Materials Sciences and Applications
Vol.4 No.10(2013), Article ID:38134,5 pages DOI:10.4236/msa.2013.410079
Corrosion Resistance of Zn and Zn-Ni Electrodeposits: Morphological Characterization and Phases Identification
![]()
1Departamento de Física e Química, Campus de Guaratinguetá, Universida de Estadual Paulista—UNESP, Guaratinguetá, Brazil; 2Faculdades Integradas Teresa D’Ávila—FATEA, Lorena, Brazil.
Email: jwjsilva@gmail.com
Copyright © 2013 Conceição A. M. Dutra et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 23rd, 2013; revised September 25th, 2013; accepted October 8th, 2013
Keywords: Corrosion; Electrodeposition; Zn; Zn Alloy
ABSTRACT
Zinc alloys coatings formed with elements of group VIIIB are promising because they display similar properties and protect steel by galvanic action. The Zn-Ni alloy is remarkable by showing improved mechanical properties and better corrosion resistance when compared to zinc coatings of similar thickness, also can be applied at higher temperatures. In this work, electrodeposits of Zn, Zn-12%Ni, obtained upon SAE 1010 steel from commercial alkali baths, were treated by blue chromatization and characterized according to mechanical properties and morphology. Studies were carried out by using measures of hardness, roughness, SEM, EDS and XRD. Among the studied electrodeposits, alloys treated by chromatization showed higher corrosion resistance and Zn-Ni electrodeposits showed higher value of roughness and hardness, while zinc coating had results similar to the steel substrate By means of XRD, it was found that electrodeposits are crystalline, being identified in Zn-Ni alloy the presence of the phases g(Ni5Zn21) and d(Ni3Zn22), which are responsible for its higher corrosion resistance.
1. Introduction
Zinc coatings protect steel both forming a physical barrier and providing a cathodic protection. The coverage corrosion resistance can be improved by using coverings of zinc alloys such as Zn-Ni, Zn-Fe, and Zn-Co [1-4]. Zinc metal has an excellent resistance and a wide application, especially as metal cover to prevent corrosion on steel exposed to water. Water usually contains dissolved salts and anions of some salts. Particularly, the anions Cl−, Br− and I− under certain conditions are able to cause local breaking of protective films and initiate pitting attack, as reported by Shreir [5]. Several reports have shown that corrosion resistance of alloys coated with Ni-Zn, within certain composition (12%-14%Ni), can be 5 - 6 times better than pure zinc of similar thickness [6,7]. Ni-Zn electrodeposits are one of the best examples of anomalous codeposition and get this rating because zinc, a less noble metal, is rather electrochemically deposited, according to Brenner definitions [8]. According to Lin’s conclusion [9], anomalous electrodeposition of Zn-Ni is caused by the slow nickel kinetics and by hydrogen evolution in nickel deposit. The corrosion protective coatings form intermediate layers between the corrosive environment and the metal substrate base. In general, they can protect the metal substrates by two main mechanisms: sacrifice and mechanisms of barrier protection [9,10]. Zn-Ni provides a sacrificial protection to steel. This alloy especially chromatized is widely used to prevent corrosion in auto industry. Corrosion resistance studies of electrodeposits of chromatized Ni-Zn alloys show that the passive layer properties are dependent on the Ni content present in the deposit and the bath composition of chromate employed. The best results were obtained for the Zn-13%Ni alloy with a green chromating bath [11] The Zn-Ni alloys which own between 8% and 20% Ni display a corrosion resistance better than the pure Zn deposits. From this nickel content, the alloy can no longer be used to protect substrates such as steel, because the alloy becomes nobler and loses its sacrifice properties. Furthermore, as the alloy is corroded, there occurs dissolution of zinc or zinc-rich phase, which would transform the initial phase, which is less noble, into another one nobler than the steel substrate, and this way, the steel substrate begins to protect superficially the Zn-Ni deposit.
2. Experimental Methods
The working electrodes were prepared using as a substrate the ordinary steel (black plate), which is the SAE 1010 steel (Standard ASTM 370) [12] 0.75 mm thick with electrodeposits of pure Zn and Zn-Ni in commercial baths based on chlorides and cyanides-free. During samples immersion, it was applied a current density of 2 A/dm2 at a temperature of about 25˚C. All processes of electrodeposition were obtained in the laboratory pilot of Enthone Electronics Brazil Ltd and being these processes and properties of Cookson Electronics Brazil Ltd. After the electrodeposition process, some samples parts were subjected to a passivating treatment bath of chrome solution. The surface analyzes were carried out by using a scanning electron microscope LEO mod. 1450 VP provided with an analyzer for energy dispersive separation of X-ray, EDS. The Zn and Zn-Ni electrodeposits were characterized by X-ray diffractometry with Cu Ka radiation and wavelength l = 1.54 Å, so it was used a DEBYEFLEX ISO 1001, RICH. SEIFERT & Co.-RONTEGENWERK.
3. Results and Discussion
3.1. Vickers Microhardness Testing
The average of Vickers microhardness values obtained from five samples for electrodeposits of zinc and its alloy are shown in Table 1.
These values analysis showed that the samples untreated and treated by chromate exhibit similar behaviors, i.e. microhardness values are coincident, indicating that the chromatization process has no interference in this parameter. The microhardness value expected for zinc coatings should be between 100 and 140 HV [13], therefore, as it can be seen from Table 1, the obtained values are in this range. When comparing the Vickers microhardness value between the electrodeposits Zn and Zn-Ni, it is observed an increase in this parameter for the alloy,
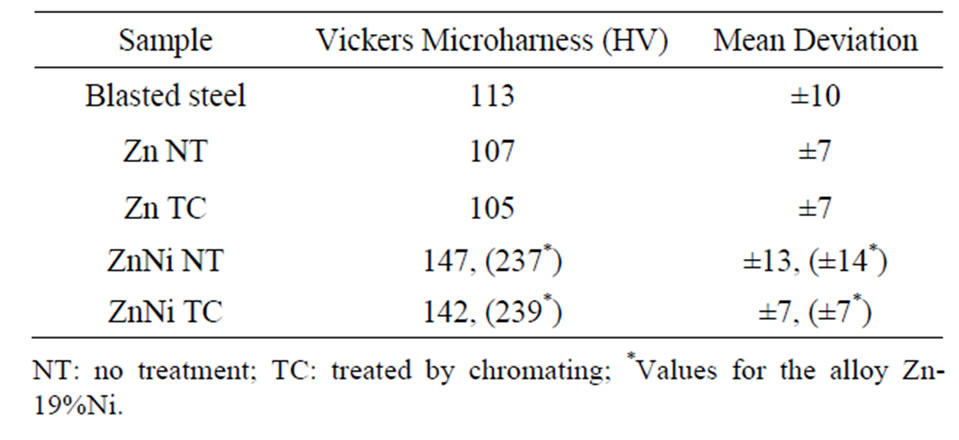
Table 1. Vickers microhardness values of Zn and Zn-Ni electrodeposits on steel.
which is justified by presence of a high Ni content in the coating (about 12% - 13%). For the alloy with higher Ni content (approximately 19%), prepared in the same initial conditions, however different batch, there was an increase in hardness (Table 1), which shows that this property directly depends on the Ni content in alloy. This behavior is in agreement with that expected [14], since electrodeposits of alloys formed with elements of group VIIIB must have higher mechanical strength, which was evidenced by an increase in hardness.
3.2. Roughness Measurements
The values of average roughness parameter (Ra) for SAE 1010 steel and for Zn and Zn-N electrodeposits untreated and treated by chromate carried out on ten samples of each material are shown in Table 2.
Analyses of this table results display that, in general, there is an increase in coatings roughness compared to the steel substrate, whereas the treatment by chemical chromate conversion has low influence in this parameter. At chromatization process, the passive layer is formed by dissolving zinc or zinc rich phases which are present in the material. Specifically, at the chemical conversion processes applied to the studied electrodeposits, layers formed on these materials surface are very fine, almost transparent. This indicates that the roughness values come from imperfections of electrodeposits, after applying the chemical conversion process.
The Ni-Zn electrodeposits have a behavior differently of other alloys from group VIIIB. It is noted higher roughness values for Zn-Ni electrodeposits, for both chromatization treated alloys and no treatment, which should be associated with the coating nodular aspect.
3.3. Morphological Characterization and Phases Identification
3.3.1. X-Ray Diffraction (XRD)
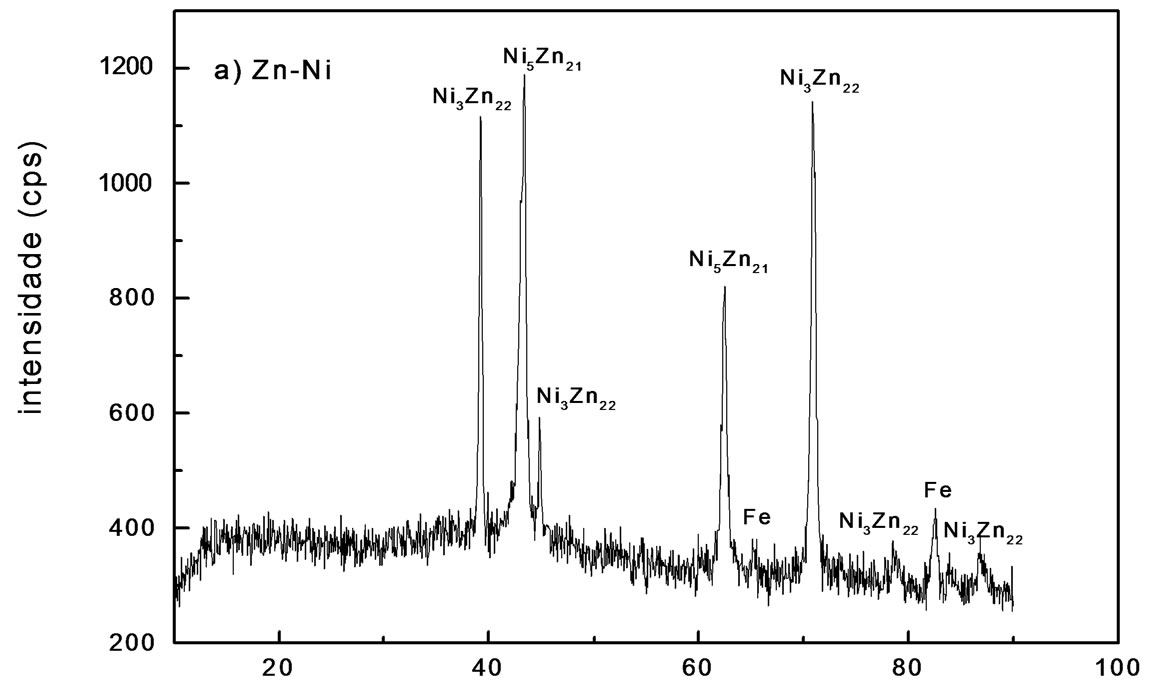
By means of this technique, some phases could be identified by displaying peaks with different relative intensities. Under the analyzed conditions, using a current density of 2.0 A∙dm−2, Zn electrodeposits consisted primarily of zinc, with iron peaks at 2.03 Å and 1.43 Å distances. Analysis carried out with pure Zn electrodeposits after
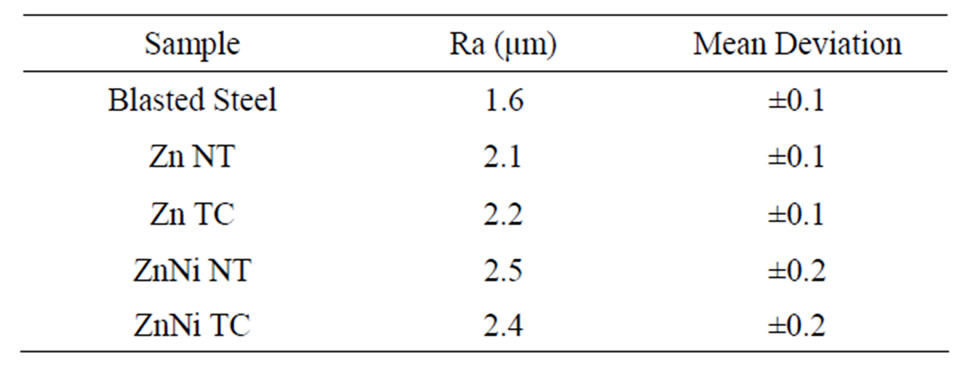
Table 2. Roughness measuring parameters of electrodeposits on steel.
chromating shows no significant changes in diffractogram, unless the change in intensity of some maximums related to zinc. Alloy showed a phase g(Ni5Zn21) or a mixture of two phases d(Ni3Zn22) and g. These results agree with reports found in literature [15]. Phases in alloys Ni-Zn varied with bath composition and some of them were identified by X-ray diffraction (Figures 1(a) and (b)).
The different phases presence in deposits depends on the Ni and Zn relative amount in bath [16]. In this study, the authors confirmed that deposits obtained from solutions containing a Ni2+/Zn2+ ratio of 0.5 and using a current density of 1.0 A∙dm−2 consisted of a two phase mixture d(Ni3Zn22) and g(Ni5Zn21). When the Ni2+/Zn2+ proportion in bath increases to 2.5, it means that deposit was predominantly phase g(Ni5Zn21). Figure 1(b) shows diffractogram for the Zn-Ni alloy after treatment with blue chromate. It is observed that decrease at peaks intensity attributed to phase d(Ni3Zn22) is due to the chemical conversion process, where passive layer is formed by zinc dissolution during this phase. Whereas phase g(Ni5Zn21) does not show the same behavior, which may be related to its higher stability.
3.3.2. Scanning Electron Microscopy (SEM)
Zn electrodeposits without treatment show good coverage on steel surface without exposing the substrate and presence of irregularities distributed over all surface. Such irregularities are, in most part, due to substrate itself after the acid pickling, pre-treatment in which the sample is subjected, before the electrodeposition process [17]. The EDS spectrum, however, reveals, in addition to zinc, the iron presence. Since the coating has a thin thickness, about 10 mm, and has irregularities, depending on the energy of electron beam used in this analysis, the innermost layers elements are also detected, as for instance Fe, the main steel substrate constituent. The glitches observed previously on Zn electrodeposits are corrected in chromatization treatment. There is a distinct change at the surface appearance after the chemical chro-
 2θ
2θ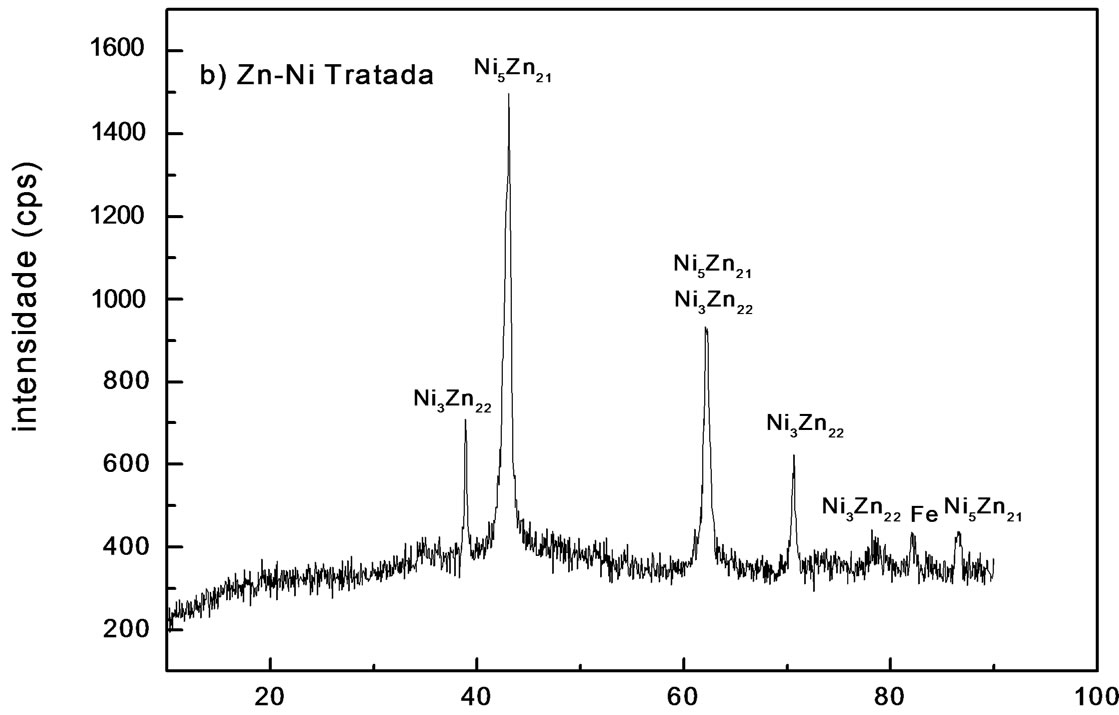 2θ
2θ
Figure 1. X-ray diffraction patterns of electrodeposit on steel, using a current of 2.0 A∙dm−2: (a) Zn-Ni; (b) Zn-Ni alloy treated.
mate conversion [18].
Images of untreated Zn-Ni electrodeposits, Figure 2, and chromatized alloy, Figure 3, resulted in very distinct superficial aspects.
The untreated Zn-Ni electrodeposits show a very irregular morphology and without substrate exposure. As a main effect of chromatization process, there appears a layer formation which makes surface more uniform and compact, resulting from chemical conversion process, in which a thin passive layer is formed from Zn dissolution.
It can also be observed cracks appearing all over the coating, particularly for alloy treated by chromating. As previously mentioned, the Ni-Zn coatings showed hardness values higher than the pure Zn coating, resulting in presence of cracking can be attributed to internal stress in the coating caused by the high content of nickel in the alloy.
In EDS analysis for Zn electrodeposits, besides Fe and Zn it is also observed small Cr amounts, coming from layer which was formed at chromatization process, despite this layer is very thin. For the untreated Zn-Ni alloy, analysis shows compositions between 11.4 and 11.7%Ni and for the shaded one, a mass ratio of 12.6%Ni and 87.4% Zn.
A rather curious observation can be seen through image of Figure 4(a), when analysis of scanning electron
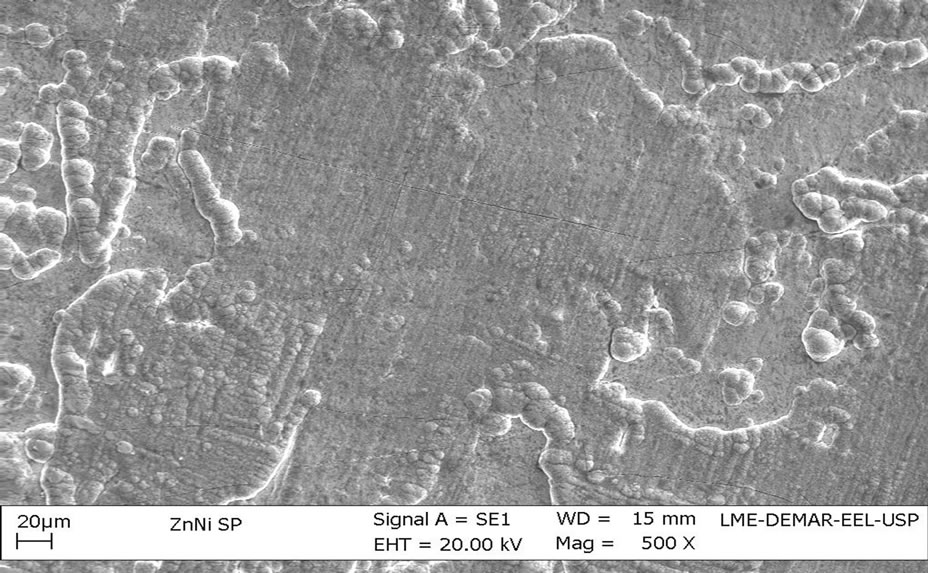
Figure 2. Image (SEM) of untreated Zn-Ni electrodeposits.
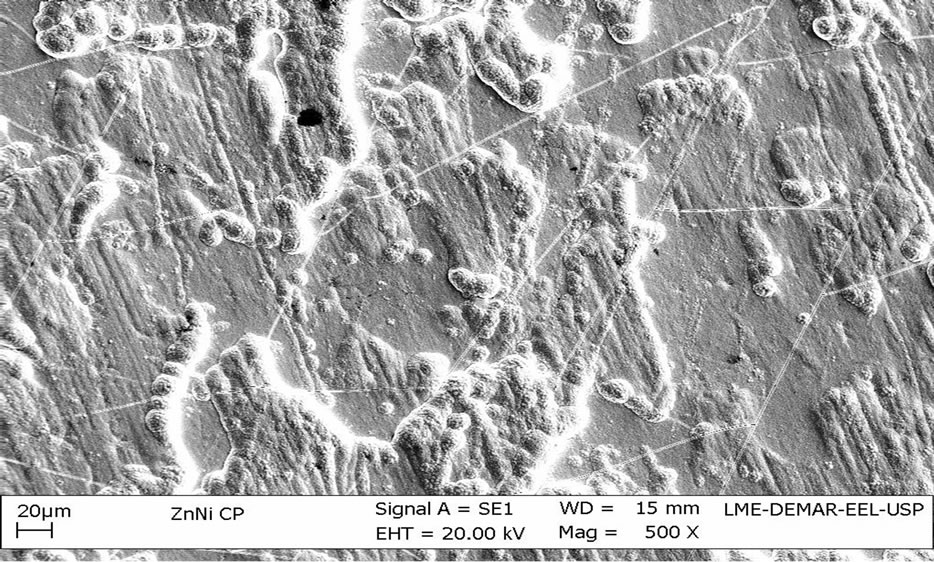
Figure 3. Image (SEM) of Zn-Ni electrodeposits treated by chromatization.
 (a)
(a)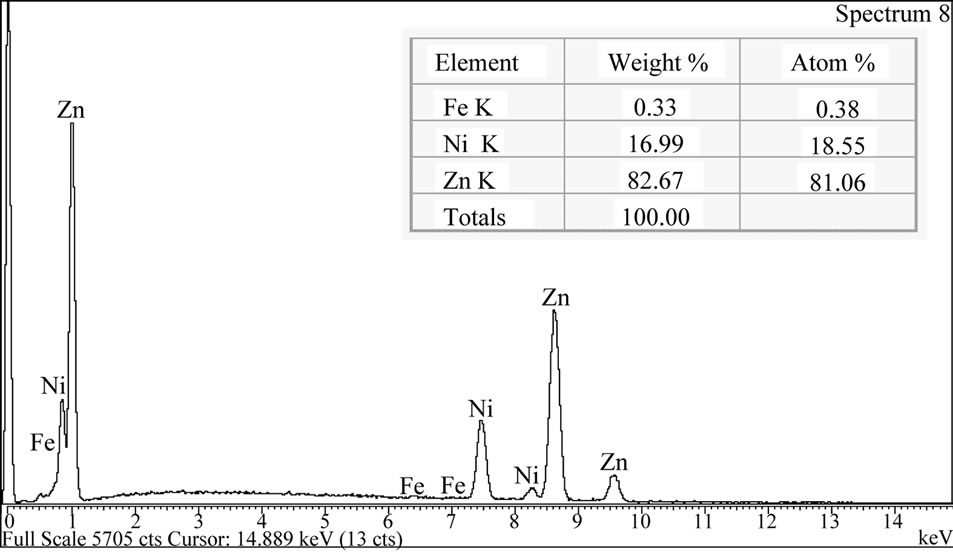 (b)
(b)
Figure 4. SEM image about Zn-19%Ni electrodeposits, chromate treated by (a) surface microscopy and (b) EDS spectrum.
microscopy is carried out with a sample whose analysis by atomic absorption acknowledges a very high Ni percentage (19.4%, m/m), being this coating composition confirmed by EDS analysis, Figure 4(b). The electrodeposits reveals presence of some dispersed nodules and on top of that, analysis by X-ray diffractometry showed g (Ni5Zn21) predominance.
4. Conclusions
From the obtained results, it can be concluded that the electrodeposition process has a great influence upon the steel corrosion resistance. Morphological characterization of Zn and Zn-Ni coatings showed that they have a high coverage degree and with no gross failures, irregular morphology of electrodeposits resulting from steel substrate failures, produced during the acid pickling.
The Zn-Ni electrodeposits display hardness higher than that from Zn, revealing that this property is directly related to the content and nature of the alloy element present in the coating. Also the alloy has greater roughness among the studied coatings, which shows this electrodeposits nodular aspect, especially when the nickel content is higher than 15% (m/m).
The Zn and Ni-Zn electrodeposits showed good crystallinity. The X-ray analysis of Zn electrodeposits shows peaks presence of Zn and Fe being iron from the steel substrate. Now, the Ni-Zn coating reveals presence of apparent peaks of great intensity related to different phases: Ni5Zn21 and Ni3Zn22. The Zn-Ni alloy shows to be more efficient and this resistance is probably attributed to presence of phase g(Ni5Zn21).
5. Acknowledgements
The authors would like to thank to COOKSON ELECTRONICS BRAZIL LTDA. and FUNDUNESP (01258/ 08-DFP process).
REFERENCES
- P. S. D. Brito, S. Patricio, L. F. Rodrigues, D. M. F. Santos and C. A. C. Sequeira, “Electrodeposition of Zn-Mn Alloys from Recycling Battery Leach Solutions in the Presence of Amines,” WIT Transactions on Ecology and Environment, Vol. 142, 2010, pp. 367-378. http://dx.doi.org/10.2495/SW100341
- D. Fiqueroa and M. J. Robinson, “The Effect of Sacrificial Coatings on Hydrogen Embrittlement and Re-Embrittlement of Ultra High Strength Steels,” Corrosion Science, Vol. 50, No. 4, 2008, pp. 1066-1079. http://dx.doi.org/10.1016/j.corsci.2007.11.023
- Z. I. Ortiz, P. Díaz-Arista, Y. Meas, R. Ortega-Borges and G. Trejo, “Characterization of the Corrosion Products of the Corrosion Products of Electrodeposited Zn, Zn-Co and Zn-Mn Alloys Coatings,” Corrosion Science, Vol. 51, No. 11, 2009, pp. 2703-2715. http://dx.doi.org/10.1016/j.corsci.2009.07.002
- M. M. Abou-Krisha, “Effect of pH and Current Density on the Electrodeposition of Zn-Ni-Fe Alloys from a Sulfate Bath,” Journal of Coatings Technology and Research, Vol. 9, No. 6, 2012, pp. 775-783.
- L. L. Shreir, “Corrosion,” Vol. 1, 2nd Edition, NewnesButterwords, London, 1976.
- S. A. Watson, “A Nickel Development Institute Review Series,” 1988. http://www.nickelinstitute.org/~/Media/Files/TechnicalLiterature/ElectrodepositedCoatingsofZinc_Nickel_14022_.pdf
- T. C. Franklin, “Some Mechanisms of Action of Additives in Electrodeposition Processes,” Surface and Coatings Technology, Vol. 30, No. 4, 1987, pp. 415-428. http://dx.doi.org/10.1016/0257-8972(87)90133-2
- A. Brenner, “Electrodeposition of Alloys,” Vol. 1 and 2 Academic Press, Principles and Practice, New York and London, 1963.
- Y. P. Lin and J. R. Selman, “Electrodeposition of Corrosion-Resistance Ni-Zn Alloy, I. Cyclic Voltammetric Study,” Journal of the Electrochemical Society, Vol. 140, No. 5, 1993, pp. 1299-1303. http://dx.doi.org/10.1149/1.2220974
- Z. F. Lodhi, J. M. C. Mol, A. Hovestad, L.’t Hoen-Velterop, H. Terryn and J. H. W. de Wit, “Corrosion Resistance of Zn-Co-Fe Alloy Coatings on High Strength Steel,” Surface & Coating Technology, Vol. 203, No. 10- 11, 2003, pp. 1415-1422.
- M. H. Sohi and M. Jalali, “Study of the Corrosion Properties of Zinc-Nickel Alloy Electrodeposits before and after Chromating,” Journal of Materials Processing Technology, Vol. 138, No. 1-3, 2003, pp. 63-66. http://dx.doi.org/10.1016/S0924-0136(03)00050-5
- American Society for Testing and Materials, ASTM a370, 2001.
- H. E. Towsend, “Coated Steel Sheets for Corrosion Resistant Automobiles,” Materials Performance, Vol. 30, No. 10, 1991, pp. 60-65.
- S. N. Lumpp, “Obtenção de Filmes Passivantes de Molibdato de Amônio e Nitrato de Cério Para Ligas de Zinco Eletrodepositadas,” Ph.D. Thesis, Campinas State University, Campinas, 2005.
- S. K. Panikkar and T. L. Rama Char, “Electroplating of Nickel from the Pyrophosphate Bath,” Journal of The Electrochemical Society, Vol. 106, No. 6, 1959, pp. 494- 499. http://dx.doi.org/10.1149/1.2427395
- W. Zhongda, L. Fedrizzi and P. L. Bonora, “Electrochemical Studies of Zinc-Nickel Codeposition in Chloride Baths,” Surface and Coatings Technology, Vol. 85, No. 3, 1996, pp. 170-174. http://dx.doi.org/10.1016/0257-8972(96)02857-5
- M. C. Pereira, J. W. J. Silva, H. A. Acciari, E. N. Codaro and L. R. O. Hein, “Morphology Characterization and Kinetics Evaluation of Pitting Corrosion of Commercially Pure Aluminium by Digital Image Analysis,” Materials Sciences and Application, Vol. 3, No. 5, 2012, pp. 287- 293. http://dx.doi.org/10.4236/msa.2012.35042
- R. B. Ribeiro, J. W. J. Silva, L. R. O. Hein, M. C. Pereira, E. N. Codaro and N. T. Matias, “Morphology Characterization of Pitting Corrosion on Sensitized Austenitic Stainless Steel by Digital Image Analysis,” ISRN Corrosion, Vol. 2013, 2013, Article ID: 905942. http://dx.doi.org/10.1155/2013/905942

