Journal of Cancer Therapy
Vol.3 No.5(2012), Article ID:23446,13 pages DOI:10.4236/jct.2012.35069
Effect of Rosehip (Rosa canina) Extracts on Human Brain Tumor Cell Proliferation and Apoptosis
![]()
1Department of Biology, North Carolina A & T State University, Greensboro, USA; 2Department of Animal Sciences, North Carolina A & T State University, Greensboro, USA; 3Department of Family and Consumer Sciences, North Carolina A & T State University, Greensboro, USA.
Email: pmmartin@ncat.edu
Received August 3rd, 2012; revised September 5th, 2012; accepted September 17th, 2012
Keywords: Rosehip; Rosa canina; Glioma; Glioblastoma Multiforme; Cell Proliferation; Apoptosis; Temozolomide; MAPK; AKT
ABSTRACT
Rosehips are blossoms from the wild rose (Rosa canina) and are commonly used as an herbal remedy. Previous reports have shown that extracts made from rosehip plants are able to reduce cell proliferation of cancer cells. In this study, we investigated the efficacy of rosehip extracts in preventing cell proliferation of three human glioblastoma cell lines A-172, U-251 MG and U-1242 MG cell lines. Each of the glioblastoma cell lines treated with rosehip extracts (1 mg/mL - 25 ng/mL) demonstrated a significant decrease in cell proliferation. The rosehip extract-mediated decrease in cell proliferation was equal to or better than the decrease of cell proliferation observed when inhibitors of the MAPK (U0126, 10 µM) or AKT (LY294002, 20 µM) signaling pathways were utilized. Additionally, pretreatment of the these cell lines with Rosehip extracts (1 mg/mL - 25 ng/mL) selectively decreased AKT, MAPK, and p70S6K phosphorylation suggesting these extracts prevent glioblastoma multiforme cell proliferation by blocking both the MAPK and AKT signaling mechanisms. Results from colorimetric cell death assays, cell cycle analysis by flow cytometry, as well as western blot studies demonstrate that rosehip extracts inhibit cell proliferation but do not promote apoptosis. Moreover, rosehip extracts were able to increase the efficacy of Temozolomide, a chemotherapeutic agent used to treat patients with glioblastomas. Surprisingly, rosehip extracts demonstrated a greater inhibition of cell proliferation than in combination with Temozolomide (100 µM) or Temozolomide as a single agent. Taken together these data suggest that rosehip extracts are capable of decreasing glioblastoma cell proliferation without promoting apoptosis and demonstrate a greater cell proliferation inhibitory effect than Temozolomide. More importantly, rosehip extracts may serve as an alternative or compliment to current chemotherapeutic regimens for glioblastomas.
1. Introduction
In the past twenty years the incidence of primary brain tumors has increased and as the population gets older this trend is expected to continue. Glioblastoma multiforme (GBM) is an aggressive neoplasm that is characterized by an elevated, aberrant, proliferative capacity that is accompanied by a diffuse pattern of brain invasion [1]. GBM is classified as a grade IV astrocytoma by the World Health Organization (WHO) and is the most malignant form of adult brain tumor [2]. Due to the deleterious properties of GBMs they are associated with extremely high mortality rates. Despite recent advances in chemotherapy, radiotherapy and surgical interventions, the clinical outcome of GBMs is poor and a better understanding of glioma biology is needed in order to develop better therapeutic options. Recent studies have investigated the anti-oncogenic properties of natural products and several of these studies have shown that natural product extracts exhibit anti-oncogenic properties against various types of cancer [3-7]. Specifically, there has been a renewed effort to examine the anti-oncogenic properties of natural products for the treatment of GBM cells [8-13]. However, the anti-oncogenic properties of rosehip extracts on GBM cells have not been widely investigated.
Rosehip extracts (rosehips) are derived from the rose plant (Rosa sp. Pl.) and can be derived from Rosa canina (Dog rose, Rosaceae, rosehip) and Rosa villous [3,14]. Rosehip extracts have been studied extensively as antiinflammatory agents as well as an alternative treatment for osteoarthritis [15-17]. Rosehip extracts have also been shown to possess a large amount of phytochemicals that include flavonoides and polyphenols that have the potential to serve as antioxidants that prevent cell proliferation and migration in breast (MCF-7), colon (HT-29) and cervical cancer (HeLa) cell lines [14,18,19]. However, neither rosehip extracts nor the bioactive components of these extracts have been extensively studied for their ability to prevent GBM cell proliferation.
AKT and MAPK signaling mechanisms are known to contribute to the development and promotion of brain tumors, especially gliomas. Genetic alterations resulting in amplification of the epidermal growth factor receptor (EGFR), p53 and phosphatase and tensin homolog (PTEN) mutations have been associated with altered AKT and MAPK signaling in GBMs [20]. AKT signaling has been associated with increased cell proliferation, invasion, angiogenesis and inhibition of apoptosis in GBMs [21-25]. Silencing AKT with siRNA oligonucleotides is able to block GBM cell proliferation and promote apoptosis [26,27]. Reseveratrol in combination with AKT silencing promotes GBM cell apoptosis [26]. Down regulation of PI3K with siRNA, an upstream kinase for AKT, also prevents GBM cell proliferation and promotes apoptosis [28]. EGFR mutations and amplification are activators of the MAPK pathway in GBMs and this pathway has been shown to increase GBM cell proliferation, invasion and migration [20]. Recently, plant extracts have shown anti-tumor properties towards GBM, however, these studies do not directly address the antitumor capacity of rosehip extracts and also do not identify which signaling mechanism(s) are interrupted [18, 19].
In this study we investigated the efficacy of rosehip extracts in three human glioblastoma cell lines to better understand the role of rosehip extracts in preventing glioblastoma cell proliferation. Additionally, we examined which cell signaling mechanisms were altered by rosehip extracts and whether rosehip extracts promoted apoptosis in glioblastoma cells. We show that rosehip extracts are able to prevent cell proliferation through a mechanism that involves inhibition of both the AKT and MAPK signaling pathways, but does not promote apoptosis. Furthermore we find that rosehip extracts prevent cell proliferation more effectively than Temozolomide, a frequently used chemotherapeutic in the treatment of glioblastoma.
2. Materials and Methods
2.1. Materials
Rosehip extracts were kind gifts from Dr. Ipek Goketpe (North Carolina A & T State University). Delbucco’s Minimal Essential Media and Minimal Essential Medium-α (MEM-α) as well as the live/dead cell viability assay were purchased from Invitrogen (USA). Fetal bovine serum (FBS) used to maintain the cells were purchased from Biowest (USA). 3-(4,5)-dimethlythiahiazo (-z-yl0-3,5-diphenytetrazoliumromide (MTT) was purchased from Calbiochem (USA). Temozolomide and tubulin primary antibody were purchased from Sigma (USA). U0126 and LY294002 were purchased from Calbiochem (USA). Antibodies against phosphorylated MAPK (Tyr 202/204), phosphorylated AKT (ser473), phosphorylated p70S6K (Thr421/Ser424), phosphorylated Rb (Ser807/811), total MAPK, Fra-1, AKT and p70S6K were purchased from Cell Signaling Technologies (Massachusetts, USA). SpectraMax M5 (Molecular Devices, USA) plate reader was used to collect MTT and live/dead data. An Accuri (Becton Dickinson, USA) Flow Cytometry was used to analyze cell cycle status.
2.2. Plant Material and Preparation of Crude Extracts
Dried Rosehip calyces and crude Rosehip extracts were supplied by Dr. Ipeke Goktepe (North Carolina A&T State University). The freeze dried calyces were grounded to powder and then stored at −20˚C. Rosehip powder (50 g) was serially extracted with 80% methanol. The mixture of freeze dried powder and 80% aqueous methanol were sonicated for 20 minutes with continual nitrogen gas purging. The extracts were filtered through Whatman No. 2 filter paper and evaporated to dryness under reduced pressure. The extracts were and stored at –20˚C. The obtained residues of the crude extracts were tested in human glioblastomas for cellular anti-proliferative activity.
2.3. Cell Culture
The human GBM cell lines U-1242 MG, U-251 MG, and A-172 were kindly supplied by Dr. Isa Hussaini (University of Virginia, and U-1242 MG) and Dr. Waldemar Debinski (Wake Forest University, A-172 and U-251 MG). The U-251 MG and A-172 cells were maintained in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, USA) supplemented with 10% FBS and 1% of penicillin/streptomycin (Fisher, USA). The U-1242 cells were maintained in Minimum Essential Media-α (MEM-α) supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were incubated at 37˚C in a humidified atmosphere containing 5% CO2. Cultures were maintained in 10 cm2 plastic dishes and serially passaged following trypsinization using a TrypLE Express Stable Trypsin-Like Enzyme with Phenol Red solution (Invitrogen, USA).
2.4. Cell Proliferation Assay
The effects of rosehip extracts on cell proliferation were determined by MTT assays. Briefly, 104 cells were seeded into a 96-well culture plate and incubated 4 - 6 h for attachment. The cells were then treated with various concentrations of the rosehip extract (1 mg/mL, 250 µg/mL, 25 µg/mL and 25 ng/mL) for 48 h. The PI3K (Phosphoinositide3-kinase) inhibitor LY294002 (20 µM) and the MEK1/MEK2 inhibitor U0126 (10 µM) were administered for 12 - 16 h (Calbiochem). Cells treated with TMZ (100 µM) were treated for 24 h prior to MTT assays. To determine the time-course response of rosehip extracts, cells were plated into 96-well plates, and rosehip was added to the culture medium and cell viability was assessed with MTT assay at 48 h after the drug treatment. Following rosehip exposure, 10 µL of MTT reagent was added to each well of a 96-well assay plate containing the cells in 100 µL of culture medium, and the plates were incubated at 37˚C in 5% CO2 for 4 h. A volume of 100 µL of a color development solution (0.04N HCL/ Isopropanol) was added for 90 minutes, after which the optical density at 570 nm was determined using a Spectra Max M5 microplate reader. The optical density was used to calculate proliferation rates after exposure to the different concentrations of rosehip relative to that of control cells with no rosehip exposure. All analyses were performed in triplicate, and the mean for each experiment was calculated.
2.5. Protein Extraction and Western Blotting
Following exposure to rosehip extracts at the various concentrations, the cells were washed with cold phosphate-buffered saline containing 0.2 mM sodium orthovanadate. The cells were then harvested and lysed on ice with 125 µL of pre-cooled Triton lysis buffer containing 1% Triton X-100, 0.2% Nonidet P-40, in the presence of 2 mm EDTA, 2 mg/mL sodium orthovanadate, and 1x protease cocktail inhibitor. Cells were centrifuged at 15,000 rpm for 1 min at 4˚C. The protein concentration of the supernatant was determined by the BCA assay (BioRad, USA) and equal amounts of protein from control and drug-treated cells were boiled for 5 min in SDS-PAGE buffer. Proteins were separated by SDS-PAGE on 8% polyacrylamide gels and subsequently transferred onto PVDF membranes. For immunoblotting, PVDF membranes were incubated with specific antibodies recognizing target proteins overnight at 4˚C. The membranes were then incubated with HRPconjugated secondary antibody (1:2000) for 1 h at room temperature and subsequently analyzed by enhanced chemiluminescence (ECL) detection system (Thermo Scientific, USA) and visualized by autoradiography. Tubulin (1:5000) was used as loading controls.
2.6. Live-Dead Viability/Cytotoxicity Assay
The viability of the cell lines following extract treatment was assessed using the LIVE-DEAD assay (Molecular Probes, USA). The assay was performed according to the manufacturer’s instructions to examine rosehip extract cytotoxicity after 48 h. The assay utilizes two fluorescent dyes to label live and dead cells. The cytoplasm of live cells was stained with 2 μm Calcein AM (green) the nucleus of dead cells stained with 4 μm EthD-1 (red). Stained cells were viewed using the Olympus IX71 inverted fluorescent microscope. Images were captured within 45 min of labeling using the Olympus DP 71 digital camera. The amount of dye in each cell was quantitatively measured using the SpectraMax M5 microplate reader.
2.7. Cell Cycle Analysis by Flow Cytometry
Cells were plated in 6 cm plates and incubated under the conditions described above. After 4 - 6 hr incubation, to allow cell attachment, the cells were rinsed and incubated in serum free media for 24 h. After 24 h, the media was discarded and the cells were treated with various concentrations of the rosehip extract (250 µg/mL and 25 µg/mL) for 48 hr. Staurosporine (1 µM) was administered for 18 h (Sigma, USA). After treatment, the cells were trypsinized and pooled, then pelleted by centrifugation at 300 × g for 6 min. Cell pellets were washed with PBS, then fixed in ice cold 70% ethanol, and stored at −20˚C for 2 hr. Fixed cells were washed with PBS followed by a 30 minute incubation at room temperature in the dark in a staining solution consisting of PBS with 20 µg/mL propidium iodide (Sigma) (Missouri, USA), 0.1% Triton X-100 and 200 µg/mL RNase A (Sigma, USA). Stained cells were analyzed on the Accuri C6 Flow Cytometer. Using DNA histograms, FCS Express 4 software (Becton Dickson, USA) was used to quantify cell cycle compartments to estimate the percentage of cells distributed in the different cell cycle phases. Each experiment was repeated 4 times and the results are indicative of 4 independent studies.
2.8. Statistical Analysis
Experiments examining cell proliferation using MTT, Live/Dead apoptotic analysis and cell cycle determination using flow cytometry were averaged and means and s.e.m. from three individual experiments were analyzed for statistical significance using a one-tailed ANOVA and the Newman-Kuels multiple comparison test to determine statistical significance.
3. Results
3.1. Rosehip Extracts Attenuate GBM Cell Proliferation and Cell Cycle Progression
We determined the rate of GBM cell proliferation in vitro by measuring the rate of MTT incorporated into three human GBM cell lines, A-172, U-251 MG and U-1242 MG cells following varying concentrations of rosehip extracts (1 mg/mL, 250 µg/mL, 25 µg/mL and 25 ng/mL). Each one of the human brain tumor cell lines assayed demonstrated a significant decrease in cell proliferation following treatment with various concentrations of rosehip extracts. Specifically, rosehip extract concentrations ranging from 25 µg/mL to 1 mg/mL significantly decrease the rate of cell proliferation in A-172 cells (Figure 1(a)). U-251 MG and U-1242 MG cells demonstrated higher sensitivity to rosehip extracts. In these cells the rosehip concentrations ranging from 25 ng/mL to 1 mg/mL show a decrease of cell proliferation (Figures 1(b) and (c)). These data suggest that crude rosehip extracts decrease the rate of cell proliferation in human brain tumor cell lines. The ability of rosehip extracts to prevent cell proliferation was compared to the ability of known PI3K/AKT (LY294002, 20 µM) and MAPK (U0126, 10 µM) inhibitors. In each of the cell lines tested the higher concentrations of rosehip extracts demonstrated an equal or greater decrease of cell proliferation than LY294002 and U0126 (Figures 1(a)-(c)). These data suggest that rosehip extracts have a greater capacity to prevent GBM cell proliferation than known inhibitors of cell proliferation.
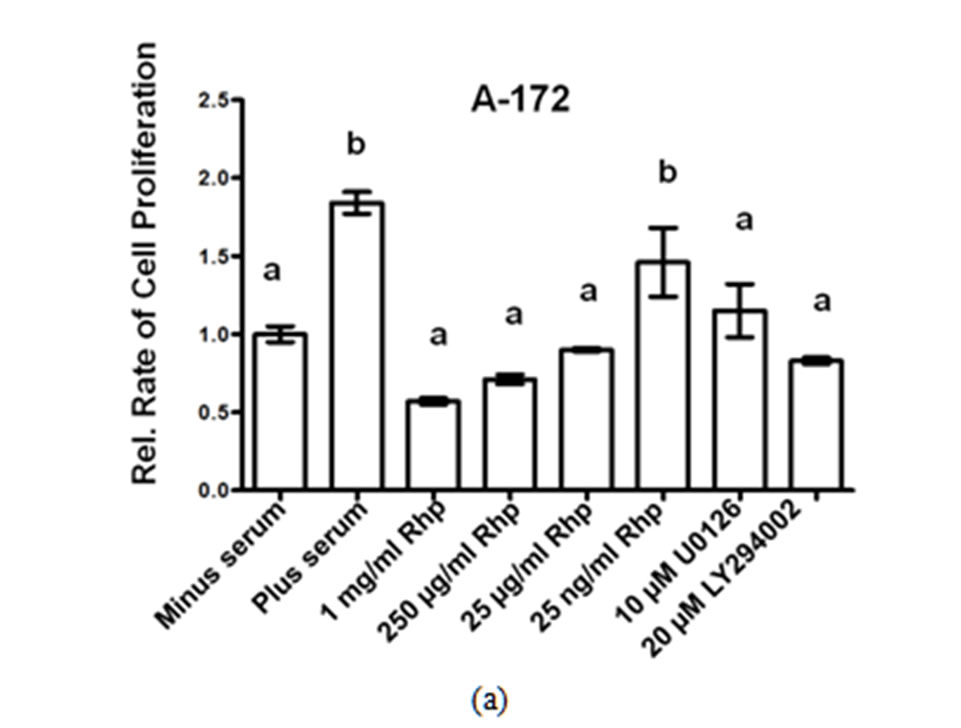
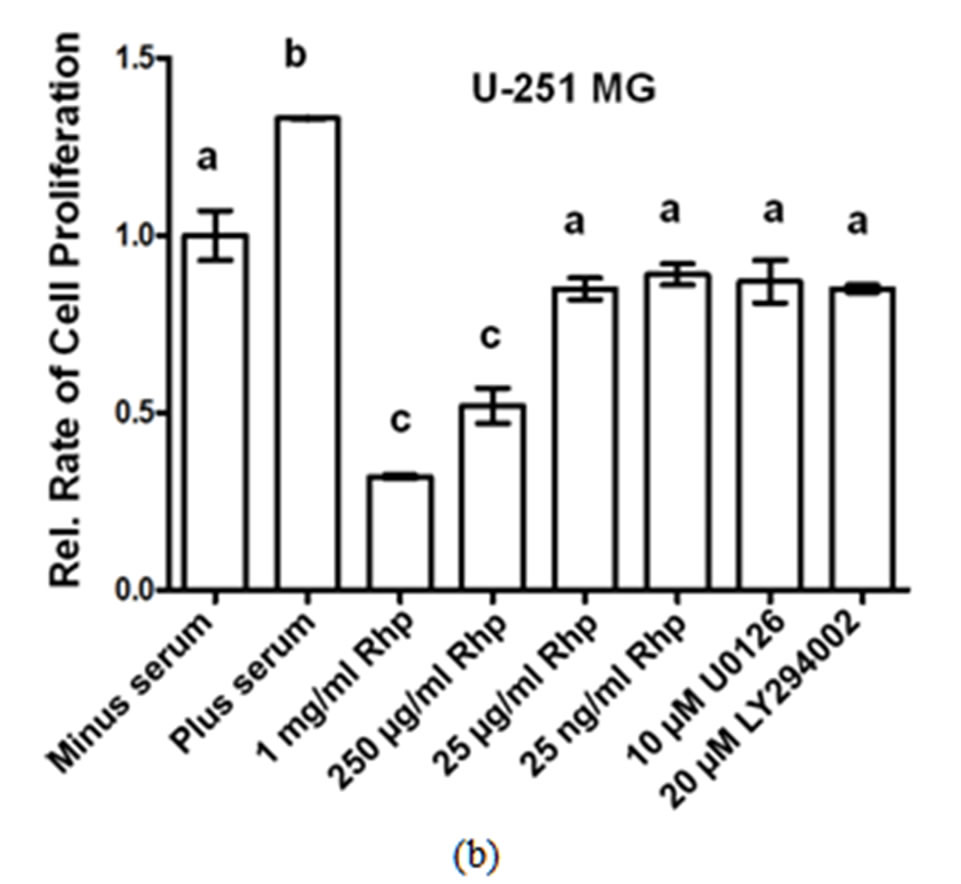
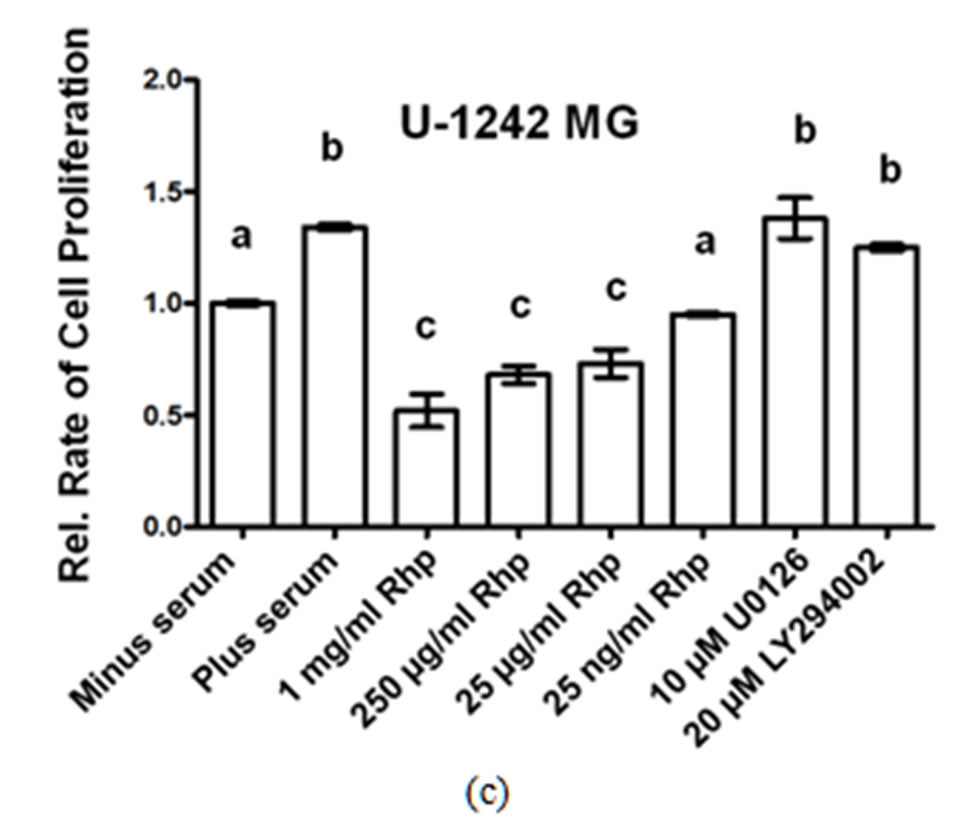
Effect of Rosehip Extract Concentration on the Rate of Cell Proliferation in Human Brain Tumor Cells. A-172, U-251MG and U-1242 MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicatedin the figure and then incubated for 48 h. The rate of cell proliferation were determined as described in the materials and methods section. Each value is presented as the mean sem of three independent determinations. The columns in each graph are presented as relative values in comparison to serum-starved cells. Bars labeled with different letters are significantly different from one another (P < 0.05). Bars labeled with the same letter are not significantly different (P > 0.05).
Figure 1. Crude rosehip extracts prevent brain tumor cell proliferation.
3.2. Rosehip Extracts Diminish AKT and MAPK Signaling Mechanisms
To investigate the intracellular mechanisms controlling rosehip extract sensitivity observed in the brain tumor cell lines, we examined the phosphorylation state of several proteins. We first determined the inhibitory effect of rosehip extracts on the phosphorylation levels of MAPK (Erk1/2; Tyr 202/04) and the total protein levels of Fra-1, a downstream target of MAPK and a regulator of GBM formation and progression [29,30]. All cell lines were serum starved for 18 h to reduce the basal phosphorylation levels, and then incubated in the presence of various concentrations of rosehip extracts (1 mg/mL, 250 µg/mL, 25 µg/mL and 25 ng/mL), and 10% FBS. Additional samples were serum starved as described above and incubated in the presence of 10% FBS and either U0126 (10 µM) or LY294002 (20 µm), known chemical inhibittors of the MAPK and AKT signaling pathways, respecttively. MAPK (Erk1/2; Tyr 202/04) phosphorylation in A-172 (Figure 2(a)), U-251 MG (Figure 3(a)), and U-1242 MG (Figure 4(a)) cells was decreased following exposure to rosehip extracts and U0126, but not LY294002. In addition to a decrease in MAPK (Erk1/2) phosphorylation, Fra-1 (total) protein levels were also decreased in response to rosehip treatment and U0126 treatment, but not LY294002. Previous studies have shown that MAPK (Erk1/2) phosphorylation results in
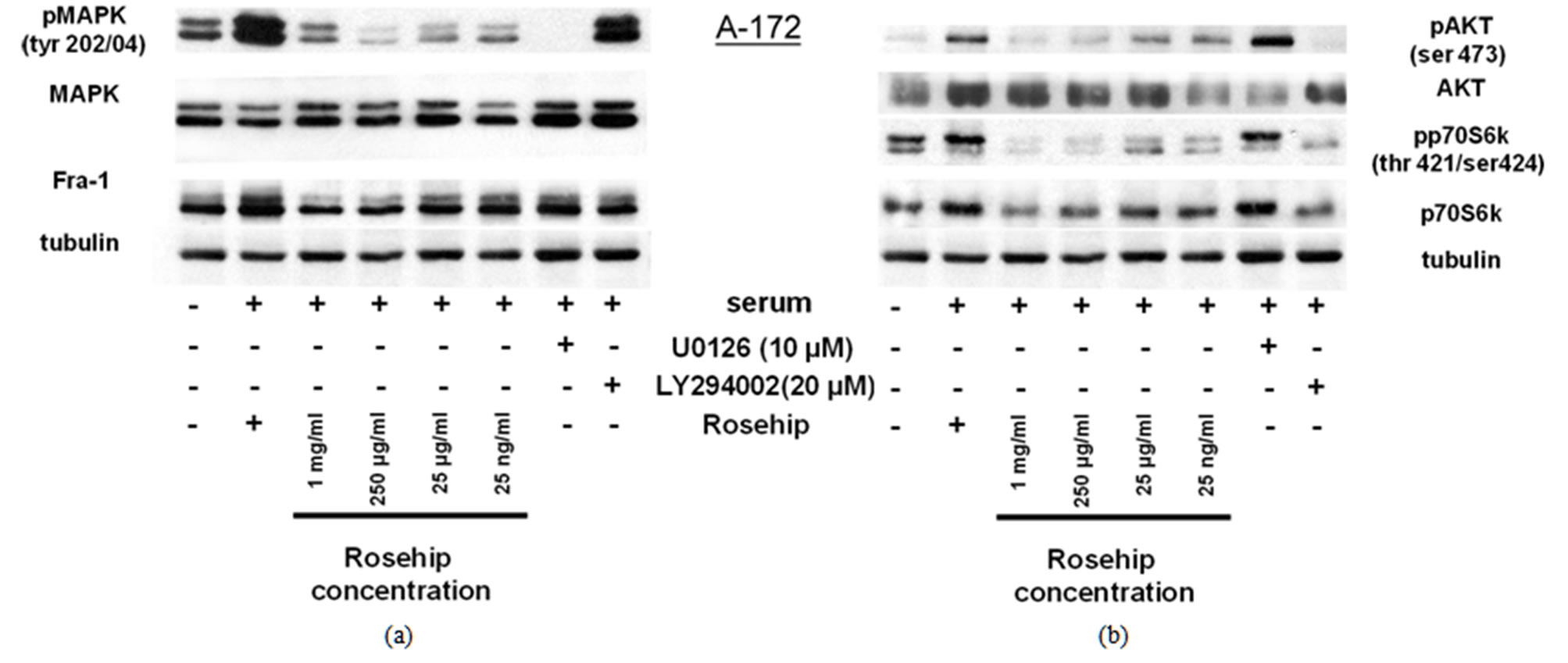
Figure 2. In A-172 cells, a GBM cell line, rosehip extracts inhibit the MAPK and AKT signaling pathways.
Inhibitory Effect of Rosehip Extract Concentration on AKT andMAPK (Erk1/2) signalingin A-172 Cells. A-172 cells were treated with rosehip extracts inthe presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. (a) The phosphorylation status of MAPK and protein expression level of Fra-1 was determined by a western blotting analysis; (b) The phosphorylation status of AKT and p70S6K was determined by a western blotting analysis.Tubulin was used as a protein loading control.
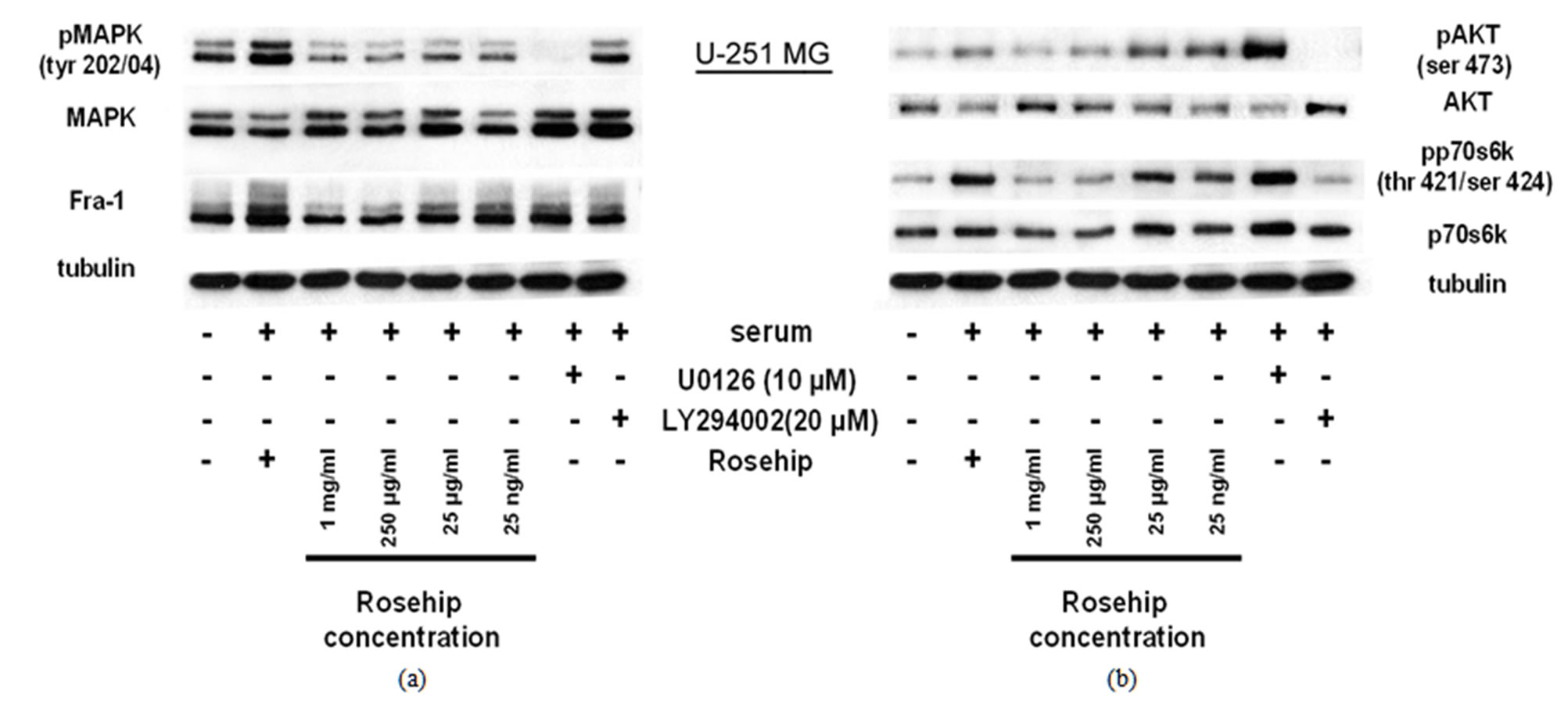
Figure 3. Rosehip extracts inhibit the MAPK and AKT signaling pathways In U-251 MG cells, a GBM cell line.
Inhibitory Effect of Rosehip Extract Concentrationon AKT andMAPK (Erk1/2) signaling in U-251MG Cells. U-251MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. (a) The phosphorylation status of MAPK (Erk1/2) and protein expression level of Fra-1 was determined by a western blotting analysis; (b)The phosphorylation status of AKT and p70S6K was determined by a western blotting analysis. Tubulin was used as a protein loading control.
increased Fra-1 protein levels and increased transcripttional activity [31-33]. Taken together these data suggest that rosehip extracts prevent MAPK (Erk1/2) phosphorylation and this inhibition of MAPK (Erk1/2) phosphorylation correlates with the reduction of cell proliferation observed in A-172 cells (Figures 1(a)-(c)).
Additional studies investigated whether rosehip extracts could decrease the activity of the AKT cell proliferation regulatory pathway. Each cell line was treated in a similar manner as described above. Rosehip extracts (all concentrations tested) prevent AKT phosphorylation (ser 473) and p70S6K (Thr 421/Ser 424) phosphorylation in A-172 cells, in a similar fashion as LY294002 (Figure 2(b)). AKT and p70S6K phosphorylation is only slightly decreased by higher concentrations of rosehip extracts (1 mg/mL and 250 µg/mL) in U-251MG cells (Figure 2(b)).
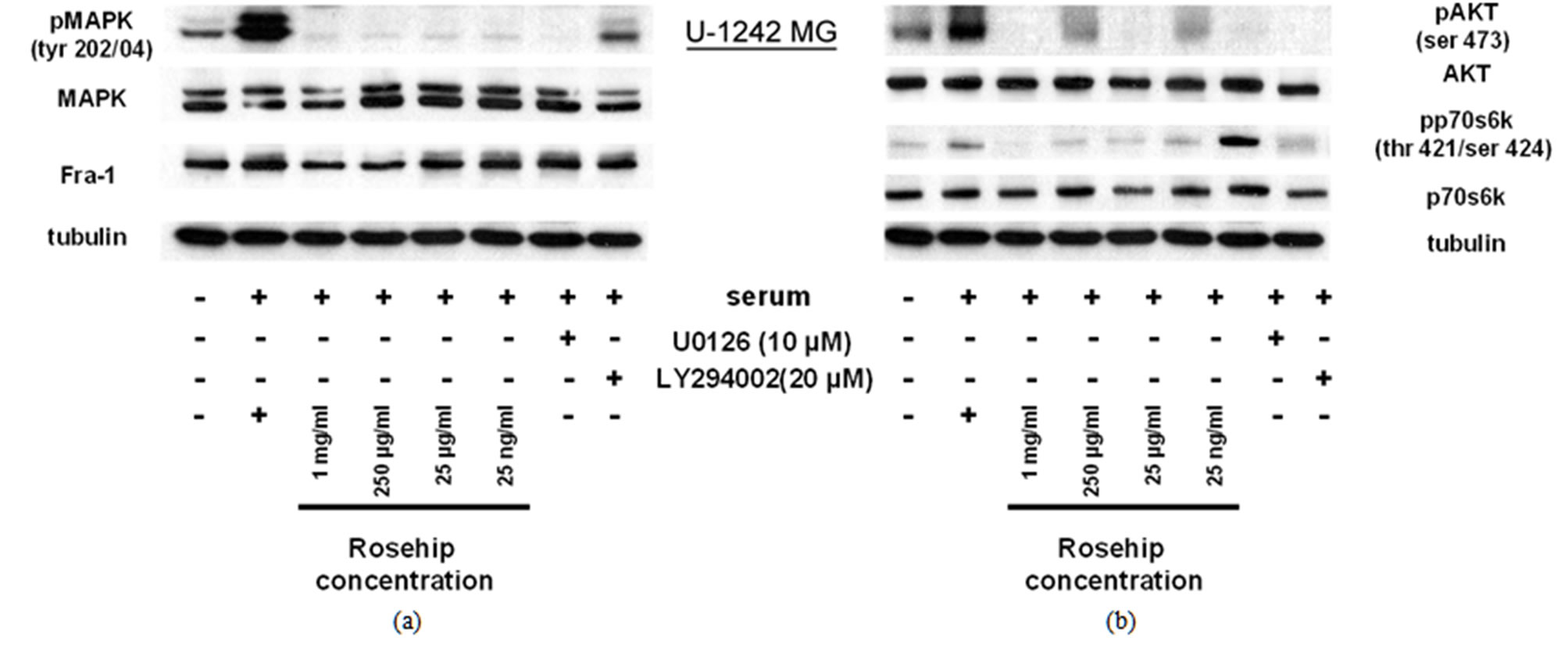
Figure 4. Rosehip extracts inhibit the MAPK and AKT signaling pathways In U-1242 MG cells, a GBM cell line.
Inhibitory Effect of Rosehip Extract Concentrationon AKT and MAPK (Erk1/2) signaling in U-1242MG Cells. U-1242MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. (a) The phosphorylation status of MAPK (Erk1/2) and protein expression level of Fra-1 was determined by a western blotting analysis; (b) The phosphorylation status of AKT and p70S6K was determined by a western blotting analysis. Tubulinwas used as a protein loading control.
In U-1242 MG cells AKT and p70S6K phosphorylation is decreased by rosehip extracts (Figure 4(b)). Specifically, rosehip extracts are able to decrease the phosphorylation of p70S6K at all concentrations tested in U-1242 MG cells (Figure 4(b)), but only at the highest tested concentrations in U-251 MG cells (Figure 3(b)). These data indicate that rosehip can also prevent the activation on the AKT cell signaling regulatory pathway in A-172 and U-251MG cells (Figures 2(b) and 3(b)). More importantly, these data indicate that rosehip extracts are able to inhibit multiple proliferation regulatory mechanisms in the cell lines assayed.
3.3 Rosehip Extracts Do Not Promote Cell Death
In order to examine whether rosehip extracts were promoting cell death in A-172, U-251 MG and U-1242 MG cell lines, cells were cultured in the presence of 250 µg/mL rosehip extract and 10% FBS and the amount of cell death was measured using a fluorescence colorimetric analysis (as described in the materials and methods section). The mean percentage of dead cells was determined following rosehip extract treatment and these values were compared to the values generated by A-172, U-251 MG and U-1242 MG cells that were cultured in 10% FBS and staurosporine (1 µM) for either 3 h (U-1242MG cells) or 24 h (A-172 and U-251MG cells) in order to induce cell death. Since in cell proliferation assays there was no significant difference observed between the 1 mg/mL and 250 µg/mL concentration of rosehip extracts we used 250 µg/mL of rosehip extracts to determine the apoptosis promoting capacity of the extracts. Each cell line tested with rosehip extracts (250 µg/mL) did not promote cell death, but staurosporine treatment did result in cell death (Figure 5). Figure 6 (these data correlate to the A-172 panel displayed in Figure 5) shows A-172 cells treated with staurosporine have a much higher amount of cell death than cells treated with rosehip extracts or cells cultured in serum containing medium without inhibitors (Figure 6, panels C, F, I). These data suggests the prevention of cell proliferation observed in cells treated with rosehip extracts is due to inhibition/decrease of cell cycle progression and not cell death.
Since rosehip extracts are able to prevent cell proliferation in the cell lines tested, but do not promote cell death we wanted to examine whether exposure to these extracts resulted in PARP cleavage, a marker of apoptosis. To test further whether rosehip is promoting apoptosis, we used a western blot to assay for PARP cleavage. PARP, a nuclear polymerase protein important for DNA repair in response to stress, is cleaved from its 116 kDA full size protein to a 89 kDA fragment, which in human cells has been associated with cell apoptosis [34]. In Figure 7 we show that rosehip extracts (all concentrations) do not induce PARP cleavage in neither the A-172, U-251MG nor U-1242MG cells (Figures 7(a)-(c)) when compared to control cells either cultured under serum starvation conditions or cultured in the presence of 10% FBS. Additionally, cells cultured with FBS (10%) and in the presence of U0126 and LY294002 do not display an increased amount of cleaved PARP (Figures 7(a)-(c)). However, A-172, U-251MG and U-1242MG cells treated with staurosporine (1 µM) demonstrate a drastic increase
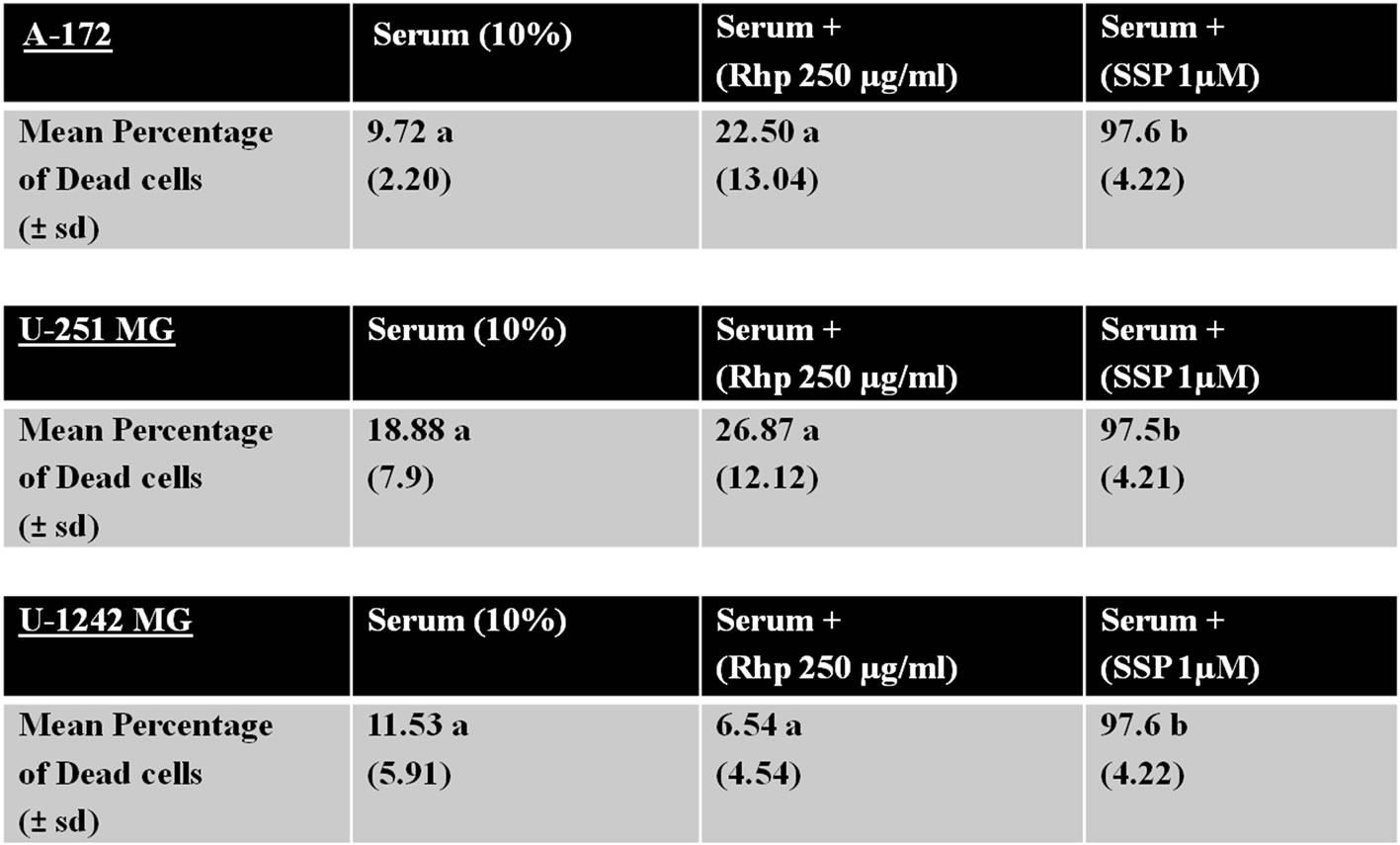
Figure 5. Rosehip extracts do not significantly increase the amount of cell death.
Effect of Rosehip Extract Concentration on Promoting Cell Death. A-172, U-251 MG and U-1242 MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. Each cell line was also exposed to staurosporine (1 μM) for 24 h (A-172 and U-251 MG cells) or 3 h (U-1242 MG cells) to promote cell death. A live/dead colorimetric assay was used to determine the number of dead cells. Mean percentages were calculated based on three independent experiments. The number in parenthesis represents the standard deviation. Values labeled with different letters are significantly different from one another (P < 0.01). Values labeled with the same letter are not significantly different from one another (P > 0.05).
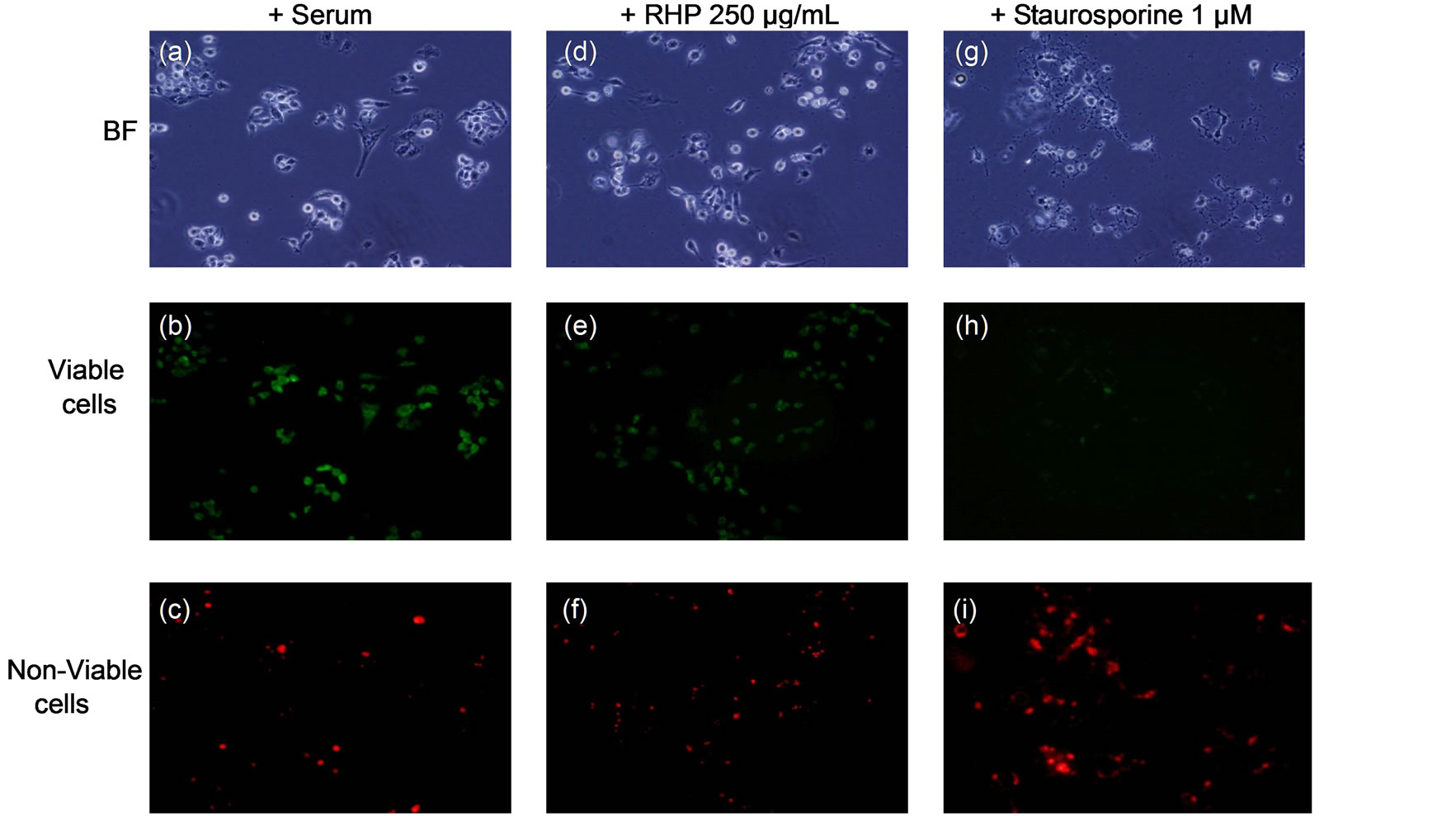
Figure 6. Rosehip extracts do not promote cell death.
Exposure to Rosehip extracts does not result in cell death. A-172 human GBM cell lines were treated with rosehip extracts for 48 h at 250 μg/mL. In addition to rosehip extracts, staurosporine (1 μM) was also used. The amount of cell death was determined using a Live/Dead colorimetircassay.
in the amount of cleaved PARP, demonstrating the affect of apoptosis on the cleavage state of PARP in these cell lines (Figures 7(a)-(c)). These data further emphasize that rosehip extracts do not promote apoptosis in the GBM cell lines assayed.
3.4. Rosehip Extracts Prevent Cell Cycle Completion in Human GBM Cell Lines
Since GBM cells treated with rosehip extracts had decreased rates of proliferation and were not undergoing
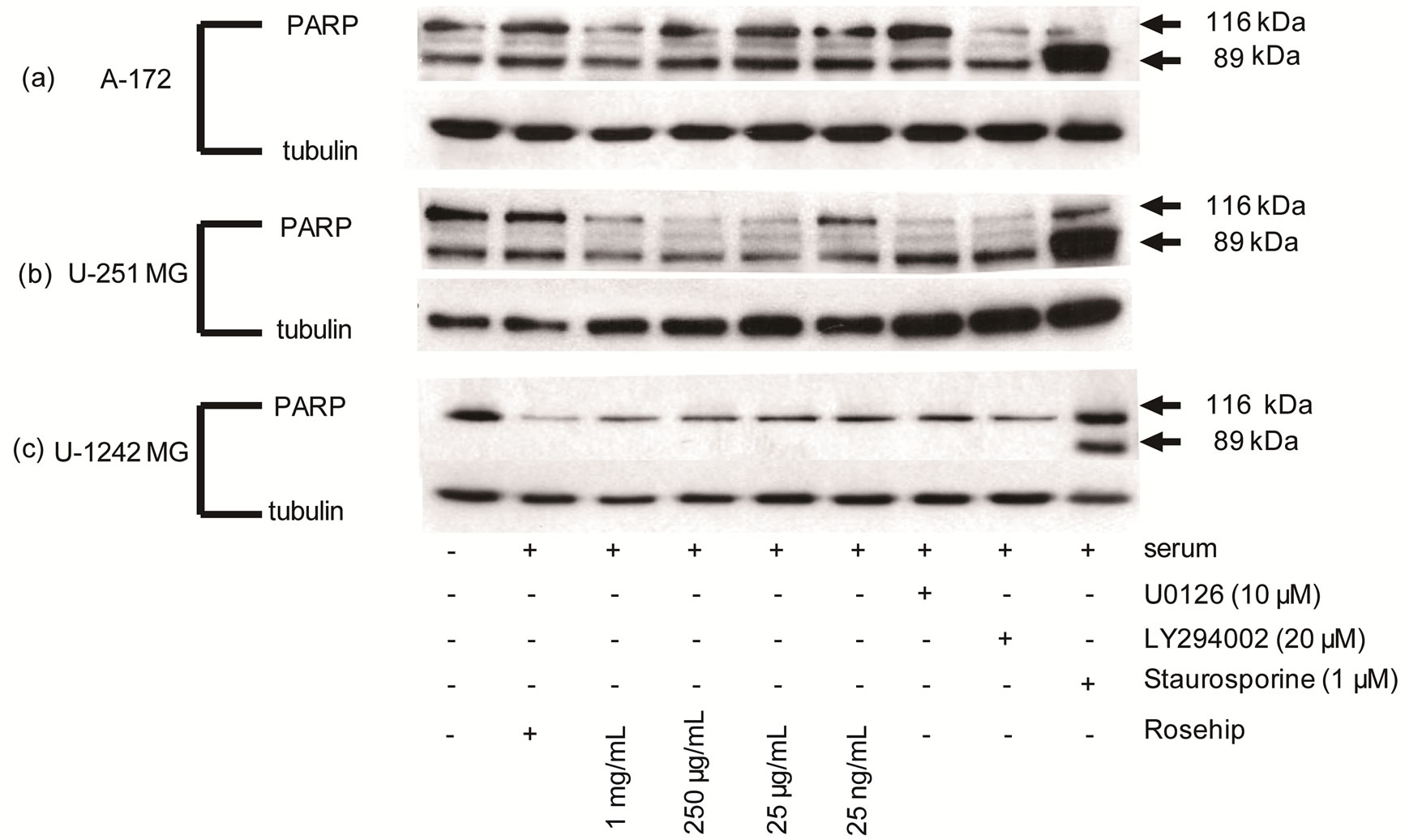
Figure 7. GBM cells exposed to rosehip extracts do not demonstrate an increased amount of PARP cleavgage.
Effect of Rosehip Extract Concentration on PARP Cleavage in Human Brain Tumor Cells. A-172, U-251 MG and U-1242 MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. Each cell line was also exposed to staurosporine (1 µM) for 24 h (A-172 and U-251 MG cells) or 3 h (U-1242 MG cells) to promote cell death and PARP cleavage. Cleaved PARP is represented by the lower band (89 kDA) and total PARP is represented by the upper band (116 kDA). (a) A-172 cells (b) U-251 MG cells and (c) U-1242 MG cells. Tubulin was used as a protein loading control.
cell death we investigated the mechanism by which these extracts were decreasing cell proliferation. Flow cytometry was used to analyze the cell cycle following exposure to rosehip extracts. A-172 cells treated with rosehip extracts at a high concentration (250 µg/mL) and a low concentration (25 µg/mL) show that cells exposed to rosehip extracts are unable to maintain a normal cell cycle (Figure 8). Specifically, rosehip extracts (both concentrations assayed) significantly prevented these cells from exiting the G0/G1 phase of the cell cycle (Figure 8). Cells treated with rosehip extracts have the same percentage of cells remaining in the G0/G1 phase of the cell cycle as serum-starved cells (Figure 8).
Since rosehip extracts were able to slow cells from exiting the G0/G1 phase of the cell cycle we determined the level of retinoblastoma (Rb) tumor suppressor protein phosphorylation (Ser 807/811) following exposure to rosehip extracts (1 mg/mL, 250 µg/mL, 25 µg/mL and 25 ng/mL). Rb phosphorylation by a CDK inactivates tumor suppressor (cell cycle inhibitory) function of Rb and promotes continuation of the cell cycle [35]. A-172 cells show a decrease of Rb phosphorylation at each concentration of rosehip extracts, whereas control serum-cultured (10% FBS) cells not exposed to rosehip extracts cells do not (Figure 9). U-251MG cells exhibit a lowered amount of Rb phosphorylation following exposure to higher concentrations of rosehip extracts (1mg/mL and
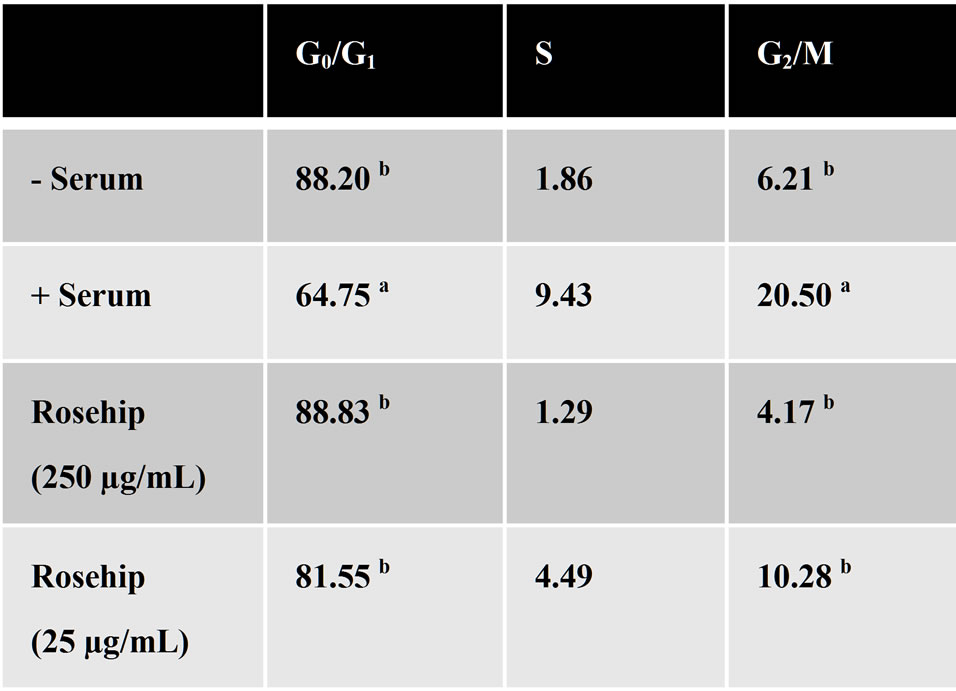
Figure 8. A-172 GBM cells treated with rosehip extracts halt the cell cycle in the G0/G1 phase.
Cell cycle modulation following exposure to rosehip extracts. A-172 cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. Cells were then fixed and stained with propidium iodide (1 mg/mL) to determine the percentage of cells in the representative portion of the cell cycle. The superscript letters (a, b) denoted significant difference between percentage values (a = P < 0.05); b = P < 0.01).
250 µg/mL); whereas U-1242MG cells only exhibited substantial decrease in Rb phosphorylation when exposed to the highest concentration of rosehip extracts (1 mg/mL) (Figure 9). These data demonstrate that rosehip extracts are able to slow the rate of cell proliferation in
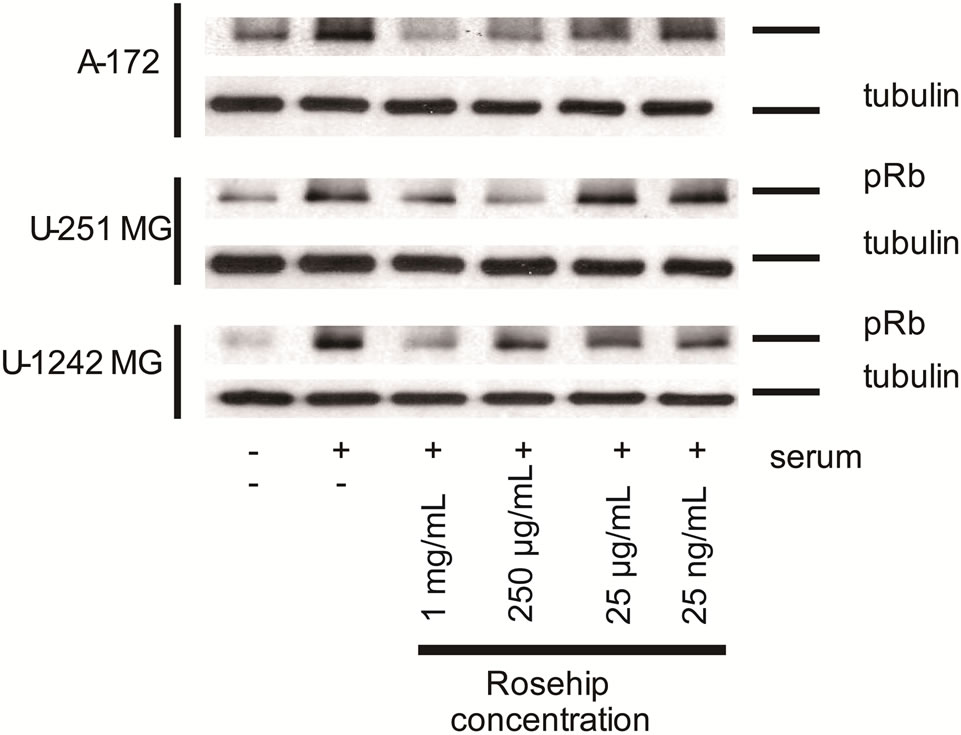
Figure 9. Rosehip extracts decrease the phosphorylation level of Retinoblastoma protein.
Effect of Rosehip Extract Concentration on Retinoblastoma Protein Phosphorylation in Human Brain Tumor Cells. A-172, U-251 MG and U-1242 MG cells were treated with rosehip extracts in the presence of 10% FBS at the concentrations indicated in the figure and incubated for 48 h. (top panel) U-251 MG cells and (middle panel) U-1242 MG cells (bottom panel) A172 cells. Tubulin was used as a protein loading control.
the GBM cell lines assayed by halting the cells in the G0/G1 phase of the cell cycle via an Rb-mediated mechanism.
3.5. Rosehip Extracts Have a Greater Anti-Proliferative Capacity than a Commonly Used Chemotherapeutic Agent
To better understand the chemotherapeutic potential of rosehip extracts against human GBM cell lines, we tested whether the antiproliferative capacity of rosehip extracts compared to that of Temozolomide (TMZ). TMZ (also known by the following brand names; Temodar, Temodal and Temcad) is one of the current first line chemotherapeutic options used in the treatment of GBMs. TMZ is an orally administered alkylating agent that can either halt the cell cycle and/or initiate cell death [36]. Using MTT assays we compared the rate of cell proliferation following exposure to either rosehip extract alone (250 µg/mL), TMZ (100 µM) alone or a combination of the rosehip extracts and TMZ [37]. We used rosehip extracts at a concentration of 250 µg/mL since our previous cell proliferation studies demonstrated that 250 µg/mL concentration of rosehip extracts significantly decreased cell proliferation (Figures 1(a)-(c)). Surprisingly, in all of the cell lines assayed, cells exposed to the rosehip extracts (250 µg/mL) demonstrated a greater decrease in cell proliferation when compared to cells exposed to TMZ (100 µM) (Figures 10(a)-(c)). These observed differences were statistically significant (P < 0.05) in A-172 and U-251 MG cell lines (Figures 10(a) and (b)). Additionally, in U-251MG cells, TMZ (100 µM) demonstrated no
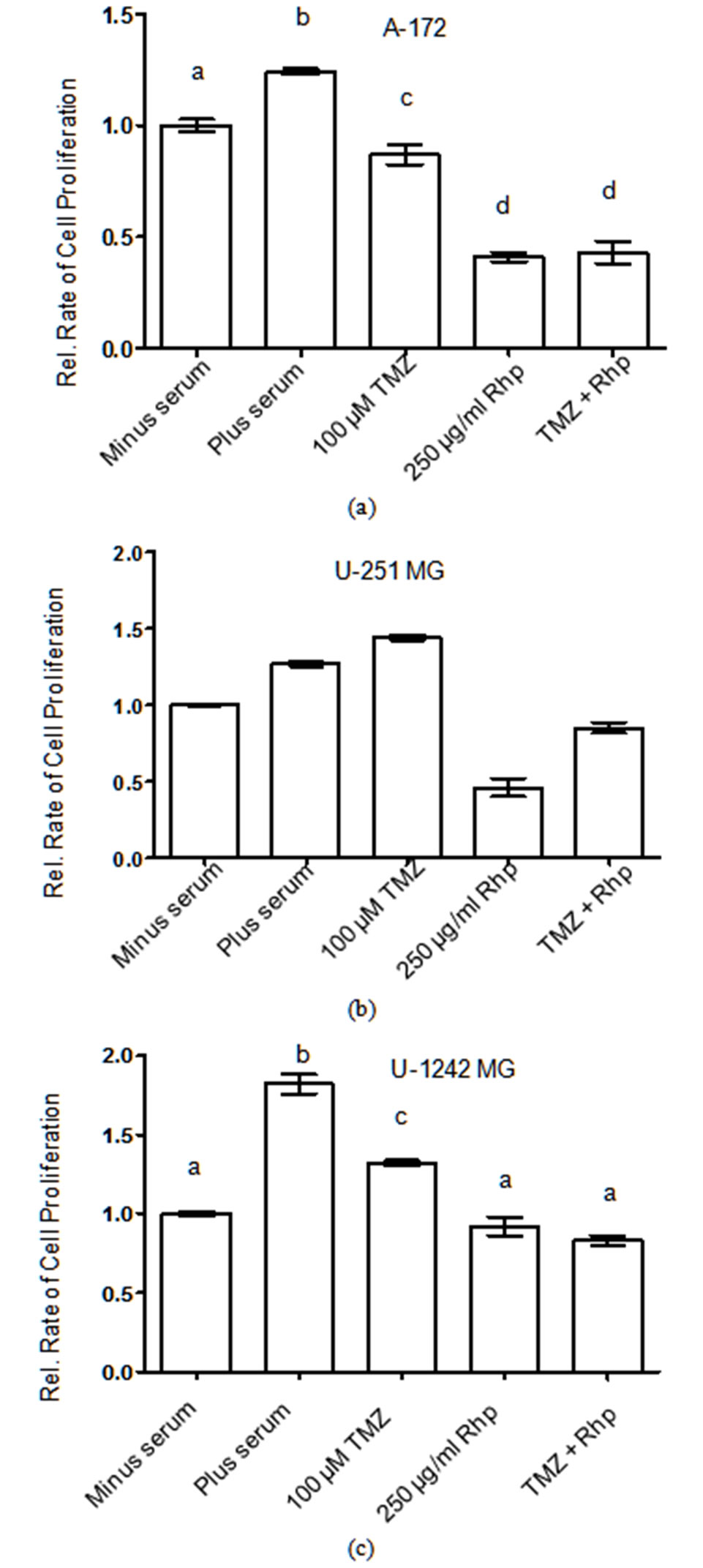
Figure 10. Rosehip extracts and temozolomide prevent brain tumor cell proliferation.
Effect of Rosehip Extract Concentration and Temozolomide on the Rate of Cell Proliferation in Human Brain Tumor Cells. A-172, U-251 MG and U-1242 MG cells were treated with rosehip extracts (250 µg/mL) in the presence of 10% FBS and/or TMZ (100 µM) as indicated in the figure and then incubated for 48 h. The rate of cell proliferation were determined as described in the materials and methods section. Each value is presented as the mean ± sem of three independent determinations. The columns in each graph are presented as relative values in comparison to serum-starved cells. Bars labeled with different letters are significantly different from one another (P < 0.05). Bars labeled with the same letter are not significantly different (P > 0.05).
inhibitory effect, whereas rosehip extracts (250 µg/mL) significantly decrease the rate of cell proliferation (Figure 10(b)). These assays also show that the combination of TMZ (100 µM) and rosehip extracts (250 µg/mL) do not synergize to decrease cell proliferation more than rosehip as a single agent (Figures 10(a)-(c)). These data demonstrate that rosehip extracts have a greater capacity to prevent in vitro GBM cell proliferation than a widely used chemotherapeutic treatment of GBMs.
4. Conclusions
The anti-cancer effects of rosehip extracts have been tested in various cancers; however, the cellular mechanisms that are affected by rosehip extracts are not clear. It has been suggested that the anti-cancer effects of rosehip extracts are related to the anti-oxidant and anti-proliferative effects of these extracts [18,19]. The present studies show that rosehip extracts prevent cell proliferation (Figure 1) and demonstrate the anti-proliferative characteristic of rosehip extracts as seen in other cancer cells [18,19]. Specifically these data show that rosehip extracts are able to prevent cell proliferation in GBM cells. The mechanistic studies presented in this study show that the anti-proliferative effects of rosehip extracts in GBM cells are associated with a decrease in AKT and MAPK signaling, which are different signaling mechanisms then observed in the anti-inflammatory and anticancer effects in previous studies [18,19]. More importantly, neither of the aforementioned studies assayed the anti-cancer effects of rosehip extracts on GBM cells nor did these studies directly test which cellular mechanisms were being negatively-regulated by the rosehip extracts. [18,19]. Our mechanistic studies (Figures 2-4) demonstrate a down regulation of Erk phosphorylation (Tyr 202/04) as well as Fra-1 protein levels a downstream target of active Erk and a regulator of gliomagenesis [29,30]. This inhibition shows that the MAPK cell signaling pathway is negatively regulated by rosehip extracts and correlates with the decrease in cell proliferative capacity of these cells following exposure to the extracts. In addition to the MAPK kinase pathway the rosehip extracts also exhibit the ability to decrease AKTmediated cell signaling. Specifically, AKT phosphorylation (ser 473), which is associated with GBM cell proliferation, survival and migration, is decreased when cells are grown in the presence of rosehip extracts (Figures 2-4). Data presented also shows that p70S6K kinase phosphorylation (Thr421/Ser424), which positively regulates glioma cell proliferation and resistance to drug therapy, is reduced after exposure to rosehip extracts (Figures 2-4) [21,24]. Together these data are the first to demonstrate that rosehip extract-mediated decrease of GBM cell proliferation is regulated by the AKT and MAPK cell regulatory mechanisms.
Tumbas and colleagues showed that a fraction of rosehip extracts containing quercetin was able to inhibit cell proliferation in Hela (cervical cancer), MCF7 (breast cancer) and HT-29 (colon cancer) cell lines [19]. Quercetin, a flavonoid, has been reported as one of the active ingredients that can be elucidated from rosehip extracts [12,13,38]. Purified quercetin has been tested against U-87MG, U-251 MG and A-172 cells [38]. These studies show that quercetin did not promote apoptosis unless it was accompanied by TRAIL; however, cell proliferation was not directly measured [38]. These data correlate with our data demonstrating that rosehip extracts do not promote GBM cell apoptosis (Figures 5-7). Specifically, in the cell lines tested, staurosporine was the only agent that induced cell death and PARP cleavage, while none of the rosehip concentrations tested increased the amount of cell death or PARP cleavage (Figures 5-7). In another report, quercetin demonstrated the ability to decrease U138MG, a human glioma cell line, cell proliferation and induce cell death via an arrest in the G2 checkpoint of the cell cycle [13]. Our result with rosehip extracts demonstrate an arrest at the G0/G1 checkpoint in the cell lines tested (Figures 8 and 9). This difference could be associated with the previously reported use of purified quercetin versus a crude extract of rosehip plants as described here [13]. It is possible that the amount of quercetin in the extract is at lower concentration levels and thus does not promote a G2 cell cycle arrest. Alternatively, it is plausible that another component of the extract may have a stronger arresting affect on the G0/G1 cell cycle checkpoint. Future studies in the laboratory will fractionate the crude rosehip extract and determine which active ingredient(s) are regulating the decrease in cell proliferation observed in our studies. Importantly, preliminary mass spectroscopy studies show that quercetin is not in the aqueous fraction of the extract (unpublished observations, PMM). Additionally, the level of retinoblastoma phosphorylation was decreased following exposure to rosehip extracts, demonstrating the cell cycle inhibitory capacity of these extracts. Decreased retinoblastoma phosphorylation has previously been observed in C6 glioma cells following exposure to caffeic acid phenethyl ester (CAPE) and natural product used by honey bees to construct their hives [39].
We extended our studies to investigate whether rosehip extracts could augment the efficacy of temozolomide, a chemotherapeutic agent used in the treatment of gliomas. Recent reports have shown that temozolomide killed brain tumor cells with greater efficiency when used in combination with epigallocatechin gallate (EGCG), a component of green tea or quercetin [12,40]. However, in our studies, rosehip extracts consistently demonstrated a greater capacity to reduce cell proliferation than temozolomide (Figure 10). Temozolomide in combination with rosehip extracts did not synergize and promote a greater reduction in cell proliferation (Figure 10).
Our studies indicate that rosehip extracts are able prevent cell proliferation in human glioblastoma cell lines without promoting apoptosis. The decreased cell proliferation observed following exposure to rosehip extracts correlates with the decreased phosphorylation of key protein kinases and proteins in both the AKT and MAPK cell proliferation regulatory mechanisms. Rosehip extracts prevent cell proliferation by stalling the cell cycle at the G0/G1 cell cycle checkpoint, but do not initiate apoptosis. Rosehip extracts also demonstrate a greater anti-proliferative potential than temozolomide, a chemotherapeutic commonly used in the treatment of gliomas. Taken together, our present data along with previous findings demonstrate an anti-oncogenic role for rosehip extracts in human glioblastoma cells.
5. Acknowledgements
This work was supported by the National Science Foundation (NSF# 1038160, O. Idassi) and The National institutes of Health Research Initiative for Scientific Enhancement (RISE) (NIH-NIGMS R25GM076162, J. Carpenter). Patrick Martin was supported by the National Institutes of Health (NIH-NCI 1P20CA138020-01 & NIH-NIGMS 5P20MD000546-07).
REFERENCES
- S. A. Grossman and J. F. Batara, “Current Management of Glioblastoma Multiforme,” Seminars in Oncology, Vol. 31, No. 5, 2004, pp. 635-644. doi:10.1053/j.seminoncol.2004.07.005
- D. N. Louis, H. Ohgaki, O. D. Wiestler, W. K. Cavenee, P. C. Burger, A. Jouvet, B. W. Scheithauer and P. Kleihues, “The 2007 WHO Classification of Tumours of the Central Nervous System,” Acta Neuropathologica, Vol. 114, No. 2, 2007, pp. 97-109. doi:10.1007/s00401-007-0243-4
- L. Barros, A. M. Carvalho and I. C. F. R. Ferreira, “Exotic Fruits as a Source of Important Phytochemicals: Improving the Traditional Use of Rosa Canina Fruits in Portugal,” Food Research International, Vol. 44, No. 7, 2011, pp. 2233-2236. doi:10.1016/j.foodres.2010.10.005
- T. Fujii and M. Saito, “Inhibitory Effect of Quercetin Isolated from Rose Hip (Rosa canina L.) against Melanogenesis by Mouse Melanoma Cells,” Bioscience Biotechnology & Biochemistry, Vol. 73, No. 9, 2009, pp. 1989- 1993. doi:10.1271/bbb.90181
- A. Kornienko and A. Evidente, “Chemistry, Biology, and Medicinal Potential of Narciclasine and Its Congeners,” Chemical Reviews, Vol. 108, No. 6, 2008, pp. 1982-2014.
- C. E. Ulbricht and W. Chao, “Phytochemicals in the Oncology Setting,” Current Treatment Options in Oncology, Vol. 11, No. 3-4, 2010, pp. 95-106.
- G. Van Goietsenoven, V. Mathieu, F. Lefranc, A. Kornienko, A. Evidente and R. Kiss, “Narciclasine as Well as Other Amaryllidaceae Isocarbostyrils Are Promising GTPase Targeting Agents against Brain Cancers,” Medicinal Research Reviews, 2012, pp. 1-17 (in press).
- M. C. Perry, M. Demeule, A. Regina, R. Moumdjian and R. Beliveau, “Curcumin Inhibits Tumor Growth and Angiogenesis in Glioblastoma Xenografts,” Molecular Nutrition & Food Research, Vol. 54, No. 8, 2010, pp. 1192- 1201.
- T. J. Turbyville, D. B. Gursel, R. G. Tuskan, J. C. Walrath, C. A. Lipschultz, S. J. Lockett, D. F. Wiemer, J. A. Beutler and K. M. Reilly, “Schweinfurthin A Selectively Inhibits Proliferation and Rho Signaling in Glioma and Neurofibromatosis Type 1 Tumor Cells in a NF1-GRDDependent Manner,” Molecular Nutrition & Food Research, Vol. 9, No. 5, 2010, pp. 1234-1243.
- E. C. Filippi-Chiela, E. S. Villodre, L. L. Zamin and G. Lenz, “Autophagy Interplay with Apoptosis and Cell Cycle Regulation in the Growth Inhibiting Effect of Resveratrol in Glioma Cells,” PLoS ONE, Vol. 6, No. 6, 2011, p. e20849. doi:10.1371/journal.pone.0020849
- N. Gagliano, G. Aldini, G. Colombo, R. Rossi, R. Colombo, M. Gioia, A. Milzaniand I. Dalle-Donne, “The Potential of Resveratrol against Human Gliomas,” Anticancer Drugs, Vol. 21, No. 2, 2010, pp. 140-150. doi:10.1097/CAD.0b013e32833498f1
- J. Jakubowicz-Gil, E. Langner, I. Wertel, T. Piersiak and W. Rzeski, “Temozolomide, Quercetin and Cell Death in the MOGGCCM Astrocytoma Cell Line” Chemico-Biological Interactions, Vol. 188, No. 1, 2010, pp. 190-203. doi:10.1016/j.cbi.2010.07.015
- E. Braganhol, L. L. Zamin, A. D. Canedo, F. Horn, A. S. Tamajusuku, M. R. Wink, C. Salbego and A. M. Battastini, “Antiproliferative Effect of Quercetin in the Human U138MG Glioma Cell Line,” Anticancer Drugs, Vol. 17, No. 6, 2006, pp. 663-671. doi:10.1097/01.cad.0000215063.23932.02
- L. Su, J. J. Yin, D. Charles, K. Q. Zhou, J. Moore and L. L. Yu, “Total Phenolic Contents, Chelating Capacities, and Radical-Scavenging Properties of Black Peppercorn, Nutmeg, Rosehip, Cinnamon and Oregano Leaf,” Food Chemistry, Vol. 100, No. 3, 2007, pp. 990-997.
- E. Rein, A. Kharami and K. Winther, “A Herbal Remedy, Hyben Vital (Stand. Powder of a Subspecies of Rosa Canina Fruits), Reduces Pain and Improves General Wellbeing in Patients with Osteoarthritis—A DoubleBlind, Placebo-Controlled, Randomised Trial,” Phytomedicine, Vol. 11, No. 5, 2004, pp. 383-391. doi:10.1016/j.phymed.2004.01.001
- E. Rein, A. Kharazmi, G. Thamsbprg and K. Winther, “A Herbal Remedy, Made from a Subspecies of Rosehip Rosa Canina, Reduces Symptoms of Knee and Hip Osteoarthritis,” Osteoarthritis and Cartilage, Vol. 12, Suppl. 2, 2004, pp. S80-S80.
- O. Warholm, S. Skaar, E. Hedman, H. M. Molmen and L. Eik, “The Effects of a Standardized Herbal Remedy Made from a Subtype of Rosa canina in Patients with Osteoarthritis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial,” Current Therapeutic ResearchClinical and Experimental, Vol. 64, No. 1, 2003, pp. 21- 31. doi:10.1016/S0011-393X(03)00004-3
- M. E. Olsson, K. E. Gustavsson, S. Andersson, A. Nilsson and R. D. Duan, “Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels,” Chemico-Biological Interactions, Vol. 52, No. 24, 2004, pp. 7264-71.
- V. T. Tumbas, J. M. Canadanovic-Brunet, D. D. Cetojevic-Simin, G. S. Cetkovic, S. M. Ethilas and L. Gille, “Effect of Rosehip (Rosa canina L.) Phytochemicals on Stable Free Radicals and Human Cancer Cells,” ChemicoBiological Interactions, Vol. 92, No. 6, 2012, pp. 1273- 1281.
- H. Ohgaki, “Genetic Pathways to Glioblastomas,” Neuropathology, Vol. 25, No. 1, 2005, pp. 1-7. doi:10.1111/j.1440-1789.2004.00600.x
- S. E. Aeder, P. M. Martin, J. W. Soh and I. M. Hussaini, “PKC-eta Mediates Glioblastoma Cell Proliferation through the Akt and mTOR Signaling Pathways,” Oncogene, Vol. 23, No. 56, 2004, pp. 9062-9069. doi:10.1038/sj.onc.1208093
- S. Brader and S. A. Eccles, “Phosphoinositide 3-Kinase Signalling Pathways in Tumor Progression, Invasion and Angiogenesis,” Tumori, Vol. 90, No. 1, 2004, pp. 2-8.
- A. B. Lassman, “Molecular Biology of Gliomas,” Curr Neurol Neurosci Rep, Vol. 4, No. 3, 2004, pp. 228-233. doi:10.1007/s11910-004-0043-3
- P. M. Martin, S. E. Aeder, C. A. Chrestensen, T. W. Sturgill and I. M. Hussaini, “Phorbol 12-Myristate 13-Acetate and Serum Synergize to Promote Rapamycin-Insensitive Cell Proliferation via Protein Kinase C-Eta,” Oncogene, Vol. 26, No. 3, 2007, pp. 407-414. doi:10.1038/sj.onc.1209791
- P. S. Mischel and T. F. Cloughesy, “Targeted Molecular Therapy of GBM,” Brain Pathology, Vol. 13, No. 1, 2003, pp. 52-61. doi:10.1111/j.1750-3639.2003.tb00006.x
- H. Jiang, X. Shang, H. Wu, S. C. Gautam, S. Al-Holou, C. Li, J. Kuo, L. Zhang and M. Chopp, “Resveratrol DownRegulates PI3K/Akt/mTOR Signaling Pathways in Human U251 Glioma Cells,” Journal of Experimental Therapeutics and Oncology, Vol. 8, No. 1, 2009, pp. 25- 33.
- P. Pu, C. Kang, J. Li, H. Jiang and J. Cheng, “The Effects of Antisense AKT2 RNA on the Inhibition of Malignant Glioma Cell Growth in Vitro and in Vivo,” Journal of Neuro-Oncology, Vol. 76, No. 1, 2006, pp. 1-11. doi:10.1007/s11060-005-3029-3
- P. Pu, C. Kang, Z. Zhang, X. Liu and H. Jiang, “Downregulation of PIK3CB by siRNA Suppresses Malignant Glioma Cell Growth in Vitro and in Vivo,” Technology in Cancer Research and Treatment, Vol. 5, No. 3, 2006, pp. 271-280.
- W. Debinski and D. M. Gibo, “Fos-Related Antigen 1 (Fra-1) Pairing with and Transactivation of JunB in GBM Cells,” Cancer Biology & Therapy, Vol. 11, No. 2, 2011, pp. 254-262. doi:10.4161/cbt.11.2.13953
- W. Debinski and D. M. Gibo, “Fos-Related Antigen 1 Modulates Malignant Features of Glioma Cells,” Molecular Cancer Research, Vol. 3, No. 4, 2005, pp. 237- 249.
- S. F. Rosenberger, J. S. Finch, A. Gupta and G. T. Bowden, “Extracellular Signal-Regulated Kinase 1/2-Mediated Phosphorylation of JunD and FosB Is Required for Okadaic Acid-Induced Activator Protein 1 Activation,” The Journal of Biological Chemistry, Vol. 274, No. 2, 1999, pp. 1124-1130. doi:10.1074/jbc.274.2.1124
- J. Basbous, D. Chalbos, R. Hipskind, I. Jariel-Encontre and M. Piechaczyk, “Ubiquitin-Independent Proteasomal Degradation of Fra-1 is Antagonized by Erk1/2 PathwayMediated Phosphorylation of a Unique C-Terminal Destabilizer,” Molecular and Cellular Biology, Vol. 27, No. 11, 2007, pp. 3936-50. doi:10.1128/MCB.01776-06
- T. Sasaki, H. Kojima, R. Kishimoto, A. Ikeda, H. Kunimoto and K. Nakajima, “Spatiotemporal Regulation of c-Fos by ERK5 and the E3 Ubiquitin Ligase UBR1, and Its Biological Role,” Molecular Cell, Vol. 24, No. 1, 2006, pp. 63-75. doi:10.1016/j.molcel.2006.08.005
- F. J. Oliver, G. de la Rubia, V. Rolli, M. C. Ruiz-Ruiz, G. de Murcia and J. M. Murcia, “Importance of Poly(ADPRibose) Polymerase and Its Cleavage in Apoptosis. Lesson from an Uncleavable Mutant,” Journal of Biological Chemistry, Vol. 273, No. 50, 1998, pp. 33533-33539. doi:10.1074/jbc.273.50.33533
- E. S. Knudsen and J. Y. Wang, “Dual Mechanisms for the Inhibition of E2F Binding to RB by Cyclin-Dependent Kinase-Mediated RB Phosphorylation,” Molecular and Cellular Biology, Vol. 17, No. 10, 1997, pp. 5771-583.
- E. S. Newlands, M. F. Stevens, S. R. Wedge, R. T. Wheelhouse and C. Brock, “Temozolomide: A Review of Its Discovery, Chemical Properties, Pre-Clinical Development and Clinical Trials,” Cancer Treatment Reviews, Vol. 23, No. 1, 1997, pp. 35-61. doi:10.1016/S0305-7372(97)90019-0
- Y. Hirose, M. S. Berger and R. O. Pieper, “p53 Effects both the Duration of G2/M Arrest and the Fate of Temozolomide-Treated Human Glioblastoma Cells,” Cancer Research, Vol. 61, No. 5, 2001, pp. 1957-1963.
- M. D. Siegelin, D. E. Reuss, A. Habel, A. Rami and A. von Deimling, “Quercetin Promotes Degradation of Survivin and Thereby Enhances Death-Receptor-Mediated Apoptosis in Glioma Cells,” Neuro-Oncology, Vol. 11, No. 2, 2009, pp. 122-131. doi:10.1215/15228517-2008-085
- H. C. Kuo, W. H. Kuo, Y. J. Lee, W. L. Lin, F. P. Chou and T. H. Tseng, “Inhibitory Effect of Caffeic Acid Phenethyl Ester on the Growth of C6 Glioma Cells in Vitro and in Vivo,” Cancer Letters, Vol. 234, No. 2, 2006, pp. 199-208. doi:10.1016/j.canlet.2005.03.046
- P. Pyrko, A. Kardosh, W. Wang, W. Xiong, A. H. Schonthal and T. C. Chen, “HIV-1 Protease Inhibitors Nelfinavir and Atazanavir Induce Malignant Glioma Death by Triggering Endoplasmic Reticulum Stress,” Cancer Research, Vol. 67, No. 22, 2007, pp. 10920- 10928. doi:10.1158/0008-5472.CAN-07-0796

