBO (toluidine blue), nor after instrumentation and irri- gation with RTF and/or TBO (logCFU/ml > 5.000). Fusobacterium nucleatum Fusobacterium nucleatum survival was examined 4 ti- mes by 1 group of researchers. In the first place they per- formed chemo-mechanical preparation and in the second place they contaminated the root canals with F. nuclea- tum. Consequently, they completed root canal prepara- tion and either used or not laser irradiation with specific solutions. The logCFU/ml ranges from 4.176 to 6.000 (Table 17) [30]. These results demonstrate no satisfying reduction of F.nucleatum after diode laser/635 nm/0.1 W irradiation combined with RTF (reduced transferred fluid) and/or TBO (toluidine blue), nor after instrumentation and ir- rigation with RTF and /or TBO (logCFU/ml > 4.176). Escherichia coli Escherichia coli survival was examined 8 times by 2 different groups of researchers. In the first place they performed chemo-mechanical preparation and in the sec- ond place they contaminated the root canals with E. coli. Consequently, they completed root canal preparation and either used or not laser irradiation with or without spe- cific solutions. The logCFU/ml ranges from 2.342 to 6.938 (Table 18). The best treatment protocols for root canal disinfect- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 1.25% NaOCl (logCFU/ml = 2.342) [28]. Instrumentation of the root canal and Er:YAG laser/ 2,940 nm/0.001 W irradiation (logCFU/ml = 2.572) [28]. Instrumentation of the root canal followed by irriga- tion with 1% NaOCl (logCFU/ml = 3.012) [25]. Instrumentation of the root canal and Er:YAG laser/2940 nm/0.003 W irradiation (logCFU/ml = 3.155) [28]. C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 135 Actinomyces naeslundii Actinomyces naeslundii survival was examined 4 ti- mes by 1 group of researchers. In the first place they per- formed chemo-mechanical preparation and in the second place they contaminated the root canals with A. naes- lundii. Consequently, they completed root canal prepara- tion and either used or not laser irradiation with or with- out specific solutions. The logCFU/ml ranges from 0 to 3.698 (Table 19) [29]. The best treatment protocols for root canal disinfec- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 5.25% NaOCl (logCFU/ml = 0). Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/10 Hz/15 sec irradiation (logCFU/ml = 3.053). Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/5 Hz/15 sec irradiation (logCFU/ml = 3.518). Instrumentation of the root canal solely (logCFU/ml = 3.698). Staphylococcus aureus Staphylococcus aureus survival was examined 8 times by 2 groups of researchers. In the first place they per- formed chemo-mechanical preparation and in the second place they contaminated the root canals with S.aureus. Consequently, they completed root canal preparation and either used or not laser irradiation with or without spe- cific solutions. The logCFU/ml ranges from 2.703 to 6.184 (Table 20). The best treatment protocols for root canal disinfec- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 1.25% NaOCl (logCFU/ml = 2.703) [28]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.001 W irradiation (logCFU/ml = 2.973) [28]. Instrumentation of the root canal followed by irriga- tion with 1% NaOCl (logCFU/ml = 3.246) [25]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.003 W irradiation (logCFU/ml = 3.348) [28]. Pseudomonas ae ruginosa Pseudomonas aeruginosa survival was examined 4 ti- mes by 1 group of researchers. In the first place they per- formed chemo-mechanical preparation and in the second place they contaminated the root canals with P. aerugi- nosa. Consequently, they completed root canal prepara- tion and either used or not laser irradiation with or with- out specific solutions. The logCFU/ml ranges from 0 to 4.544 (Table 21) [29]. The best treatment protocols for root canal disinfec- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 5.25% NaOCl (logCFU/ml = 0). Instrumentation of the root canal and Nd: YAG laser/ 1064 nm/10 Hz/15 sec irradiation (logCFU/ml = 3.695). B) Anal ysis of the Polymicrobial Studies Results Microbial survival after polymicrobial infection of root canals was examined 30 times by 6 groups of re- searchers. In the first place they performed chemo-me- chanical preparation and in the second place they conta- minated the root canals with some of the following mi- croorganisms; E. coli, S. aureus, A. naeslundii, P. aerugi- nosa, S. anginosus, E. fa ecalis, F. nucleatum, A. israelii, P. gingivalis and P. int ermed ia. Consequently, they com- pleted root canal preparation and either used or not laser irradiation with or without specific solutions. The log- CFU/ml ranges from 0 to 6.548 (Table 22). The best treatment protocols for root canal disinfec- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 5.25% NaOCl (logCFU/ml = 0) [29]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.8 W irradiation (logCFU/ml = 1.924) [27]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/1.5 W irradiation (logCFU/ml = 2.113) [27]. Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/1.5 W irradiation (logCFU/ml = 2.477) [27]. Instrumentation of the root canal and Ho:YAG laser/ 2130 nm/0.8 W irradiation (logCFU/ml = 2.491) [27]. Instrumentation of the root canal followed by irriga- tion with 1.25% NaOCl (logCFU/ml = 2.522) [28]. Instrumentation of the root canal and Ho:YAG laser/ 2130 nm/1.5 W irradiation (logCFU/ml = 2.531) [27]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.001 W irradiation (logCFU/ml = 2.772) [28]. Instrumentation of the root canal followed by irriga- tion with 1% NaOCl (logCFU/ml = 3.138) [25]. Instrumentation of the root canal and Nd: YAG laser/ 1064 nm/0.8 W irradiation (logCFU/ml = 3.204) [27]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.003 W irradiation (logCFU/ml = 3.251) [28]. Instrumentation of the root canal and diode laser/665 nm/1 W irradiation combined with MB as photosensiti- zing agent and PBS (Phosphate-Buffered Saline) broth (logCFU/ml = 3.341) [34]. Instrumentation of the root canal and diode laser/665 nm/1 W irradiation combined with MB as photosensiti- zing agent (logCFU/ml = 3.344) [34]. Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/10 Hz/15 sec irradiation(logCFU/ml = 3.374) C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 136 [29]. Instrumentation of the root canal combined with MB as photosensitizing agent (logCFU/ml = 3.814) [34]. Instrumentation of the root canal combined with MB as photosensitizing agent and PBS (Phosphate-Buffered Saline) broth (logCFU/ml = 3.906) [34]. Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/5 Hz/15 sec irradiation (logCFU/ml = 3.993) [29]. C) Analysis of the Mean Averages of Microbial Survival in General The survival of 10 microorganisms well connected with endodontic problems: E. faecalis, S. anginosus, F. nuclea- tum, A. israelii, P. gingivalis, P. intermedia, A. naes- lundii, P. aeruginosa, E. coli, and S. aureus was exam- ined by 10 groups of researchers regarding the bacteri- cidal effect of 30 different treatment protocols on them. In the first place the majority of them performed chemo- mechanical preparation and in the second place they contaminated the root canals with one or more microor- ganisms. Then, they completed the root canal prepara- tion and either used or not laser irradiation with or without specific solutions. The logCFU/ml ranges from 0 to 7.940 (Table 23). The best treatment protocols for root canal disinfec- tion in descending order are as follows: Instrumentation of the root canal followed by irriga- tion with 5.25% NaOCl (logCFU/ml = 0) [29]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.8 W irradiation (logCFU ml = 1.924) [27]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/1.5 W irradiation (logCFU/ml = 2.133) [27]. Instrumentation of the root canal and diode laser/665 nm/1 W irradiation with the combined effect of Me- thylene Blue (MB) as photosensitizing agent (logCFU/ ml = 2.490) [33,34]. Instrumentation of the root canal and Ho:YAG laser/ 2130 nm/0.8 W irradiation (logCFU/ml = 2.491) [27]. Instrumentation of the root canal followed by irriga- tion with 1.25% NaOCl (logCFU/ml = 2.522) [28]. Instrumentation of the root canal and Ho: YAG laser/ 2130 nm/1.5 W irradiation (logCFU/ml = 2.531) [27]. Instrumentation followed by irrigation with 2.5% NaOCl (logCFU/ml = 2.602) [31]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.001 W irradiation (logCFU/ml = 2.772) [28]. Instrumentation of the root canal with the combined effect of Methylene Blue (MB) as photosensitizing agent (logCFU/ml = 3.002) [33,34]. Instrumentation of the root canal and diode laser/665 nm/1 W irradiation (logCFU/ml = 3.033) [33,34]. Instrumentation followed by irrigation with 1% NaOCl (logCFU/ml = 3.138) [25]. Instrumentation of the root canal and Nd:YAG laser/ 1064 nm 0. W irradiation (logCFU/ml = 3.204) [27]. Instrumentation of the root canal and Er:YAG laser/ 2940 nm/0.03 W irradiation (logCFU/ml = 3.251) [28]. Instrumentation of the root canal and Nd:YAG laser/ 1064 nm/10 Hz/15 sec irradiation (logCFU/ml = 3.374) [29]. Instrumentation of the root canal and Nd:YAG laser / 1064 nm/5 Hz/15 sec irradiation (logCFU/ml = 3.993) [29]. 4. DISCUSSION Generally, it is well known that certain microorganisms are related to specific pathological situations in endodon- tics. Being aware of them is necessary so as to be able to consider the importance of the disinfection capacity of each treatment plan. Consequently, it has been proved that: 1) With symptomatic endodontic disease and apical bone resorption T. denticola is associated [1]. 2) In endodontically infected teeth without a sinus tr- act E. faecalis and Strept. anginosus were mostly found [2]. 3) In teeth with necrotic pulp P. gingivalis, P. endo- dontalis, P. intermedia, and P. nigrescens were identified more frequently [4]. 4) With root canal treatment failures E. faecalis is as- sociated [1,3]. 5) In endodontically infected teeth with sinus tracts P. gingivalis and F. nucleatum were mostly identified [2]. This systematic review identified 10 in vitro studies that examined the effectiveness of treatment protocols, among classical methods and/or various laser applica- tions in root canal disinfection. The results of these 10 studies indicated that the treatments which provide the best bactericidal ability regarding each bacterial solely and all of them were, in descending order of efficacy: 4.1. Enterococcus faecalis Instrumentation of the root canal and diode laser/665 nm/ 1 W irradiation with the combined effect of Methylene Blue (MB) as photosensitizing agent seemed to be better in root canal disinfection than instrumentation of the root C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 137 canal and diode laser/665 nm/1 W irradiation. In addi- tion, the last method was slightly better than instrument- tation of the root canal combined with MB as photosen- sitizing agent and significantly better than instrumenta- tion followed by irrigation with 2.5% NaOCl (sodium hypochloride). Regarding the instrumentation and diode laser/830 nm/ 3 W irradiation where no CFU/ml was found, this is due to the lack of sensitivity of the methodology used to de- tect low concentrations of viable cells (contact with au- thor). 4.2. Streptococcus anginosus Instrumentation of the root canal and diode laser/635 nm/0.1 W irradiation combined with RTF (reduced tr- ansferred fluid) and/or TBO (toluidine blue), as well as instrumentation with RTF and/or TBO demonstrated neither accepted nor satisfying reduction of S. anginosus (logCFU/ml = 5.000, logCFU/ml = 6.079, logCFU/ml = 6.113 and logCFU/ml = 6.204, respectively). 4.3. Fusobacterium nucleatum Instrumentation of the root canal and diode laser/635 nm/0.1 W irradiation combined with RTF (reduced trans- ferred fluid) and/or TBO (toluidine blue), as well as in- strumentation with RTF and/or TBO showed insufficient disinfection of F. nucleatum. (logCFU/ml = 4.176, log- CFU/ml = 6, logCFU/ml = 5.939 and logCFU/ml = 5.986, respectively). 4.4. Escherichia coli Instrumentation of the root canal followed by irrigation with 1.25% NaOCl seemed to be better in root canal disinfection than instrumentation of the root canal and Er:YAG laser/2940 nm/0.001 W irradiation. Furthermore, instrumentation of the root canal followed by irrigation with 1% NaOCl was less effective than the previous treat- ments, but slightly better than instrumentation of the root canal and Er:YAG laser/2940 nm/0.003 W irradiation. 4.5. Actinomyces naeslundii Instrumentation of the root canal followed by irrigation with 5.25% NaOCl seems to be the best in root canal di- sinfection as with this concentration no viable cells of Actinomyces naeslundii are detected. This method is used and mentioned only by one group of researchers [29].Concerning the other treatments, they are signifi- cantly worse and in fact instrumentation of the root canal and Nd:YAG laser/1064 nm/10 Hz/15 sec irradiation is slightly better than the same method but with 5 Hz irra- diation or instrumentation solely. 4.6. Staphylococcus aureus Instrumentation of the root canal followed by irrigation with 1.25% NaOCl was better enough than instrumenta- tion and Er:YAG laser/2940 nm/0.001 W irradiation and seemed to be better in root canal disinfection than in- strumentation of the root canal followed by irrigation with 1%NaOCl and instrumentation with Er:YAG laser/ 2940 nm/0.003 W irradiation. 4.7. Pseudomonas aeruginosa Instrumentation of the root canal followed by irrigation with 5.25% NaOCl was the best in root canal disinfec- tion, as no CFU/ml of Pseudomonas aeruginosa was found. This method was conducted by one group of re- searchers [29]. Regarding instrumentation and Nd:YAG laser/1064 nm/10 Hz/15 sec irradiation, it was moder- ately worse than the previous one. 4.8. Crobial Studies Results Instrumentation of the root canal followed by irrigation with 5.25% NaOCl seemed to be the best in root canal disinfection as no viable cells were detected. However, this concentration of NaOCl is not used in vivo because it actively attacks living tissue without contributing sig- nificantly to treatment [35]. Furthermore, instrumenta- tion and Er:YAG laser/2940 nm/0.8 W irradiation was well successful and slightly better than the same treat- ment but with 1.5 Watt irradiation, which was also better than instrumentation of the root canal and Nd:YAG la- ser/1064 nm/1.5 W irradiation. Also, instrumentation of the root canal and Ho: YAG laser/2130 nm/0.8 W irra- diation was effective enough, as well as instrumentation of the root canal followed by irrigation with 1.25% NaOCl and instrumentation followed with Ho:YAG la- ser/2130 nm/1.5 W irradiation. Finally, instrumentation of the root canal and Er:YAG laser/2940 nm/0.001 W irradiation was less effective than the previous methods. All the other methods seemed to be worse in root canal disinfection. 4.9. Microbial Survival in General Instrumentation of the root canal followed by irrigation with 5.25% NaOCl seemed to be the best in root canal disinfection as no viable cells were detected. Instrumen- tation and Er:YAG laser/2940 nm/0.8 W irradiation was well successful and slightly better than the same treat- ment but with 1.5 Watt irradiation. Instrumentation fol- lowed with diode laser/665 nm/1 W irradiation with the combined effect of Methylene Blue (MB) as photosensi- tizing agent showed the same results when compared to instrumentation of the root canal and Ho:YAG laser/ 2130 nm/0.8 W irradiation. Also good results show in- strumentation of the root canal followed by irrigation with 1.25% NaOCl and Ho:YAG laser/2130 nm/1.5 W irradiation which both are slightly better than instru- C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 138 mentation followed by irrigation with 2.5% NaOCl and Er:YAG laser/2940 nm/0.001 W irradiation. All the other methods seemed to be worse in root canal disinfection. 5. CONCLUSIONS There are treatment protocols with the assistance or not of laser irradiation that can eliminate E. faecalis, E. coli and S. aureus inside the root canal. However, there is a serious number of S. anginosus, F. nucleatum, A. naes- lundii and P. aeruginosa that remain inside the root canal even after laser irradiation. In vitro, NaOCl 5% seems to be the strongest solution in root canal disinfection. Con- cluding, it seems that new research is needed in order to set a treatment protocol effective in the root canal disin- fection from all bacteria mentioned above that are re- lated to endodontic origin pathology. REFERENCES [1] Foschi, F., Cavrini, F., Montebugnoli, L., Stashenko, P., Sambri, V. and Prati, C. (2005) Detection of bacteria in endodontic samples by polymerase chain reaction assays and association with defined clinical signs in Italian pa- tients. Oral Microbiology and Immunology, 20, 289-295. doi:10.1111/j.1399-302X.2005.00227.x [2] Sassone, L., Fidel, R., Faveri, M., Fidel, S., Fiqueiredo, L. and Feres, M. (2008) Microbiological evaluation of pri- mary endodontic infections in teeth with and without si- nus tract. Intern ational Endodontic Journal, 41, 508-515. doi:10.1111/j.1365-2591.2008.01397.x [3] Gomes, B., Pinheiro, E., Gadê-Neto, C., Sousa, E., Fer- raz, C., Zaia, A., et al. (2004) Microbiological examina- tion of infected dental root canals. Oral Microbiology and Immunology, 19, 71-76. doi:10.1046/j.0902-0055.2003.00116.x [4] Gomes, B., Jacinto, R., Pinheiro, E., Sousa, E., Zaia, A., Ferraz, C., et al. (2005) Porphyromonas gingivalis, Por- phyromonas endodontalis, Prevotella intermedia and Pre- votella nigrescens in endodontic lesions detected by cul- ture and by PCR. Oral Microbiology and Immunology, 20, 211-215. doi:10.1111/j.1399-302X.2005.00214.x [5] Sousa-Neto, M., Marchesan, M., Pécora, J., Junior, A., Silva-Sousa, Y. and Saquy, P. (2002) Effect of Er:YAG laser on adhesion of root canal sealers. Journal of Endo- dontics, 28, 185-187. doi:10.1097/00004770-200203000-00010 [6] Schoop, U., Kluger, W., Dervisbegovic, S., Goharkhay, K., Wernisch, J., Georgopoulos, A., et al. (2006) Innova- tive wavelengths in endodontic treatment. Lasers in Sur- gery and Medicine, 38, 624-630. doi:10.1002/lsm.20331 [7] Jha, D., Guerrero, A., Ngo, T., Helfer, A. and Hasselgren, G. (2006) Inability of laser and rotary instrumentation to eliminate root canal infection. Journal of the American Dental Association, 137, 67-70. [8] Garcez, A., Ribeiro, M., Tegos, G., Núñez, S., Jorge, A. and Hamblin, M. (2007) Antimicrobial photodynamic therapy combined with conventional endodontic treat- ment to eliminate root canal biofilm infection. Lasers in Surgery and Medicine, 39, 59-66. doi:10.1002/lsm.20415 [9] Blum, J., Michailesco, P. and Abadie, M. (1997) An evaluation of the bactericidal effect of the Nd:YAP laser. Journal of Endodontics, 23, 583-585. doi:10.1016/S0099-2399(06)81127-1 [10] Dostálová, T., Jelínková, H., Housová, D., Sulc, J., Ne- meć, M., Dusková, J., et al. (2002) Endodontic treatment with application of Er:YAG laser waveguide radiation disinfection. Journal of Clinical Laser Medicine and Surgery, 20, 135-139. doi:10.1089/104454702760090218 [11] Fegan, S. and Steiman, H. (1995) Comparative evalua- tion of the antibacterial effects of intracanal Nd:YAG la- ser irradiation: An in vitro study. Journal of Endodontics, 21, 415-417. doi:10.1016/S0099-2399(06)80827-7 [12] Fonseca, M., Júnior, P., Pallota, R., Filho, H., Denardin, O., Rapoport, A., et al. (2008) Photodynamic therapy for root canals infected with Enterococcus faecalis. Pho- tomedicine and Laser Surgery, 26, 209-213. doi:10.1089/pho.2007.2124 [13] Gordon, W., Atabakhsh, V., Meza, F., Doms, A., Nissan, R., Rizoiu, I., et al. (2007) The antimicrobial efficacy of the erbium, chromium:yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal dentin walls infected with Enterococcus faecalis. Journal of the Ameri- can Dental Association, 138, 992-1002. [14] Gutknecht, N., Nuebler-Moritz, M., Burghardt, S. and Lampert, F. (1997) The efficiency of root canal disinfec- tion using a holmium:yttrium-aluminum-garnet laser in vitro. Journal of Clinical Laser Medicine and Surgery, 15, 75-78. [15] Hardee, M., Miserendino, L., Kos, W. and Walia, H. (1994) Evaluation of the antibacterial effects of intraca- nal Nd:YAG laser irradiation. Journal of Endodontics, 20, 377-380. doi:10.1016/S0099-2399(06)80294-3 [16] Kreisler, M., Kohnen, W., Beck, M., Al-Haj, H., Christof- fers, A., Götz, H., et al. (2003) Efficacy of NaOCl/H2O2 irrigation and GaAlAs laser in decontamination of root canals in vitro. Lasers in Surgery and Medicine, 32, 189-196. doi:10.1002/lsm.10148 [17] Moritz, A., Gutknecht, N., Goharkhay, K., Schoop, U., Wernisch, J. and Sperr, W. (1997) In vitro irradiation of infected root canals with a diode laser: Results of micro- biologic, infrared spectrometric, and stain penetration examinations. Quintessence International, 28, 205-209. [18] Moshonov, J., Orstavik, D., Yamauchi, S., Pettiette, M. and Trope, M. (1995) Nd:YAG laser irradiation in root canal disinfection. Endodontics & dental traumatology, 11, 220-224. doi:10.1111/j.1600-9657.1995.tb00492.x [19] Perin, F., França, S., Silva-Sousa, Y., Alfredo, E., Saquy, P., Estrela, C., et al. (2004) Evaluation of the antimicro- bial effect of Er:YAG laser irradiation versus 1% sodium hypochlorite irrigation for root canal disinfection. Aus- tralian Endodontic Journal, 30, 20-22. doi:10.1111/j.1747-4477.2004.tb00162.x [20] Ramsköld, L., Fong, C. and Strömberg, T. (1997) Ther- mal effects and antibacterial properties of energy levels required to sterilize stained root canals with an Nd:YAG laser. Journal of Endodontics, 23, 96-100. doi:10.1016/S0099-2399(97)80253-1 [21] Schoop, U., Goharkhay, K., Klimscha, J., Zagler, M., C opyright © 2011 SciRes. OJST  J. Theodosopoulou et al. / Open Journal of Stomatology 1 (2011) 126-139 Copyright © 2011 SciRes. 139 OJST Wernisch, J., Georgopoulos, A., et al. (2007) The use of the erbium, chromium:yttrium-scandium-gallium-garnet laser in endodontic treatment: the results of an in vitro study. Journal of the American Dental Association, 138, 949-955. [22] Schoop, U., Moritz, A., Kluger, W., Patruta, S., Gohark- hay, K., Sperr, W., et al. (2002) The Er:YAG laser in en- dodontics: results of an in vitro study. Lasers in Surgery and Medicine, 30, 360-364. doi:10.1002/lsm.10054 [23] Soukos, N., Chen, P., Morris, J., Ruggiero, K., Abernethy, A., Som, S., et al. (2006) Photodynamic therapy for en- dodontic disinfection. Journal of Endodontics, 32, 979- 984. doi:10.1016/j.joen.2006.04.007 [24] Vezzani, M., Pietro, R., Silva-Sousa, Y., Brugnera-Junior, A. and Sousa-Neto, M. (2006) Disinfection of root canals using Er:YAG laser at different frequencies. Photomedi- cine and Laser Surgery, 24, 499-502. doi:10.1089/pho.2006.24.499 [25] Folwaczny, M., Mehl, A., Jordan, C. and Hickel, R. (2002) Antibacterial effects of pulsed Nd:YAG laser ra- diation at different energy settings in root canals. Journal of Endodontics, 28, 24-29. doi:10.1097/00004770-200201000-00006 [26] Bergmans, L., Moisiadis, P., Teughels, W., Van Meerbeek, B., Quirynen, M. and Lambrechts, P. (2006) Bactericidal effect of Nd:YAG laser irradiation on some endodontic pathogens ex vivo. International Endodontic Journal, 39, 547-557. doi:10.1111/j.1365-2591.2006.01115.x [27] Moritz, A., Schoop, U., Goharkhay, K., Jakolitsch, S., Kluger, W., Wernisch, J., et al. (1999) The bactericidal effect of Nd:YAG, Ho:YAG, and Er:YAG laser irradiation in the root canal: An in vitro comparison. Journal of Clinical Laser Medicine and Surgery, 17, 161-164. [28] Mehl, A., Folwaczny, M., Haffner, C. and Hickel, R. (1999) Bactericidal effects of 2.94 μm Er:YAG-laser ra- diation in dental root canals. Journal of Endodontics, 25, 490-493. doi:10.1016/S0099-2399(99)80288-X [29] Piccolomini, R., D’Arcangelo, C., D’Ercole, S., Catamo, G., Schiaffino, G. and De Fazio, P. (2002) Bacterologic evaluation of the effect of Nd:YAG laser irradiation in experimental infected root canals. Journal of Endodon- tics, 28, 276-278. doi:10.1097/00004770-200204000-00004 [30] Bergmans, L., Moisiadis, P., Huybrechts, B., Van Meer- beek, B. and Quirynen, M.P.L. (2008) Effect of photo-acti- vated disinfection on endodontic pathogens ex vivo. In- ternational Endodontic Journal, 41, 227-239. doi:10.1111/j.1365-2591.2007.01344.x [31] Wang, Q., Zhang, C. and Yin, X. (2007) Evaluation of the bactericidal effect of Er,Cr:YSGG, and Nd:YAG la- sers in experimentally infected root canals. Journal of Endodontics, 33, 830-832. doi:10.1016/j.joen.2007.03.017 [32] De Souza, E., Cai, S., Simionato, M. and Lage-Marques, J. (2008) High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surgery, Oral Medi- cine, Oral Pathology, Oral Radiology & Endodontics, 106, 68-72. doi:10.1016/j.tripleo.2008.02.032 [33] Foschi, F., Fontana, C., Ruggiero, K., Riahi, R., Vera, A., Doukas, A., et al. (2007) Photodynamic inactivation of enterococcus faecalis in dental root canals in vitro. La- sers in Surgery and Medicine, 39, 782-787. doi:10.1002/lsm.20579 [34] Fimple, J., Fontana, C., Foschi, F., Ruggiero, K., Song, X., Pagonis, T., et al. (2008) Photodynamic treatment of endodontic polymicrobial infection in vitro. Journal of Endodontics, 34, 728-734. doi:10.1016/j.joen.2008.03.011
|