Journal of Biomedical Science and Engineering
Vol.5 No.4(2012), Article ID:18516,9 pages DOI:10.4236/jbise.2012.54028
Biodegradable stent
![]()
1Department of Molecular Science and Technology, Ajou University, Suwon, Korea (South)
2Department of Polymer Engineering, Pukyong National University, Busan, Korea (South)
3Office of R&D Program Management, Korea Evaluation Institute of Industrial Technology, Seoul, Korea (South)
Email: #moonskim@ajou.ac.kr
Received 11 January 2012; revised 31 January 2012; accepted 23 February 2012
Keywords: stent; biomaterials; biodegradable; bioabsorbable
ABSTRACT
The bare metal stent (BMS) used in the blood vessel caused the restenosis after the operation due to formation and proliferation of neointimal. Recently, as a method to overcome the problems of BMS, drug eluting stent (DES) is developed and being applied to human body which has drug reducing restenosis applied on the metal surface. DES has the advantage of greatly reducing the restenosis after the operation; however, metal stent remains in the body after the drug is released causing issues such as late thrombosis and restenosis so that currently the attention is increasing for biodegradable materials that reduce restenosis and thrombosis by degrading as a certain amount of time passes after the drug is released by the stent material. In this review, the study trend of biodegradable stent will be explained.
1. INTRODUCTION
In 1977, Gruentzig of Switzerland successfully executed percutaneous transluminal coronary balloon angioplasty in stricture lesion to open up a new era of coronary artery [1]. This method dilates the balloon to widen the stricture lesion, which brings about the improvement of blood flow with the dilatational balloon to expand the narrowed coronary artery. Acute obliteration during or right after the operation, however, may cause death or acute myocardial infarction, because 30% - 40% of restenosis within 6 months occur from constriction and neointimal hyperplasia of coronary artery after the balloon dilatation [2,3].
In the early 1990s, bare metal stent (BMS) was operated on the stricture lesion of coronary artery with the stent on the balloon and wire mesh on the inner wall of coronary artery to expand the coronary artery [4]. The insertion of BMS had lower restenosis occurrence rate than balloon expansion, and the stent played the role of supporting the blood vessel inner wall to prevent the contraction of the coronary artery reducing the restenosis rate to 20% - 30%. Stent insertion reduced the critical shortfall of balloon expansion which was the stricture of coronary artery with acute recoil, but mechanical blood vessel damage was caused in the expansion process and neointimal was formed to cause in stent restenosis [5,6]. In order to prevent in stent restenosis, many researches were conducted, and sirolimus originally developed as immunosuppressant and paclitaxel used as anticancer drug were reported to effectively suppress formation of neointimal [7,8]. The problem was, however, systemic injection of such drugs interfere with maintaining the local drug concentration in the stent, and is prone to serious side effects. In order to overcome such problems, the research to insert the drug on the stent surface and locally release the drug was conducted. As the result, drug-eluting stent (DES) was born, but this also has the issue of drug release control and being unable to maintain appropriate drug concentration.
In order to overcome such limitations of the existing stents, the development of biodegradable DES was suggested, which is expected to bring a dramatic change in treatment of coronary arterial diseases. This review intends to discuss the design of the stent using biodegradable biomaterial and the technological development trends.
2. BIODEGRADABLE STENT MATERIALS
For stent materials, whether to expand with a balloon or if the self-expansion using shaped memory characteristics shall be employed should be considered. Balloon expandable stents must be able to maintain the form once expanded, and the stent using self-expansion must have sufficient elasticity to expand. The ideal characteristics of a stent material are shown in Table 1. In this section, the research trend of biodegradable stent production using polymer, metal, and ceramic is introduced.
2.1. Polymer
The synthetic polymer of poly-L-lactic acid (PLLA) is biodegradable biomaterial with its repetition unit hydrolyzed into lactic acid, and eventually decomposed to water and carbon dioxide and absorbed to metabolism. In Igaki and Tamai groups, the PLLA of molecular weight 183 kDa was used to develop a stent of thickness 170 μm in the zig-zag helical coil form strut. The stent of Igaki and Tamai group is self-expansion by heat and when applied to clinical experiments, the operation on coronary artery lesion reported restenosis rate of 10.5% after 6 months [9]. After such successful cases, the researches on stents using biodegradable polymers were actively conducted.
Abbott vascular group also used PLLA to produce a BVS (bioresorbable vascular scaffold) stent with interconnected linear bridges with the thickness of 150 μm strut in the form of zig-zag hoops, which expands using a balloon. The stent surface is coated with poly-D,L-lactic acid (PDLLA) and everolimus (a rapamycin derivative) to develop DES [10]. BVS stent used everolimus to effectively reduce inflammatory reaction.
Blindt group developed a double-helical type stent with paclitaxel using PDLLA of molecular weight of 240 - 250 kDa [11]. The stent was produced using controlled expansion of saturated polymers so that it has excellent mechanical stability, and as the result of 3 months of experiments in vivo, the comparison of the stent with (49% ± 4%) and without (71% ± 4%) paclitaxel reported that coronary stenosis was significantly reduced.
The tyrosine-derived polycarbonate was used to develop a stent with the slide and lock design and strut thickness of 150 μm [12]. The slide and lock design stent has excellent radial strength with a locking structure when expanded. Also, due to its radiopacity, the materialization with X-ray and fluoroscopy is reported to be possible.
Niels Grabow group blended PLLA and poly(4-hydroxybutyrate) in the mass ratio of 78/22% to produce a stent in the slotted tube form [13]. This stent has elastic modulus and tensile strength reduced by 52% and 20% respectively compared to PLLA alone, but the elongation
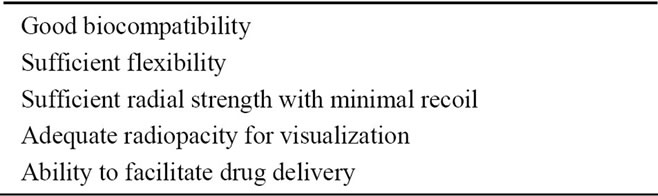
Table 1. Ideal properties of biodegradable stent materials.
to break increased by 16 times. Also, stent was prepared in the water of 37˚C for 5 minutes, and then was quickly expanded within a minute using the balloon under the pressure of 8 bar. And in vitro biodegradation, the molecular weight after 48 weeks was reported to have dropped to 82%.
In Liu group, poly(ε-caprolactone) (PCL) of molecular weight of 80 kDa was used to produce a stent that expands by balloon; the components of this stent were geometrically designed and assembled as self-lock so that it is reported to have excellent resistibility against the pressure of the external blood vessels when expanding. The surface of the stent was applied with poly (D,L-lactic acid-co-glycolic acid) (PLGA) and paclitaxel at room temperature using the spray coating method. The release of paclitaxel was confirmed for over 60 days in vitro [14]. Also, PLLA and PLGA were used in the bi-layer format of elastic memory to develop a self-expanding stent at 37˚C [15].
And Lauto group developed a self-expanding stent using chitosan, a natural polymer, in an experimental stage. The chitosan film was used to produce in the helical coil form, which was reported to completely expand within 3 minutes [16].
2.2. Metal
BMS is a permanent implant which causes not only stent thrombosis and chronic injury but also the risk of increasing the formation of neointimal. Thus, researches on development of biodegradable metal stent material were actively conducted, and recently pure iron and magnesium alloy are receiving much attention as the biodegradable stent material [17].
Pure iron (over 99.5%) has high elasticity modulus and excellent radial strength to make a stent with thin strut [18]. And iron is theoretically expected to easily break during or after the expansion due to its yield strength and tensile strength being similar, but as the result of in vivo experiment, the strut with the thickness of 100 - 120 μm was expanded with the pressure of 3 - 10 atm and no destruction of stent was observed at all [19]. Iron is oxidized to ferrous iron and ferric iron by the phagocytosis of nearby tissues. Ferrous iron reduces the proliferation of smooth muscle cells in vitro tissue culture so as to suppress the formation of neointimal and it was reported that local toxicity does not occur [20].
Another biodegradable metal of magnesium alloy was first used as the orthopedic implant, but recently it is receiving much attention as the cardiovascular implant [21]. AE21 and WE43 among magnesium alloys are emerging as the cardiovascular stent material [22,23]. AE21 is composed of 97% magnesium, 2% aluminum, and 1% of rare earth metals (Ce, Pr, Nd), and WE43 is composed of >85% magnesium, <5% yttrium, <5% of zirconium, and <5% of rare earth metals. The biodegradation of magnesium involves hydrolysis of magnesium chloride in physiological environment, decomposing to the hydrogen gas and magnesium hydroxide, and the biodegradation period is about 60 - 90 days. Magnesium has generally low elasticity modulus and low radial strength so that it is difficult to support blood vessel walls. Therefore, the thickness of the strut increases. Also, it has low ductility and therefore is easy to break, and its radiolucent quality makes it impossible to materialized with X-ray and fluoroscopy; however, recently the stent that can be materialized using intravascular ultrasound and MRI was reported [24]. Biotronik produced a stent with magnesium alloy of WE43 and clinical experiment was conducted, but the results were disappointing [25]. Currently, such biodegradable metal material requires further research on the development of processing technology and the interaction of biology and material.
2.3. Ceramic
Besides polymer and metal, ceramic also has the potential possibility as the stent material. Iridium oxide is a ceramic material with excellent biocompatibility, and supplies hydrogen peroxide when coated on the stent surface [26]. It was reported that the iridium oxide sprayed on the metal stent releases hydrogen peroxide which converts to water and oxygen to reduce inflammatory reaction and catalyzes the proliferation of endothelial cells [27]. The nonconducting and amorphous hydrogenated silicon-carbide (SiC) is well known for its antithrombogenic characteristics [28]. The stent sprayed with SiC is receiving much attention as the material to reduce restenosis by reducing the accumulation of platelets, leukocytes, and monocytes [29]. Currently ceramic materials are mostly used as the stent coating materials rather than the material of the stent struts [30].
3. MECHANICAL PROPERTY FOR BIODEGRADABLE STENTS
The biodegradable stent materials mentioned in the above section are under research to add the unique characteristics of each material and elastic/biodegradable characteristics. There are many biodegradable stent materials reported. In this section, the elastic/biodegradable characteristics of polyester-based material among the biodegradable stent materials shall be explained.
3.1. Polymer Mechanical Property
The polymer receiving much attention as the biodegradable stent material is mainly polyester. PLLA composed on L isomer forms the secondary combination between polymer chains so that the crystallinity is greater than PDLLA composed of D and L isomers [31]. High-crystallinity polymer generally has strong union between molecules so that it can easily break at room temperature, but has the advantages of high tensile strength and diverse processing methods compared to low-crystallinity polymer. Also, polymer chain has excellent mobility so that under certain force, the chains optimize themselves and arrange. If the viscoelastic polymers are quickly increased, the rearranging time of the chains are shorter so that they become brittle; on the other hand, if they are slowly increased, the chains are rearranged so that they can be easily deformed. The physiochemical characteristics of the commercial biodegradable polymers are shown in Table 2 as follows [32-46]. Also, the glass transition temperature (Tg), an important physical characteristics in the polymers, was summarized. Tg is a special characteristic for amorphous material such as polymer, and refers to the characteristic of phase transition from glass phase to rubber phase according to the temperature. As the temperature rises, the molecules divide the kinetic energy due to the heat so that the union between the molecules is destroyed and the mobility of the chain increases. Especially Tg is related to the strength of polymer at the room temperature or the body temperature. PLLA with Tg of 60˚C - 65˚C exists as glass phase near the body temperature. Thus, PLLA is thought of as a brittle polymer. PLLA, which has the highest Tg among the general biodegradable polymers, has high tensile strength and would be an appropriate choice for the researchers who are looking for a brittle material.
3.2. Polymer Degradation
The molecular chains of polyester stent materials should contain easily bond-cleavage segments sensitive to water or enzyme, that is to say, hydrolytically or enzymatically degradable stent can be designed. Natural polyesters are enzymatically degradable, while synthetic polyesters tend to hydrolytically degrade [47]. Meanwhile, the dominantly utilized materials in the most manufactured stents are synthetic polyesters. Hydrolytic degradation of synthetic polyesters occurs in the middle of the chain or at the end of the chain. The random scission where the decomposition occurs in the middle of the chain is dominantly observed in the early stage of polymer decomposition, and as the number of polymer chains increase as the polymer decomposes, the chain-end scission is more dominant where the hydrolysis occurs at the end of the chain. The products of the decomposition depend on the molecular structure of the polymer, and decomposition products with excellent biocompatibility are promoted.
PLLA is decomposed to lactic acid, poly (glycolic acid) (PGA) to glycine, and PCL to organic acid such as
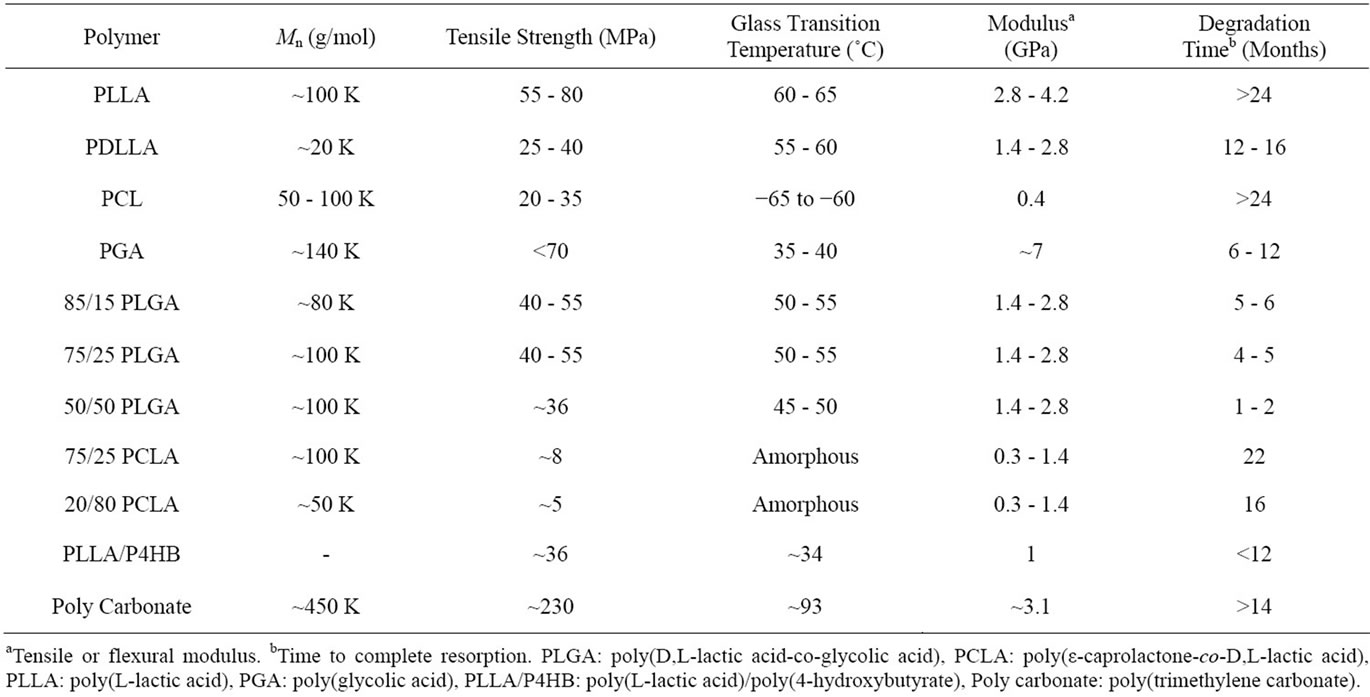
Table 2. Properties of biodegradable polymers [32-46].
6-hydroxyhexanoic acid; most decomposition products are converted to carbon dioxide and water through metabolism reactions. Factors contributing to the biodegradation of polymers include molecular weight, molecular structure, hydrophobicity, pH, crystallinity, and melting point; generally the contact with water or water infiltration accelerates the biodegradation. The polymer of crystalline structure with well-organized molecules delays the internal ester unit and water reaction, and thus the biodegradation speed will be slowed down. For example, PCL is a hydrophobic polymer with high crystallinity with biodegradation period of 1 - 2 years. And polymers such as PLGA are amorphous and less hydrophobic than PCL so that the biodegradation period is a few months.
4. DRUG FOR BIODEGRADABLE STENTS
Methods of using drugs were suggested to suppress the inflammatory reaction caused by stent and the formation and proliferation of the neointimal occurred by proliferation of smooth muscle cells. When applying biodegradable stents, various drugs are included to locally release them over a long term to reduce the restenosis (Table 3). Depending on the composition of drugs and polymer stents, the release of the drugs and timing can be controlled. Drugs that suppress the excessive proliferation of smooth muscle cells to reduce the restenosis include heparin, paclitaxel, and sirolimus, and there are many other drugs that are expected to be used for reduction of restenosis [48]. Heparin effectively reduces thrombosis and formation of neointimal, and paclitaxel and sirolimus mainly interrupts the formation of neointimal with the effects of antiproliferative. In this section, the characteristics of representative drugs applicable to biodegradable stents shall be introduced.
4.1. Heparin
Heparin is a type of acidic polysaccharide with sulfate, mainly used as a material reforming the blood vessel surface as an anticoagulant. Heparin combines with antithrombin III in the blood to suppress the activation of thrombin and other blood coagulation factors (X, XII, XI, IX), exhibiting anti-thrombosis reactions and anti-proliferation reactions to directly suppress platelets and migration of smooth muscle cells [49]. Such characteristics are believed to reduce the occurrence of restenosis, and in reality, many researches using heparin-coated and containing stents are being conducted. The method of introducing heparin into the stent includes physical adhesion, ionic bond, and copolymerization, but the physical adhesion and ionic bond have lower stability than copolymerization so that they are easily removed from the surface. Thus, it is used by copolymerization with polymers such as poly(vinyl alcohol) and poly(methyl methacrylate) [50]. Beside such, there is a method of using biodegradable PLGA microsphere to release heparin [51] and poly(ethylene glycol) is added in the production of polymer-heparin film as the plasticizer to control the release of heparin from biodegradable PLGA [52]. The heparin-containing stent was reported to effectively reduce the formation of neointimaland thrombosis through
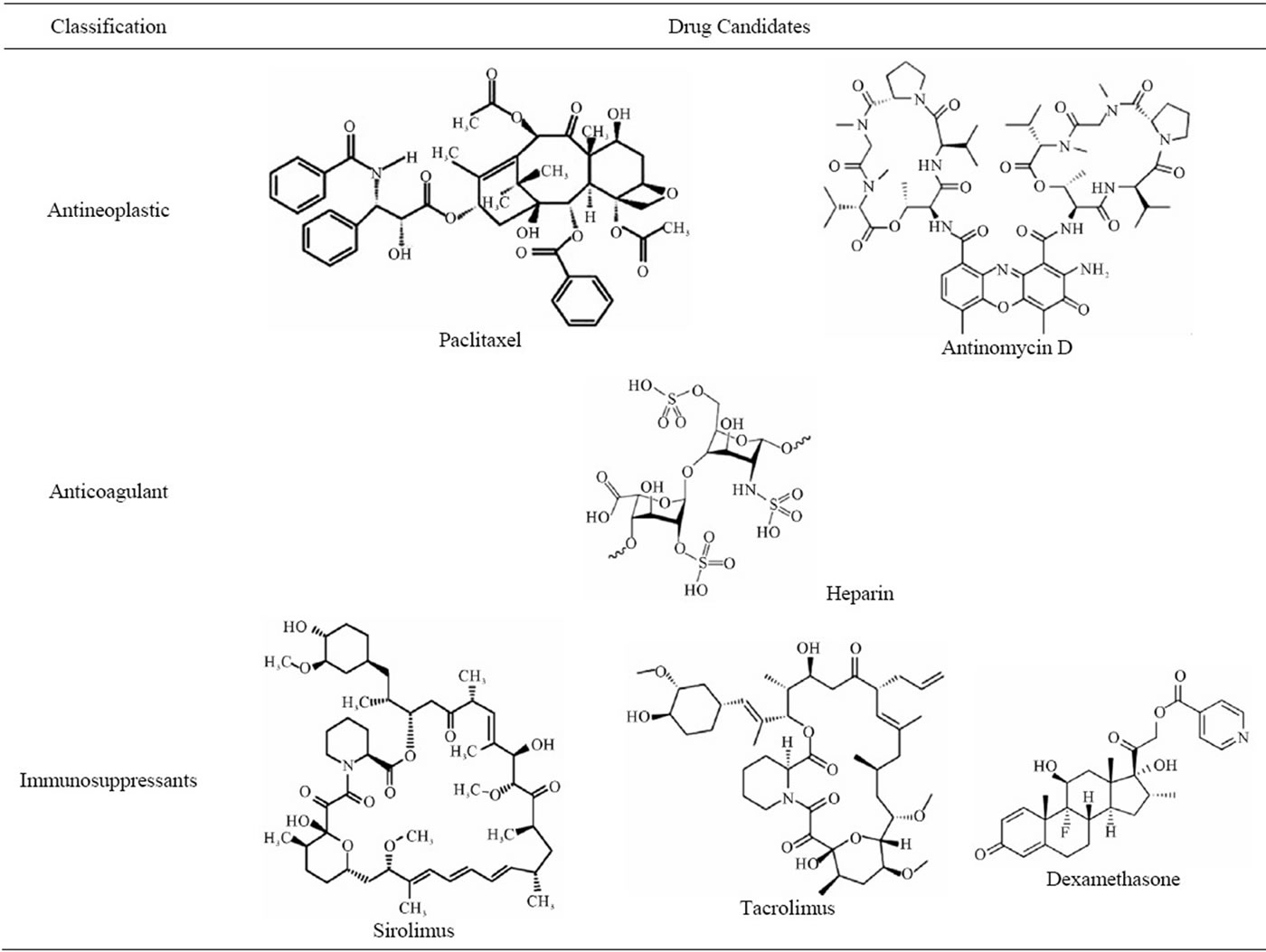
Table 3. Potential drug candidates for biodegradable stents.
animal experiments [53].
4.2. Sirolimus
Sirolimus is a naturally occurring macrocyclic lactone type, which is a drug first approved by FDA in 1999 for the prevention effects of rejection after kidney implant [54]. As one of the immunosuppressants, it binds with the intracellular receptor protein of blood vessel smooth muscle cells to disturb cell cycle, suppressing the migration and proliferation of smooth muscle cells of blood vessels [55]. Sirolimus, also as an immunosuppressant, contributes to the prevention of restenosis by suppressing the inflammatory reaction after blood vessel damage. Cypher of Johnson & Johnson is a DES with the mixture of poly(ethylene-co-vinylacetate) and poly(n-butyl methacrylate) in the ratio 67:33 sprayed on the stent surface where sirolimus and parylene C were sprayed, first approved as the DES stent by FDA in 2003 [56].
4.3. Paclitaxel
Paclitaxel is widely used as an anticancer drug, especially being recognized for its effects on breast cancer and ovarian cancer [57-59]. Paclitaxel encourages the polymerization of tubulin which has an important influence on the cell proliferation process, in turn abnormally stabilizing microtubule and rendering it dysfunctional to suppress cell proliferation. Such influence suppresses the cellular replication of smooth muscle cells and destructs the cells [60]. Also, paclitaxel has strong liphophilicity so that it is easy to be absorbed in the cell, and has the advantage that the medicinal effect can last long within the cell. Boston Scientific, following Taxus and Cypher, applied paclitaxel in mixture to the poly(styrene-b-isobutylene-b-styrene) triblock copolymer as a DES approved by FDA in 2004.
5. CONCLUSION
The history of coronary artery interventional procedure begun by coronary artery balloon dilatation in 1977 was the continuation of much effort and challenge, and overcoming such. Afterwards, from BMS to DES, the coronary artery interventional procedure has achieved brilliant advancements, but still issues such as thrombosis and restenosis are present. For DES, encouraging results were reported in the early-mid period clinical experiments compared to the metal stents [61]; however, the results with the concerns for late thrombosis due to the stent remaining in the body after the release of the drug are being suggested [62,63]. While there is no accurately clarified mechanism regarding the phenomenon, excessive inflammatory reaction is caused due to the drugs and healing process is blocked to cause the formation of new inner membrane. Beside such, various complex reasons exist and currently the theoretical knowledge and understanding on the pathological physiology are falling short. As a rational method to overcome the faced issues, the DES which completely decomposes by using biodegradable material should be developed. The biodegradable BVS stent currently in research was recently applied to 45 patients in 2 clinical occasions, and the encouraging result of 4.4% of heart-related side effects and no thrombosis was announced [64]. Also, unlike the DES using metal, it has the advantage of leaving no implant in the body after the artery is healed. Such completely decomposable biodegradable stent is expected to become a new standard of the treatment of coronary artery diseases. In the future, the development of advanced biodegradable materials and fusion among metal, polymer, and ceramic materials shall overcome the limitations of the existing DES and overcome the thrombosis and restenosis currently suggested as problems.
![]()
![]()
REFERENCES
- Grüntzig, A.R., Senning, A. and Siegenthaler, W.E. (1979) Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. New England Journal of Medicine, 301, 61-68. doi:10.1056/NEJM197907123010201
- Topol, E.J., Leya, F., Pinkerton, C.A., Whitlow, P.L., Hofling, B., Simonton, C.A., Masden, R.R., Serruys, P.W., Leon, M.B., Williams, D.O., King, S.B., Mark, D.B., Isner, J.M., Holmes, Jr, D.R., Ellis, S.G., Lee, K.L., Keeler, G.P., Berdan, L.G., Hinohara, T. and Califf, R.M. (1993) A comparison of directional atherectomy with coronary angioplasty in patients with coronary artery disease. New England Journal of Medicine, 329, 212-227. doi:10.1056/NEJM199307223290401
- John, A. and Bittl, M.D. (1996) Advanced in coronary angioplasty. New England Journal of Medicine, 335, 1290-1302. doi:10.1056/NEJM199610243351707
- O’Laughlin, M.P., Perry, S.B., Lock, J.E. and Mullins, C.E. (1991) Use of endovascular stents in congenital heart disease. Circulation, 83, 1923-1939.
- Fischman, D.L., Leon, M.B., Baim, D.S., Schatz, R.A., Savage, M.P., Penn, I., Detre, K., Veltri, L., Ricci, D., Nobuyoshi, M., Cleman, M., Heuser, R., Almond, D., Teirstein, P.S., Fish, R.D., Colombo, A., Brinker, J., Moses, J., Shaknovich, A., Hirshfeld, J., Bailey, S., Ellis, S., Rake, R. and Goldberg, S. (1994) A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. New England Journal of Medicine, 331, 496-501. doi:10.1056/NEJM199408253310802
- Serafino, L.D., Scudiero, L., De Laurentis, M., Ilardi, F., Magliulo, F., Carotenuto, G., Perrino, C. and Esposito, G. (2011) Drug-Eluting Stent for the treatment of early fistula failure. Journal of Biomedical Science and Engineering, 2, 196-200.
- Colombo, A., Drzewiecki, J., Banning, A., Grube, E., Hauptmann, K., Silber, S., Dudek, D., Fort, S., Schiele, F., Zmudka, K., Guagliumi, G. and Russell, M.E. (2003) Randomized study to assess the effectiveness of slowand moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation, 108, 788- 794. doi:10.1161/01.CIR.0000086926.62288.A6
- Holmes, Jr, D.R., Leon, M.B., Moses, J.W., Popma, J.J., Cutlip, D., Fitzgerald, P.J., Brown, C., Fischell, T., Wong, S.C., Midei, M., Snead, D. and Kuntz, R.E. (2004) Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation, 109, 634-640. doi:10.1161/01.CIR.0000112572.57794.22
- Tamai, H., Igaki, K., Kyo, E., Kosuga, K., Kawashima, A., Matsui, S., Komori, H., Tsuji, T., Motohara, S. and Uehata, H. (2000) Initial and 6-month results of biodegradable poly-L-lactic acid coronary stents in humans. Circulation, 102, 399-404.
- Abbott. (2007) The world’s first clinical trial of a fully bioabsorbable drug eluting coronary stent. Proceedings of the Transcatheter Cardiovascular Therapeutics 19th Annual Scientific Symposium, Washington DC, 20-25.
- Vogt, F., Stein, A., Rettemeier, G., Krott, N., Hoffmann, R., Vom Dahl, J., Bosserhoff, A.K, Michaeli, W., Hanrath, P., Weber, C. and Blindt, R. (2004) Long-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stent. European Heart Journal, 25, 1330-1340. doi:10.1016/j.ehj.2004.06.010
- Daemen, J. and Serruys, P.W. (2007) Drug-eluting stent update 2007: Part I. A survey of current and future generation drug-eluting stents: Meaningful advances or more of the same? Circulation, 116, 316-328. doi:10.1161/CIRCULATIONAHA.106.621342
- Grabow, N., Bünger, C.M, Schultze, C., Schmohl, K., Martin, D.P., Williams, S.F., Sternberg, K. and Schmitz, K.P. (2007) A biodegradable slotted tube stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for rapid balloon-expansion. Annals of Biomedical Engineering, 35, 2031-2038. doi:10.1007/s10439-007-9376-9
- Liu, S.J., Chiang, F.J., Hsiao, C.Y., Kau, Y.C. and Liu, K.S. (2010) Fabrication of balloon-expandable self-lock drug-eluting polycaprolactone stents using micro-injection molding and spray coating techniques. Annals of Biomedical Engineering, 38, 3185-3194. doi:10.1007/s10439-010-0075-6
- Venkatraman, S.S., Tan, L.P., Joso, J.F.D., Boey, Y.C. and Wang, X. (2006) Biodegradable stents with elastic memory. Biomaterials, 27, 1573-1578. doi:10.1016/j.biomaterials.2005.09.002
- Lauto, A., Ohebshalom, M., Esposito, M., Mingin, J., Li, P.S., Felsen, D., Goldstein, M. and Poppas, D.P. (2001) Self-expandable chitosan stent: Design and preparation. Biomaterials, 22, 1869-1874. doi:10.1016/S0142-9612(00)00371-9
- Wissam, A. J., Sameer, K., Ioakim, G., Feras, A., Kunal, C., Mark, D. and Amjad, K. (2011) Self-expanding metal stenting for malignant colonic tumours: A prospective study. Journal of Biomedical Science and Engineering, 2, 151-154.
- Peuster, M., Hesse, C., Schloo, T., Fink, C., Beerbaum, P. and Von Schnakenburg, C. (2006) Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials, 27, 4955-4962. doi:10.1016/j.biomaterials.2006.05.029
- Peuster, M., Wohlsein, P., Brügmann, M., Ehlerding, M., Seidler, K., Fink, C., Brauer, H., Fischer, A. and Hausdorf, G. (2001) A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into new zealand white rabbits. Heart, 86, 563-569. doi:10.1136/heart.86.5.563
- Mueller, P.P., May, T., Perz, A., Hauser, H. and Peuster, M. (2006) Control of smooth muscle cell proliferation by ferrous iron. Biomaterials, 27, 2193-2200. doi:10.1016/j.biomaterials.2005.10.042
- Staiger, M.P., Pietak, A.M., Huadmai, J. and Dias, G. (2006) Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials, 27, 1728-1734. doi:10.1016/j.biomaterials.2005.10.003
- Heublein, B., Rohde, R., Kaese, V., Niemeyer, M., Hartung, W. and Haverich, A. (2003) Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart, 89, 651-656. doi:10.1136/heart.89.6.651
- Di Mario. C., Griffiths, H., Goktekin, O., Peeters, N., Verbist, J., Bosiers, M., Deloose, K., Heublein, B., Rohde, R., Kasese, V., Ilsley, C. and Erbel, R. (2004) Drugeluting bioabsorbable magnesium stent. Journal of Interventional Cardiology, 17, 391-395. doi:10.1111/j.1540-8183.2004.04081.x
- Eggebrecht, H., Rodermann, J., Hunold, P., Schmermund, A., Böse, D., Haude, M. and Erbel, R. (2005) Novel magnetic resonance-compatible coronary stent: The absorbable magnesium-alloy stent. Circulation, 112, E303- E304. doi:10.1161/01.CIRCULATIONAHA.104.521641
- Zartner, P., Cesnjevar, R., Singer, H. and Weyand, M. (2005) First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheterization Cardiovascular Interventions, 66, 590-594. doi:10.1002/ccd.20520
- Zhao, Z.H., Sakagami, Y. and Osaka, T. (1998) Toxicity of hydrogen peroxide produced by electroplated coatings to pathogenic bacteria, Canadian Journal of Microbiology, 44, 441-447. doi:10.1139/w98-030
- Mario, C.D., Grube, E., Nisanci, Y., Reifart, N., Colombo, A., Rodermann, J., Mullerb, R., Ummanc, S., Liistroa, F., Montorfanoa, M. and Alte, E. (2004) MOONLIGHT: A controlled registry of an iridium oxide-coated stent with angiographic follow-up. International Journal of Cardiology, 95, 329-331. doi:10.1016/j.ijcard.2003.10.007
- Bolz, A. and Schaldach, M. (1990) Artificial heart valves: Improved blood compatibility by PECVD a-SiC:H coating, Artificial Organs, 14, 260-269. doi:10.1111/j.1525-1594.1990.tb02967.x
- Unverdorben, M., Sippel, B., Degenhardt, R., Sattler, K., Fries, R., Abt, B., Wagner, E., Koehler, H., Daemgen, G., Scholz, M., Ibrahim, H., Tews, K-H., Hennen, B., Berthold, H.K. and Vallbracht, C. (2003) Comparison of a silicon carbide-coated stent versus a noncoated stent in human beings: The tenax versus NIR stent study’s long-term outcome. American Heart Journal, 145, E17. doi:10.1067/mhj.2003.90
- Sgura, F.A., Mario, C.D., Liistro, F., Montorfano, M., Colombo, A. and Grube, E. (2002) The lunar stent characteristics and clinical results. Herz, 27, 514-517. doi:10.1007/s00059-002-2363-x
- Brown, D.A., Lee, E.W., Loh, C.T. and Kee, S.T. (2009) A new wave in treatment of vascular occlusive disease: Biodegradable stents-clinical experience and scientific principles. Journal of Vascular and Interventional Radiology, 20, 315-325. doi:10.1016/j.jvir.2008.11.007
- Zilberman, M. and Eberhart, R.C. (2006) Drug-eluting bioresorbable stents for various applications. Annual Review of Biomedical Engineering, 8, 153-180. doi:10.1146/annurev.bioeng.8.013106.151418
- Serrano, M.C., Chung, E.J. and Ameer, G.A. (2010) Advances and applications of biodegradable elastomers in regenerative medicine. Advanced Functional Materials, 20, 192-208. doi:10.1002/adfm.200901040
- Xue, L., Dai, S. and Li, Z. (2010) Biodegradable shapememory block co-polymers for fast self-expandable stents. Biomaterials, 31, 8132-8140. doi:10.1016/j.biomaterials.2010.07.043
- Park, S.B., Kang, K.H., Park, H.J., Park, J.S., Heo, S.H., Kim, H., Choy, Y.B. and Heo, C.Y. (2011) An evaluation of poly(L-lactic acid) plate and screw system for fixation of mandible fracture in rabbit model. Tissue Engineering and Regenerative Medicine, 8, 398-405.
- Woodruff, M.A. and Hutmacher, D.W. (2010) The return of a forgotten polymer—Polycaprolactone in the 21st century. Progress in Polymer Science, 35, 1217-1256. doi:10.1016/j.progpolymsci.2010.04.002
- Kim, M.S., Kim, J.H., Min, B.H., Chun, H.J., Han, D.K. and Lee, H.B. (2011) Polymeric scaffolds for regenerative medicine. Polymer Reviews, 51, 1-30. doi:10.1080/15583724.2010.537800
- Gunatillake, P.A. and Adhikari, R. (2003) Biodegradable synthetic polymers for tissue engineering. European Cell and Materials, 5, 1-16.
- Barrett, D.G. and Yousaf, M.N. (2009) Design and applications of biodegradable polyester tissue scaffolds based on endogenous monomers found in human metabolism. Molecules, 14, 4022-4050. doi:10.3390/molecules14104022
- Yasuda, H., Yamamoto, K., Nakayama, Y., Tsutsumi, C., Lecomte, P., Jerome, R., McCarthy, S. and Kaplane, D. (2004) Comparison of Sm complexes with Sn compounds for syntheses of copolymers composed of lactide and ε-caprolactone and their biodegradabilities. Reactive & Functional Polymers, 61, 277-292. doi:10.1016/j.reactfunctpolym.2004.06.007
- Tiainen, J., Veiranto, M., Suokas, E., Tormala, P., Waris, T., Ninkoviv, M. and Ashammakhi, N. (2002) Bioabsorbable ciprofloxacin-containing and plain self-reinforced polylactide-polyglycolide 80/20 screws: Pullout strength properties in human cadaver parietal bones. Journal of Craniofacial Surgery, 13, 427-433. doi:10.1097/00001665-200205000-00013
- Middleton, J.C. and Tipton, A.J. (1998) Synthetic biodegradable polymers as medical devices. Medical Plastics and Biomaterials Magazine.
- Pillai, C.K. and Sharma, C.P. (2010) Review paper: Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. Journal of Biomaterials Applications, 25, 291-366. doi:10.1177/0885328210384890
- Yang, S., Leong, K.F., Du, Z. and Chua, C.K. (2001) The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering, 7, 679-689. doi:10.1089/107632701753337645
- Zhang, Z., Kuijer, R., Bulstra, S.K., Grijpma, D.W. and Feijen, J. (2006) The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials, 27, 1741-1748. doi:10.1016/j.biomaterials.2005.09.017
- Ramchandani, M., Pankaskie, M. and Robinson, D. (1997) The influence of manufacturing procedure on the degradation of poly(lactide-co-glycolide) 85:15 and 50:50 implants. Journal of Controlled Release, 43, 161-173. doi:10.1016/S0168-3659(96)01481-2
- Sudesh, K., Abe, H. and Doi, Y. (2000) Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Progress in Polymer Science, 25, 1503-1555. doi:10.1016/S0079-6700(00)00035-6
- Gingras, A.C., Raught, B. and Sonenberg, N. (2004) mTOR signaling to translation. Current Topics in Microbiology and Immunology, 279, 169-197. doi:10.1007/978-3-642-18930-2_11
- Ragosta, M., Karve, M., Brezynski, D., Humphries, J., Sanders, J.M., Sarembock, I.J., Gimple, L.W. and Powers, E.R. (1999) Effectiveness of heparin in preventing thrombin generation and thrombin activity in patients undergoing coronary intervention. American Heart Journal, 137, 250-257. doi:10.1053/hj.1999.v137.91541
- Goosen, M.F. and Sefton, M.V. (1983) Properties of a heparin-poly(vinyl alcohol) hydrogel coating. Journal of Biomedical Materials Research, 17, 359-373. doi:10.1002/jbm.820170212
- Yang, Z., Birkenhauer, P., Julmy, F., Chickering, D., Ranieri, J.P., Merkle, H.P., Lüscher, T.F. and Gander, B. (1999) Sustained release of heparin from polymeric particles for inhibition of human vascular smooth muscle cell proliferation. Journal of Controlled Release, 60, 269-277. doi:10.1016/S0168-3659(99)00078-4
- Tan, L.P., Venkatraman, S.S., Sung, P.F. and Wang, X.T. (2004) Effect of plasticization on heparin release from biodegradable matrices. International Journal of Pharmaceutics, 283, 89-96. doi:10.1016/j.ijpharm.2004.06.022
- Sheth, S., Dev, V., Jacobs, H., Forrester, J.S., Litvack, F. and Eigler, N.L. (1995) Prevention of subacute stent thrombosis by polymer-polyethylene oxide-heparin coating in the rabbit carotid artery. Journal of the American College of Cardiology, 25, 348A-349A. doi:10.1016/0735-1097(95)92903-I
- Marx, S.O., Jayaraman, T., Go, L.O. and Marks, A.R. (1995) Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circulation Research, 76, 412-417.
- Poon, M., Marx, S.O., Gallo, R., Badimon, J.J., Taubman, M.B. and Marks, A.R. (1996) Rapamycin inhibits vascular smooth muscle cell migration. Journal of Clinical Investigation, 98, 2277-2283. doi:10.1172/JCI119038
- Moses, J.W., Leon, M.B., Popma, J.J., Fitzgerald, P.J., Holmes, D.R., O’Shaughnessy, C., Caputo, R.P., Kereiakes, D.J., Williams, D.O., Teirstein, P.S., Jaeger, J.L. and Kuntz, R.E. (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. New England Journal of Medicine, 349, 1315-1323. doi:10.1056/NEJMoa035071
- Lee, J.Y., Kim, D.Y., Kim, G.H., Kang, K.M., Min, B.H., Lee, B., Kim, J.H. and Kim, M.S. (2011) Enhanced stability of nano-emulsified paclitaxel. Journal of Biomedical Science and Engineering, 4, 350-354.
- Lee, J.Y., Kim, K.S., Kang, Y.M., Kim, E.S., Hwang, S.J., Lee H.B., Min, B.H., Kim, J.H. and Kim, M.S. (2010) In vivo efficacy on subcutaneous tumor growth of Ptxloaded injectable in situ gel. International Journal of Pharmaceutics, 392, 51-56. doi:10.1016/j.ijpharm.2010.03.033
- Kim, G.H., Lee, J.Y., Kang, Y.M., Kang, K.N., Kim, E.S., Kim, D.Y., Kim, J.H. and Kim, M.S. (2011) Preparation and characterization of self-emulsified docetaxel. Journal of Nanomaterials, 2011, 6-11. doi:10.1155/2011/860376
- [61] Liistro, F. and Bolognese, L. (2003) Drug-eluting stents. Heart Drug, 3, 203-213. doi:10.1159/000075706
- [62] Ong, A.T.L., Hoye, A., Aoki, J., van Mieghem, C.A., Rodriguez Granillo, G.A., Sonnenschein, K., Regar, E., McFadden, E.P., Sianos, G., van der Giessen, W.J., de Jaegere, P. P., de Feyter, P., van Domburg, R.T. and Serruys, P.W. (2005) Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after baremetal, sirolimus, or paclitaxel stent implantation. Journal of the American College of Cardiology, 45, 947-953. doi:10.1016/j.jacc.2004.09.079
- [63] Ong, A.T., McFadden, E.P., Regar, E., de Jaegere, P.P., van Domburg, R.T. and Serruys, P.W. (2005) Late angiographic stent thrombosis (LAST) events with drugeluting stents. Journal of the American College of Cardiology, 45, 2088-2092. doi:10.1016/j.jacc.2005.02.086
- [64] Iakovou, I., Schmidt, T., Bonizzoni, E., Ge, L., Sangiorgi, G.M., Stankovic, G., Airoldi, F., Chieffo, A., Montorfano, M., Carlino, M., Michev, I., Corvaja, N., Briguori, C., Gerckens, U., Grube, E. and Colombo, A. (2005) Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Journal of the American Medical Association, 293, 2126-2130. doi:10.1001/jama.293.17.2126
- [65] Bruyne, B.D. (2010) Absorb cohort B trial: 6-Month clinical and angiographic results of the evaluation of the bioresorbable everolimus-eluting vascular scaffold (BVS) in the treatment of patients with de novo native coronary artery lesions. Proceedings of the Euro PCR 2010 Conference, Paris, France, 6.
NOTES
*Doo Yeon Kwon and Jae Il Kim are equal first authors in this work.
#Corresponding author.

