Health
Vol.5 No.11(2013), Article ID:39461,7 pages DOI:10.4236/health.2013.511242
Blood glucose response to aerobic exercise training programme among patients with type 2 diabetes mellitus at the University of Nigeria Teaching Hospital, Enugu South-East, Nigeria*
![]()
1Department of Medical Rehabilitation, Faculty of Health Sciences and Technology, University of Nigeria, Enugu Campus, Enugu, Nigeria
2Department of Medical Rehabilitation, University of Nigeria, Enugu Campus, Enugu, Nigeria
3Biomedical Technology Department, School of Health Technology, Federal University of Technology, Owerri, Nigeria; #Corresponding Author: siklam_86@yahoo.co.uk
4Physiotherapy Department, University of Nigeria Teaching Hospital, Ituku Ozalla, Nigeria
5Department of Medical Rehabilitation, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Awka, Nigeria
6Nursing Department, University of Nigeria Teaching Hospital, Ituku Ozalla, Nigeria
Copyright © 2013 Charles Ikechukwu Ezema et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 28 August 2013; revised 28 September 2013; accepted 19 October 2013
Keywords: Type 2 Diabetes Mellitus; Blood Glucose; Aerobic Exercise
ABSTRACT
Background and Objective: Control of all types of diabetes involves maintaining normal or near normal blood glucose levels through the appropriate therapy: insulin, oral hypoglycaemic agents, diet, and exercise. The aim of this study was to investigate the blood glucose response to aerobic exercise training among subjects with type 2 diabetes mellitus at University of Nigeria Teaching Hospital (UNTH), Enugu, Nigeria. Methods: Age matched randomized controlled trial design was used; subjects with diagnosis of type 2 diabetes mellitus attending the diabetes clinic of the UNTH participated in the study. Fifty four subjects (N = 54) with type 2 diabetes mellitus (fasting blood sugar [FBS] of between 110 & 225 mg/dl) were age matched and randomized to two groups: exercise (n = 30) and control (n = 24) groups. The exercise group involved in an 8 weeks continuous training (60% - 79% HR max) of between 45 and 60 min, 3 times per week, while the control group remained sedentary. SBP, DBP, VO2max and FBS were assessed. Analysis of co-variance and Pearson correlation tests were used in data analysis. Results: Findings of the study revealed a significant effect of exercise training program on, SBP, DBP, FBS and VO2max at p < 0.05. Changes in VO2max significantly and negatively correlated with changes in FBS (r = −0.266) at p < 0.01. Conclusion: It was concluded that aerobic exercise programme is an effective adjunct in controlling blood glucose level among type 2 diabetic subjects.
1. INTRODUCTION
The etiology of type 2 diabetes mellitus (T2DM) is unknown, but several studies indicate that the disease results from a combination of genetic susceptibility and external risk factors [1,2]. According to this multifactorial model, genetically predisposed subjects will not necessarily develop overt disease unless they are also exposed to particular environmental factors [1,3]. Important risk factors for the development of type 2 diabetes mellitus, apart from obesity, include a family history of diabetes, increased age, hypertension, lack of physical exercise, and ethnic background [1,2].
World Health Organisation (WHO) data in 2000 showed that approximately 117 million people had diabetes mellitus (DM) worldwide and it was postulated that this number might rise to 370 million by the year 2030 [4]. The WHO report also revealed that a larger percentage of this increase will occur in developing countries especially Africa due to population growth, ageing, unhealthy diets, obesity and sedentary lifestyle [4]. Treatment of diabetes includes a combination of exercise, proper diet, medication, and daily self-care [5]. Aerobic exercise has consistently been shown to improve glucose control, enhance insulin sensitivity, and improve cardiovascular risk factors such as visceral adiposity, lipid profile, arterial stiffness, and endothelial function [6].
Consistent with all the evidences on the benefits of aerobic exercise, the American Diabetes Association (ADA) recommends that individuals with type 2 DM (T2DM) perform at least 150 min of moderate-intensity aerobic exercise and/or at least 90 min of vigorous aerobic exercise per week [7]. Although a lifestyle modification of this nature could have substantial impact on the metabolic and cardiovascular health of this population. [6].
Most of these studies investigating diabetes and associated factors are conducted using white, caucasian and other non pure black African subjects. However, studies [2,8-10] have shown interracial, interpersonal and ethnic variation in susceptibility of T2DM. It has also been reported that genetics plays a major role in a person’s VO2max and that heredity accounts for up to 25% - 50% of the variance seen between individuals [11]. It is also unclear whether genetic and associated factors could affect response to exercise in T2DM subjects of pure black (Nigeria) African origin. Therefore, the purpose of the present study was to investigate the effect of the continuous training program on blood pressure and fasting blood sugar (FBS) of pure black African subjects with T2DM.
2. MATERIALS AND METHODS
2.1. Subjects
The subjects comprised of 54 (27 male and 27 female) adults with diagnosis of type 2 diabetes mellitus who were attending diabetes clinic of University of Nigeria Teaching Hospital [UNTH], Enugu. Their age ranged between 40 and 55 years. Subject were fully informed about the experimental procedures, risk and protocol, after which they gave their informed consent in accordance with the American College of Sports Medicine guidelines (ACSM) [12], regarding the use of human subjects as recommended by the human subject protocol. Ethical approval was granted by the research and ethics committee of the UNTH, Enugu.
2.2. Research Design
In the present study, age matched randomized independent groups design was used to determine the influence of the continuous training program on cardiovascular parameters and FBS. All procedures were conducted at the Medical Rehabilitation department of University of Nigeria, Enugu Campus (UNEC), Enugu, Nigeria. Subjects’ ages were arranged in ascending order (50 to 70 years) and then assigned to exercise and control groups in an alternating pattern (age-matched). The exercise group involved in a continuous training program for 8 weeks; while the control group remained sedentary during the period. At the end of the training and sedentary period a post-test procedure was administered to all subjects.
2.3. Inclusion Criteria
Only those who volunteered to participate in the study were recruited, they were all stable, without any cardiac complications. Their blood pressure (BP < 140/90) was within normal range. Only those treated with or on diet and/or oral agents were recruited. They were sedentary and have no history of psychiatry or psychological disorders or abnormalities.
2.4. Exclusion Criteria
Obese or underweight (BMI between 20 and 30 kg/m2), smokers, alcoholic, uncontrolled hyperglycemia (>250 mg/dl) and hypertension (Resting BP > 200/115) other cardiac, renal, respiratory disease and subjects on insulin therapy were excluded. Those involved in vigorous physical activities and above averagely physically fit were also excluded.
A total of 61 type 2 DM subjects satisfied the necessary study criteria. Subjects were aged matched and randomly grouped into experimental (31) and control (30) groups.
2.5. Pre-Training Procedure
Physiological Measurement: Subjects resting heart rate (HR), SBP, and DBP were monitored from the right arm as described by Musa et al. [13] using an automated digital electronic BP monitor (Omron digital BP monitor, Model 11 EM 403c; Tokyo Japan).
Anthropometric Measurement: Subjects’ physical characteristics (weight [kg] and height [m]) and body composition (body mass index [BMI] (kg/m2)) assessment was done in accordance with standardized anthropometric protocol [14].
Fasting Plasma Blood Glucose: Subject’s pre and post training fasting plasma glucose level was measured using the Accucheck glucometer before the exercise training and after the 8 weeks exercise training under the supervision of a medical laboratory scientist from the Medical Laboratory Science Department of UNTH, Enugu.
Stress Test: The Young Men Christian Association (YMCA) sub-maximal cycle ergometry test protocol was used to assess subject’s aerobic power as described by ACSM [15], Golding et al. [16]. The YMCA protocol uses two to four 3-minutes stages of continuous exercise. Two HR-power output data points was needed (two steady state HR) of between 110 and 150 beat/min. The bicycle seat height was adjusted and the subjects’ knee slightly flexed when the pedal was in the down position. Exercise test started with a 2 to 3 minutes warm up at zero resistance in order to acquaint the subjects with the cycle ergometer. According to Brook et al. [17]; Pollock and Wilmore [18] middle aged, less fit, cardiac patient generally begins at 100 or 150 to 300 kg·m·min−1 (17 w or 25 w to 50 w respectively) with power increments of 5 - 25 watts per stage.
The first 3-minutes work rate was set between 100 and 150 kgm (17 - 25 watts), (1 watt = 6 kg·m·min−1). The pedal speed was set at 50 rpm (revolutions per minute) by setting the metronome at 100 bpm (beats per minute), HR was measured within the last minute of each stage. When an HR of above 110 bpm was obtained in the first 3 minutes, then only one additional 3 minute stage was performed by increasing the workload by either 30 or 150 kgm. If the second stage HR was less than 110 bpm, then 3rd or 4th stage 3 minutes was performed at additional workload of 30 or 150 kgm up to 300 kgm in order to obtained two HR between 110 and 150 bpm. These two HR should not differ by more than 5 bpm, when they did, the test was extended by another minute or until a stable value was obtained. At the end of the test, another 2 to 3 minutes recovery period (cool down) at zero resistance pedaling was administered.
The two steady state HR was plotted against the respective workload on the YMCA graph sheet. A straight line was draw through the two points and extended to the subjects predicted maximum HR (220-Age). The point at which the diagonal line intersects the horizontal (predicted HR max) line represents the maximal working capacity for the subject (HR max). A perpendicular line was dropped from this point to the baseline where the maximal physical workload capacity was read in kg·m·min−1, which was used to predict the subjects VO2max. This procedure was done for both pre and posttest stress test [19].
2.6. Training Procedure
Exercise group (group 1): After a 10-minutes warm up (pedaling at zero resistance), subjects in the exercise group exercised on a bicycle ergometer at a moderate intensity of between 60% - 79% of their HR max [19,20]. The starting workload was 100 kgm (17 watts) which was increased at a pedal speed of 50 rpm to obtain a HR max 60% was increased in the first two weeks to and level up at 79% HR max throughout the remaining part of the training period. The initial of exercise session was increased from 45 minutes in the first two weeks of training to and levelled up at 60 minutes throughout the remaining part of the training. After each training session, 10 minutes cool down was established by pedalling at zero resistance. Exercise session of three times per week was also maintained throughout the 8 weeks training period.
The control group (group 2): Subjects in the control group were instructed not to undertake any organized/ structured physical activity apart from the activity of daily living during the 8 weeks period of study.
2.7. Post Training Procedure
Post training SBP, DBP, VO2max, stress test and FBS were conducted as earlier described in the pretest procedures using standardized protocols, techniques and methods. All preand post-test measurements were recorded on a data sheet.
Fifty four subjects (30 from continuous, and 24 from control group) completed the eight weeks training program. Seven subjects (1 from exercise, and 6 from control group) had dropped out because of noncompliance and incomplete data; therefore, the data of 54 subjects were used in the statistical analysis (Figure 1).
Statistical Analysis: Following data collection, the measured variables were statistically analyzed. The descriptive statistics (Means & standard deviations) of the subjects physical characteristics, blood pressure, VO2 max and FBS were determined. Analysis of covariance (ANCOVA) was used to assess treatment outcomes. In the ANCOVA, the post-test values were the outcome variables and baseline characteristics that as covariates. Pearson product moment correlation test was used to determine the relationship between the estimated changes in VO2max (changed score) and FBS. All statistical analysis was performed using the statistical package for the social science (SPSS), (Version 16.0 Chicago IL, USA). The probability level for all the above tests was set at 0.05 to indicate significance.
3. RESULTS
The subject’s Mean ± SD was 47.35 ± 4.55; age ranged between 40 and 55 years. Mean (SD) age and body mass index (BMI) of subjects in exercise group were 47.53 (4.68) years, and 22.48 (2.89) kgm−2 respec-
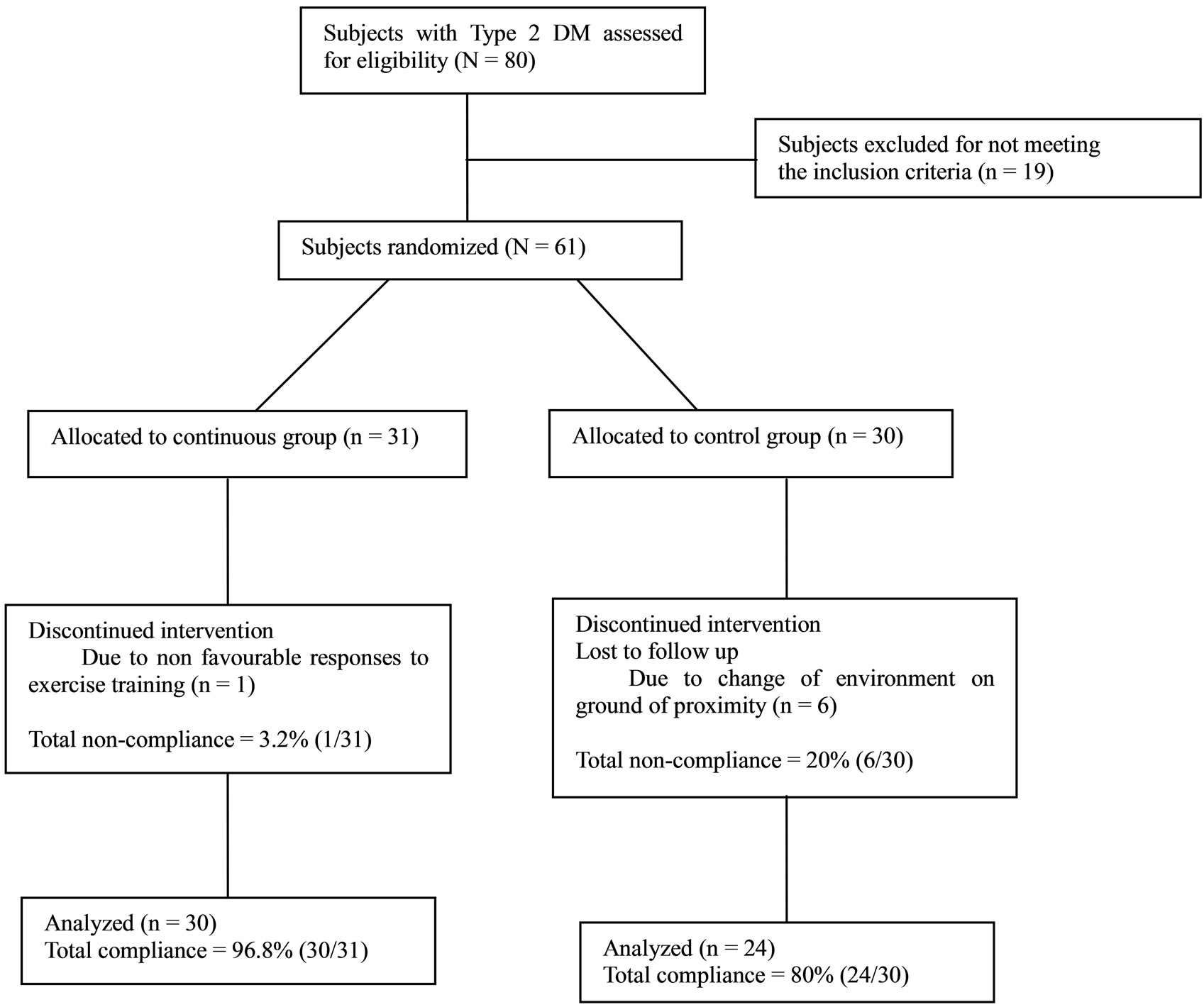
Figure 1. Study design flow chart.
tively, while for the control group mean (SD) age and BMI were 47.13 (4.48) years and 23.37 (5.31) kgm−2 respectively. Detailed physical characteristics’ of subjects are depicted in Table 1.
ANCOVA tests and groups’ pre and post treatment mean BP (SD) mmHg; FBS (mg/dl) and VO2max (ml kg−1·min−1) are depicted in Table 2. ANCOVA analysis indicated significant difference in groups’ pre and post treatment SBP (F = 31.377, p = 0.000) DBP (F = 9.004, p = 0.007) FBS (F = 26.597, p = 0.000) and VO2max (F = 6.643, p = 0.013).
There was a significant negative correlation between changes between VO2max and changes in FBS (r = −0.266) at p < 0.01 (Figure 2).
4. DISCUSSION
Findings from the present study revealed a significant decrease in SBP, DBP, FBS and increase in VO2max in the exercise group over control group. Also, result of the present study indicated a significant negative correlation between changes in VO2max and changes in FBS. The favourable changes resulting from aerobic training in both SBP and DBP demonstrated in the present study is
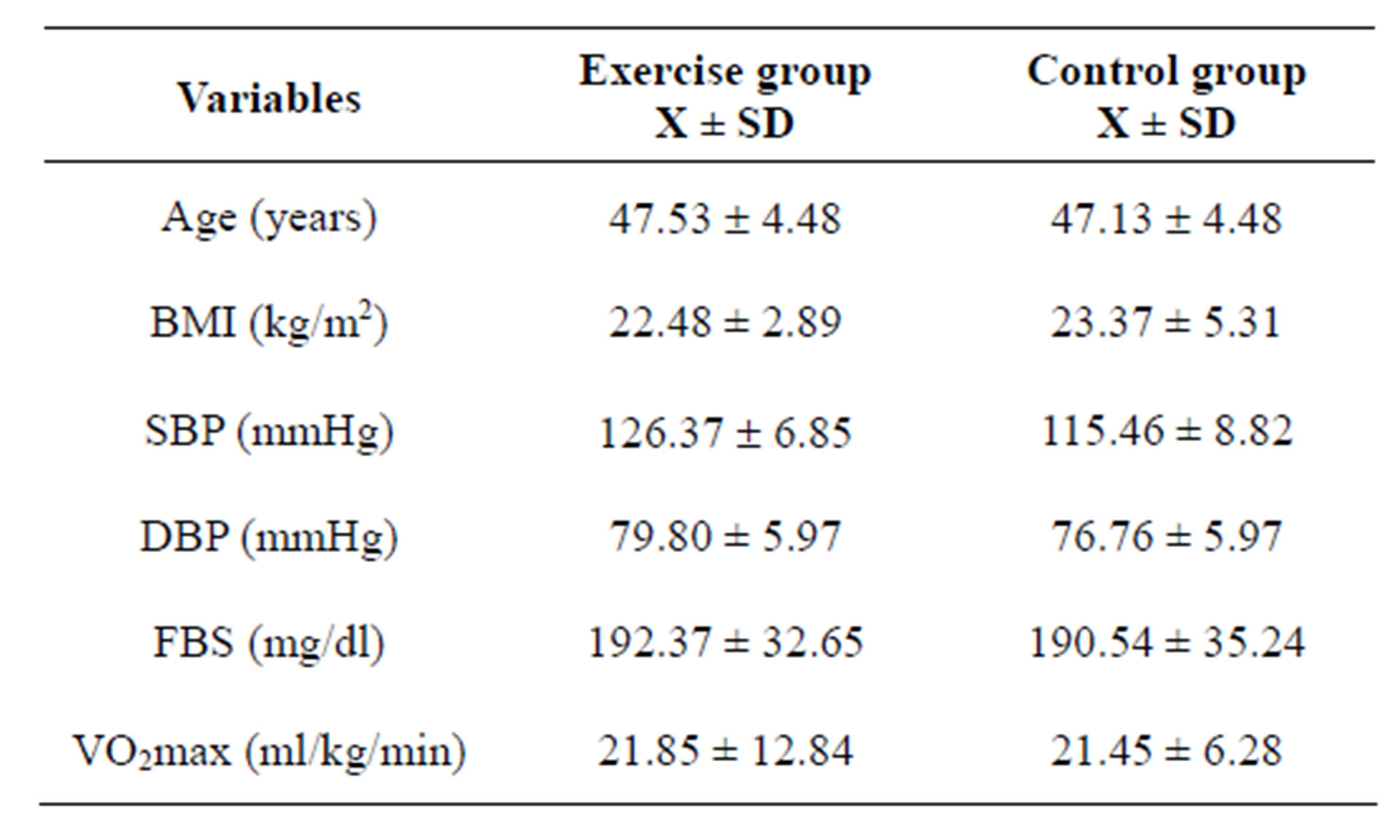
Table 1. Groups mean ± SD values for baseline and physical characteristics (N = 54).
consistent with several other studies [20-23].
A similar study was conducted by Kadoglou et al. [24] in 2007. They investigated the effect of aerobic exercise training on glucose control in diabetes mellitus. A total of 60 overweight individuals with type 2 DM, but without vascular complications, were randomly assigned to either a 6-month aerobic exercise training programme (four times/week, 45 - 60 min/session), designated as exercise group, or to the control group. All participants were on
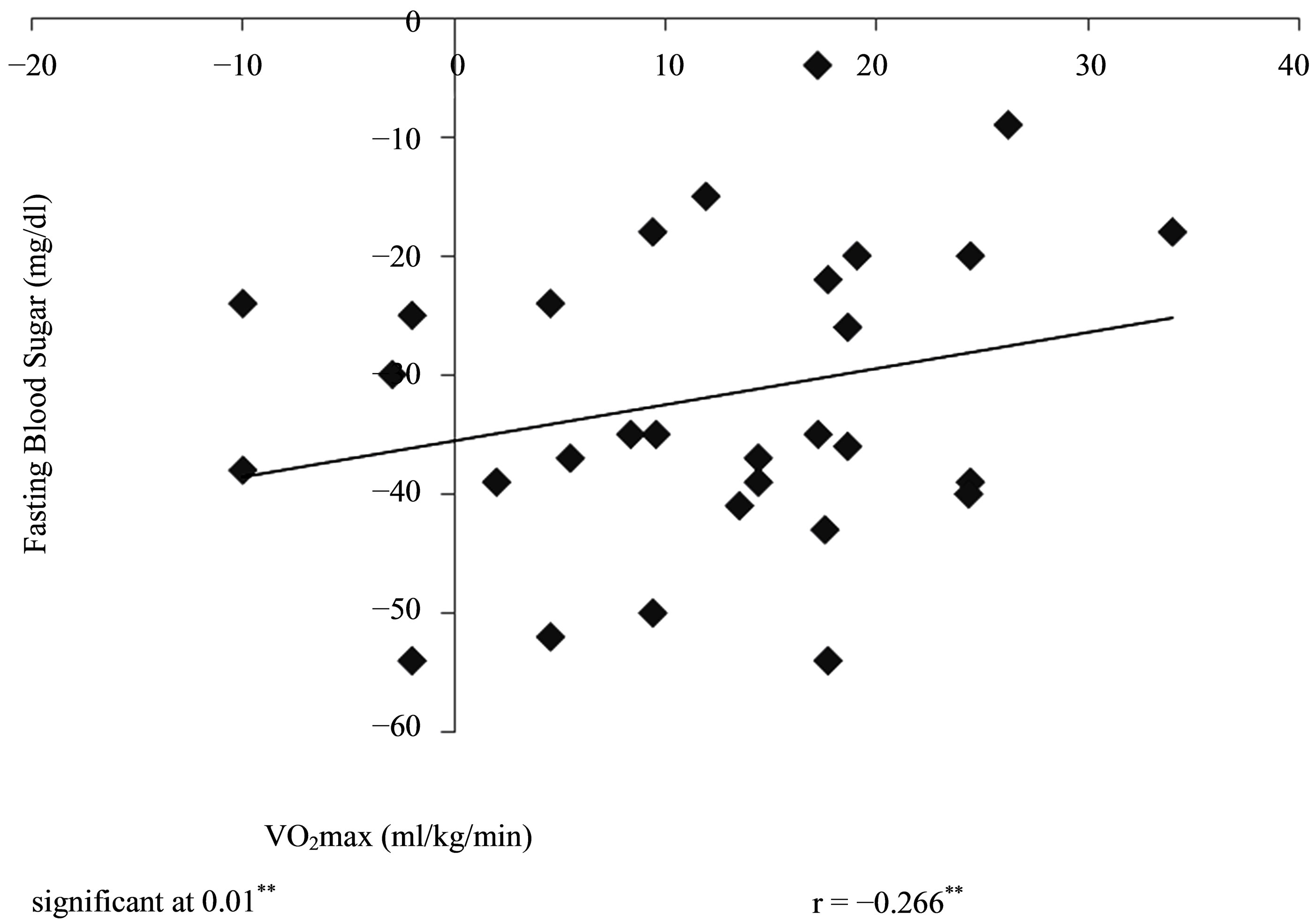
Figure 2. Correlation between training changes in VO2max and FBS (N = 30).
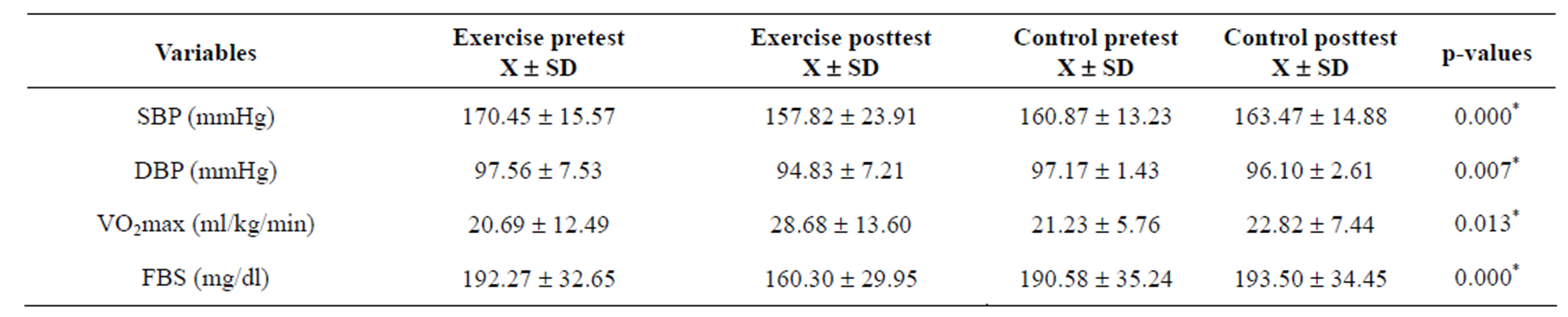
Table 2. ANCOVA test and groups mean ± SD for pretest and posttest values (N = 54).
*Significant, p < 0.05.
an oral antidiabetic regimen. Blood pressure (BP) and glycaemic profile were measured at baseline and at the end of the study. They reported that in comparison with baseline and control group, exercise-treated patients improved glucose control, exercise capacity (VO2 peak) and exhibited decreased insulin resistance and systolic BP considerably (p < 0.05). They conclusion Aerobic exercise training without significant weight loss improves metabolic profile and exerts anti-inflammatory effects in patients with type 2 DM.
Another related study was investigated by Gordon et al. [25]. They conducted a prospective randomized study to investigate the efficacy of Hatha yoga and aerobic exercise over control. In their study, 77 subjects with type 2 diabetic in the Hatha yoga exercise group that were matched with a similar number of type 2 diabetic patients in the conventional aerobic exercise and control groups. Fasting blood glucose (FBG) was determined at baseline and at two consecutive three monthly intervals. They reported significant reduction in the concentrations of FBG in the Hatha yoga and conventional aerobic exercise groups after six months and decreased by 29.48% and 27.43% respectively (p < 0.0001). They concluded and demonstrated the efficacy of Hatha yoga exercise and conventional aerobic exercises on fasting blood glucose in patients with type 2 diabetes and suggest that Hatha yoga exercise and conventional aerobic exercise may have therapeutic preventative and protective effects on diabetes mellitus.
Boulé et al. [26] in a Meta-analysis review, investigated the effects of aerobic xercise on glycemic control in T2DM. They selected studies that evaluated the effects of exercise interventions (duration 8 weeks) in adults with T2DM. Fourteen (11 randomized and 3 nonrandomized) controlled trials were included. They concluded that exercise training reduces glycosylated hemoglobin (HbA1c) by an amount that should decrease the risk of diabetic complications compared with control groups.
The mechanism of blood glucose reduction by aerobic training could be linked to three major pathways: the acute stimulation of muscle glucose transport pathway; acute enhancement of insulin action; and long term upregulation of the insulin signaling pathway resulting from regular exercise training [27-29]. The probable reason for the significant reduction in FBS in the exercise group over the control group might not be unconnected to the fact that aerobic bouts of exercise training may help train the motor units, enhancing the anaerobic and aerobic energy systems. This may lead to more effective utilization of fats and carbohydrates [30].
5. CONCLUSION
The present study supports the recommendations of moderate intensity (continuous) training program as an adjunct non invasive management of T2DM and dual therapeutic down regulation effects on blood pressure.
6. CONSENT
Written informed consent was obtained from all the subjects for their participation and publication of this study.
7. ACKNOWLEDGEMENTS
The authors acknowledged all the staff of the Physiotherapy Department and Diabetes Clinic, University of Nigeria Teaching Hospital, Ituku Ozalla, Enugu, Nigeria.
REFERENCES
- Vantilburg, J.H.O., Sandkuijl, L.A., Strengman, E., Vansomeren, H., Rigters-Aris, C.A.E., Pearson, P.L., VanHaeften, T.W. and Wijmenga, C. (2003) A genome-wide scan in type 2 diabetes Mellitus provides independent replication of a susceptibility locus on 18p11 and suggests the existence of novel loci on 2q12 and 19q13. The Journal of Clinical Endocrinology & Metabolism, 88, 2223-2230. http://dx.doi.org/10.1210/jc.2002-021252
- DeFronzo, R.A. and Ferrannini, E. (1991) Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care, 14, 173-194. http://dx.doi.org/10.2337/diacare.14.3.173
- Valsania, P. and Micossi, P. (1994) Genetic epidemiology of non-insulin-dependent diabetes. Diabetes/Metabolism Reviews, 10, 385-405. http://dx.doi.org/10.1002/dmr.5610100404
- Akindele, M.O., Kozdo, P. and Mustapha, N. (2006) Cardiovascular responses of type 2 diabetes patients to intermittent and continuous aerobic exercises. The Nigerian Journal of Rehabilitation, 11, 2079-2083.
- Polikandrioti, M. (2009) Exercise and diabetes mellitus. Exercise and Diabetes Mellitus, 3, 130-131.
- Eves, N.D. and Plotnikoff, R.C. (2006) Resistance training and type 2 diabetes: Considerations for implementation at the population level. Diabetes Care, 29, 1933- 1941. http://dx.doi.org/10.2337/dc05-1981
- Sigal, R.J., Kenny, G.P. and Wasserman, D.H. (2004) Castaneda-Sceppa C: Physical activity/exercise and type 2 diabetes. Diabetes Care, 27, 2518-2539. http://dx.doi.org/10.2337/diacare.27.10.2518
- Elbein, S.C. (2002) Perspective. The search for genes for type 2 diabetes in the post-genome era. Endocrinology, 143, 2012-2018. http://dx.doi.org/10.1210/en.143.6.2012
- Horenstein, R.B. and Shuldiner, A.R. (2004) Genetics of diabetes. Reviews in Endocrine and Metabolic Disorders, 5, 25-34. http://dx.doi.org/10.1023/B:REMD.0000016122.84105.75
- Sabra, M., Shuldiner, A.R. and Silver, K. (2004) Candidate genes for type 2 diabetes mellitus. In: Le Roith, D., Taylor, S.I. and Olefsky, J.M., Eds., Diabetes Mellitus: A Fundamental and Clinical Text. 3rd Edition, Lippincott Williams & Wilkins, Philadelphia, 1003-1012.
- Bouchard, C., Dionne, F.T., Simoneau, J.A. and Boulay, M.R. (1992) Genetics of aerobic and anaerobic performances. Exercise and Sport Sciences Reviews, 20, 27-58.
- American College of Sports Medicine, Johnson, E.P., Ed., ACSM’s guidelines for exercise testing and prescription. 6th Edition, Lippincott Williams & Wilkins, Philadelphia, 2000.
- Musa, D.I., Ibrahim, D.M. and Toriola, A.L. (2002) Cardiorespiratory fitness and risk factors of CHD in preadolescent Nigerian girls. Journal of Human Movement Studies, 42, 455-450.
- International Society for the Advancement of Kinanthropometry (2001) International standards for anthropometric assessment. ISAK, Patche Fstroom.
- American College of Sports Medicine (1995) ASCM’s guidelines for exercise testing and prescription. 5th Edition, Williams & Wilkins, Baltimore.
- Golding, L.A., Meyers, C.R. and Sinniny, W.E. (1989) Way to physical fitness. The complete carnote to fitness testing and instruction, 3rd Edition, Human Kinetics Publishers, Champaign.
- Brooks, G.A., Fahey, T.D. and White, T.P. (1996) Exercise physiology, human bioenergetics and its application. 2nd Edition, May Field Publishing Company, Mountain View.
- Pollock, M.L. and Wilmore, J.H. (1990) Exercise in health and disease; evaluation and prescription for prevention and rehabilitation. 2nd Edition, WB Saunders Company, Philadelphia.
- American College of Sports Medicine (1993) Position stand: Physical activity, physical fitness, and hypertension. Medicine & Science in Sports & Exercise, 25, 1-10.
- Lamina, S., Okoye, C.G. and Dagogo, T.T. (2009) Therapeutic effect of an interval exercise training program in the management of erectile dysfunction in hypertensive patients. The Journal of Clinical Hypertension, 11, 1-5. http://dx.doi.org/10.1111/j.1751-7176.2009.00086.x
- Lamina, S. (2010) Effects of continuous and interval training programs in the management of hypertension: A randomized controlled trial. The Journal of Clinical Hypertension, 12, 841-849. http://dx.doi.org/10.1111/j.1751-7176.2010.00315.x
- Westhoff, T.H., Franke, N., Schmidt, S., Vallbracht-Israng, K., Meissner, R. and Yildirim, H. (2007) Too old to benefit from sports? The cardiovascular effects of exercise training in elderly subjects treated for isolated systolic hypertension. Kidney and Blood Pressure Research, 30, 240-247. http://dx.doi.org/10.1159/000104093
- Laterza, M.C., de Matos, L.D., Trombetta, I.C., Braga, A.M., Roveda, F. and Alves, M.J. (2007) Exercise training restores baroreflex sensitivity in never trained hypertensive patients. Hypertension, 49, 1298-1306. http://dx.doi.org/10.1161/HYPERTENSIONAHA.106.085548
- Kadoglou, N.P.E., Lliadis, F., Angelopoulou, N., Perrea, D., Ampatzidis, G., Liapis, C.D. and Alevizos, M. (2007) The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. The European Journal of Cardiovascular Prevention & Rehabilitation, 14, 837-843. http://dx.doi.org/10.1097/HJR.0b013e3282efaf50
- Gordon, L.A., Morrison, E.Y., McGrowder, D.A., Young, R., Fraser, Y.T., Zamora, E.M., Alexander-Lindo, R.L. and Irving, R.R. (2008) Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complementary and Alternative Medicine, 13, 8-21.
- Boulé, N.G., Haddad, E., Kenny, G.P., Wells, G.A. and Sigal, R.G. (2001) Effects of exercise on glycemic control and body mass in Type 2 diabetes mellitus. A metaanalysis of controlled clinical trials. JAMA, 286, 1218- 1227. http://dx.doi.org/10.1001/jama.286.10.1218
- Youngren, J.F. (2010) Exercise and the regulation of blood glucose. http//www. Diabetesmanager.pbworks.com/w/page
- Gao, J., Ren, J., Gulve, E.A. and Holloszy, J.O. (1994) Additive effect of concentrations and insulin on GLUT-4 translocxation into the sarcolemma. Journal of Applied Physiology, 77, 1597-1601.
- Cartee, G.D., Douen, A.G., Ramial, T., Klip, A. and Holloszy, J.O. (1991) Stimulation of glucose transport in skeletal muscle by hypoxia. Journal of Applied Physiology, 709, 1593-16900
- Wilmore, J.A. and Costil, D.L. (2005) Physiology of sport and exercise. 3rd Edition, Human Kinetics Book, Champaign.
NOTES
*Competing interests: The authors have no conflict of interest in this work.
Authors’ Contributions: CIE, SL & REN were responsible for conducting the literature review, designing the study, and writing the manuscript. REN collected all medical reports of the patients. UAE, AAA, MJN & SL were responsible for performing the statistical analysis, interpreting the data and drafting the manuscript. CIE & SL were responsible for supervising the conduct of the experiment. SL, MJN & CIE revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.

