Advances in Bioscience and Biotechnology
Vol.3 No.7(2012), Article ID:24835,5 pages DOI:10.4236/abb.2012.37109
Optimization of micropropagation and Agrobacterium-mediated gene transformation to spinach (Spinacia oleracea L.)
![]()
1Young Researchers Club, Khorasgan Branch, Islamic Azad University, Isfahan, Iran
2Khorasgan Branch, Islamic Azad University, Isfahan, Iran
3Department of Tissue Culture & Gene Transformation, Agricultural Biotechnology Research Institute of Iran (ABRII), Karaj, Iran
4Horticultural Department, University of Guilan, Rasht, Iran
Email: *d.naderi@khuisf.ac.ir
Received 2 August 2012; revised 5 September 2012; accepted 10 October 2012
Keywords: Spinacia oleracea; Transformation; Gus Gene; Transient Expression; Stable Expression
ABSTRACT
Spinach is one of the dioecious plant which is considered as a model plant in genetic and molecular studies of sex determination because of its special characteristics such as low chromosome number and short life cycle. An efficient protocol for Spinacia oleracea Agrobacterium-mediated gene transformation was developed. The leaf disks, roots, hypocotyls and cotyledons of this plant were inoculated with LBA4404. LBA4404 carrying pCAMBIA3301 binary vector with 35SCaMV gus-int and 35SCaMV bar cassettes. Effects of two preparation condition (induction of vir genes and noninduction) were considered. Also effects of different number days of co-cultivation and pre-culture of explants were examined. After co-cultivation, the explants were transferred to regeneration medium containing 250 mg·L–1 Carbeniciline. Transient expression efficiency was calculated based on the number of blue spots per explants one week after inoculation. Based on the results of transient expression, stable transformation was carried out. After formation of callus the histochemical GUS assay was carried out on some parts of them and other parts were leaved for being regenerated.
1. INTRODUCTION
Spinach (Spinacia oleracea L.) is a dioecious plant that is native to West Asia and probably Iran and at present is widely cultivated in the world as one of the most popular vegetables and known as a rich source of iron, vitamins and minerals [1]. The major purposes of spinach breeding are to develop cultivars with resistances to various pests and diseases, high temperature resistance, late flowering, and low concentrations of nitrate and oxalate. Although a large number of cultivars have been developed through conventional breeding programs, it is still difficult to produce cultivars with some of such traits, because of the lack of the available germplasm. Thus, it is now expected to apply genetic manipulation to spinach for improvement of this important vegetable crop [1].
In many cases, the production of transgenic plants is prevented due to inability to regenerate plants from those tissues susceptible to transformation thus an efficient regeneration system is a prerequisite for the production of genetically modified spinach plants in crop improvement programs [2].
Since the first report in 1973 [3], when only one regenerating callus was obtained, spinach was believed to be recalcitrant to in vitro regeneration. Many papers dealing with the in vitro regeneration of spinach appeared, and it became increasingly clear that genotype is one of the most important factors affecting the regeneration capacity of spinach [4-10]. However, in addition to the high variability observed in the regeneration capacity of different spinach cultivars, high individual variability within a population of the same spinach cultivar has been reported [10].
Reports on spinach regeneration through somatic embryogenesis and organogenesis have indicated that the type of explants plays an important role so root segments are superior in somatic embryogenesis while cotyledons are efficient in organogenesis [6,7,9,11]. Leaf discs, hypocotyls, root segments, leaf-derived protoplasts, thin cell layers from hypocotyls and roots have also been utilized in organogenesis [4,5,8,9,11-14].
In all the cited studies, gibberellic acid (GA3), combined with an auxin, was found to be necessary for induction of embryogenic callus. In a recent study, researcher found that A 31-kDa basic protein, designated BP31, accumulated at much higher levels in the embryogenic callus formed in the presence of GA3 than in the non-embryogenic callus formed in the absence of GA3 [15].
Genetic transformation systems have been described for many plant species. The technology involves the delivery of defined foreign genes into plant cells, obtaining integration of the genes into plant genomes and observing expression of the genes in the regenerated plants. This successful application of plant transformation technology is due to development of techniques which allow plant regeneration from cells or tissues. Genes are introduced either with the help of Agrobacterium tumefaciens or through direct delivery of DNA [16]. However, the most popular and efficient method in dicotyledonous plants is Agrobacterium mediated transformation.
Cervera et al. and Cao et al. have suggested that, low efficiency of transformation in citrange explants was due to an insufficient length of co-cultivation [17,18]. However, they have also reported that it was more difficult to eliminate Agrobacterium after longer periods of co-cultivation. Further, they have also reported that the 5-day culture period resulted in over growth of Agrobacterium and a subsequent decrease in the co-cultivation was the most effective for increasing the frequency of transient GUS-expression in citrange explants. Therefore, the period of co-cultivation should be optimized.
Length of co-cultivation time employed varies widely from a few hours to a few days depending upon the species, explant and culture condition.
Shuangxia et al. examined various aspects of transformation to improve the efficacy of producing transformants in cotton and found that shoot co-cultivation duration of 48 hours was optimal for developing a highly efficient method of transforming embryogenic callus [19]. Moralejo et al. have described a procedure for genetic transformation of Eucalyptus globulus (Labill) and they have studied the influence of explants pre-cultivation and reported that when seedlings were precultured for 4 - 6 days, the level of GUS transient expression was significantly greater than that of control (i.e. without preculture) and that the seedlings precultured for 6 days seemed to be more suitable for stable integration of transgenes [20]. They further reported that the improvement of DNA uptake could be due to stimulation of cell division by the hormones in the pre-cultivation medium, since mitotic cells would be more susceptible to Agrobacterium (or) would have a higher level of transcription. Preculturing of the explants on the regenerating medium prior to inoculation of Agrobacterium increased the frequency of transformants in pigeon pea to 62 percent from cotyledonary node and 27 percent from shoot tip [21], 28.8 percent from callus and 1.2 percent from shoots regenerated [22].
Shuangxia et al. by using acetosyringone developed a reliable and high efficiency system of transforming embryogenic callus mediated by Agrobacterium tumefaciens in cotton [19].
The GUS gene served as both selectable as well as screenable marker. The efficiency of the transformation was reported to be two-fold higher than with Kanamycin. Localization of GUS expression is also detected by histochemical assay [23].
A pre-requisite for transferring genes into plants is the availability of efficient transformation protocol. Although successful Agrobacterium mediated transformation have been reported earlier, the transformation efficacy was found to vary within and between plant species. It is therefore very important to identify conditions suitable to both high rates of transformation and genotype independent regeneration methods.
Current investigations were aimed at developing a protocol to increase the regeneration and transformation efficiency in spinach.
2. MATERIALS AND METHODS
“Varamin88” cultivar spinach seeds (Seed and plant improvement institute, Karaj, Iran) were washed with water (60˚C) for 5 min and ethanol 70% for 5 min and then rinsed three times with sterile distilled water. Seeds were then immersed in 50% commercial bleach for 10 min and finally rinsed with sterile distilled water and blotted dry on a piece of sterile filter-paper. The seeds were planted in 90 mm Petri dishes (20 seeds per dish) containing 25 mL basal plant growth regulator (PGR)- free medium for germination. Non-contaminated seedlings were picked and replanted on new Petri dishes (five seedlings per dish) containing the same medium, and grown for a few additional days, until the seedlings developed 4 - 5 leaves and the root system was well developed. The basal medium contained mineral solution [24], 20 g/L sucrose, 100 mg/L myo-inositol, 2 mg/L thiamine, 2 mg/L pyridoxine, 5 mg/L nicotinic acid and 2 mg/L adenine, all obtained from Sigma-Aldrich (St. Louis, MO, USA). Media were gelled with 0.8% (w/v) agar (Torlak, Belgrade, Serbia) and the pH was adjusted to 5.6 using pH-meter. Media were sterilized in an autoclave at 121˚C for 20 min.
We used hypocotyles, cotyledons and roots as explants in this experiment. Explants were separated aseptically from 10 days old seedlings and placed on basal medium containing 10 mg·L–1 GA3 and 2 mg·L–1 Kn combined with one of three concentrations of IAA or NAA (5, 10, or 15 mg·L–1). Regenerated shoots 1 - 3 cm in length were separated from the cotyledons and used for rooting experiments.
The root, leaf, hypocotyls and cotyledon explants were aseptically excised from 10-day old seedling for infection with A. tumefaciens. The Agrobacterium LBA-4404 was maintained on Lambert medium containing kanamycin 50 mg/mL and 50 mg/mL rifampicin incubated at 28˚C temperature for 24 hours. The explants were gently shaken in the bacterial suspension for 15 minutes and blotted dry on a sterile filter paper. They were transferred to the MS medium and co-cultivated under dark condition for two days. After Co-cultivation explants were transferred to the regeneration medium containing MS with 250 mg/L Carbenicillin. For standardization of transformation protocol different factors including explants types, acetosyringone (100 mM), pre-culture period on medium containing MS with 10 mg·L–1 NAA and cocultivation period are considered. In order to estimate the effects of various factors on Agrobacterium mediated transformation, the number of blue spot per callus and percent of callus with blue spot were counted and used as GUS gene activity indices that was estimated histochemically.
Data were subjected to ANOVA in SAS (ver. 9.2, SAS Institute, Inc., Cary, NC). Means were separated using the Tukey test.
3. RESULTS AND DISCUSSION
ANOVA determined that explant and different regeneration medium and their two ways interaction had significant effect on spinach regeneration. Interaction between explant and regeneration medium on percent of regenerate explants showed that the highest percent of regeneration was obtained in root explants cultured in medium with 10 mg/L NAA and the lowest value was related to hypocotyls explants with no regenerate explants in all medium (Table 1). Interaction between explant and regeneration medium also revealed that cotyledons explants response positively to increasing NAA concentration while don’t response to IAA.
Papers dealing with the in vitro regeneration of spinach indicated that genotype is one of the most important factors affecting the regeneration capacity of spinach [4-10]. So our cultivar that improved in seed and plant improvement institute has a suitable regeneration capacity and we can use this cultivar for transformation program. In addition reports on spinach regeneration through somatic embryogenesis and organogenesis previously indicated that the type of explants plays an important role so root segments are superior in somatic embryogenesis while cotyledons are efficient in organogenesis [6,7,9,11]. However in our study root explants was superior but without transformed explants so we propose the using cotyledon explants which show the higher percent of callus with blue spot and number of blue spot per explants (Figure 1) as transformation indicator (Table 2). In other hand cotyledons are good explants as meristem cells are easily formed on their base, which can be directed towards shoot primordium development with the correct hormonal balance [9].
NAA had a significant positive effect on regeneration. In all the cited studies, gibberellic acid (GA3), combined with an auxin, was found to be necessary for induction of embryogenic callus [15].
Against Shuangxia et al. [19], using acetosyringone don’t have positive effect on transforming embryogenic callus mediated by Agrobacterium tumefaciens in spinash and decrease number of spot per plant and percent

Table 1. Effect of different explant and hormones on callus production percent.
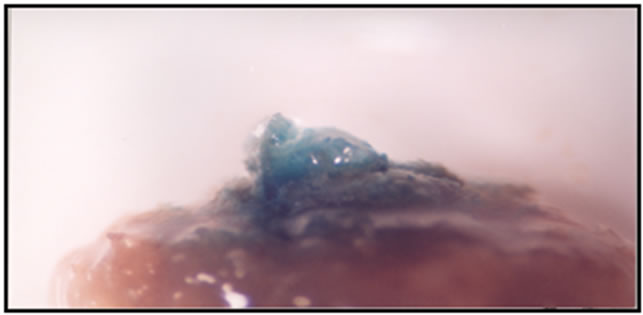

Figure 1. Number of spot per plant and percent of explants with blue spot were counted as a transformation indicator.
of explants with blue spot in comparison with control (Table 3). In similar way pre-culture also don’t show any positive effect on transforming (Table 4) while Moralejo et al. have studied the influence of explants pre-cultivation and reported that when seedlings were precultured for 4 - 6 days, the level of GUS transient expression was significantly greater than that of control [20].
In compatible to Cervera et al. and Cao et al. length of co-cultivation have significant positive effect on efficiency of transformation in spinach [17,18]. By increasing co-cultivation duration number of spot per plant and percent of explants with blue spot were increased (Table 5). Shuangxia et al. found that shoot co-cultivation duration of 48 hours was optimal for developing a highly efficient method of transforming embryogenic callus [19].
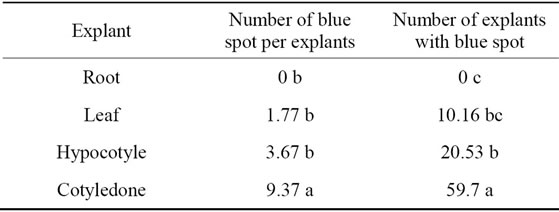
Table 2. Effect of explant on number of blue spot per explants and number of explants with blue spot.

Table 3. Effect of acetosyringone on number of blue spot per explants and number of explants with blue spot.

Table 4. Effect of pre-culture duration on number of blue spot per explants and number of explants with blue spot.
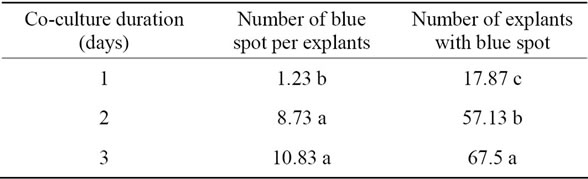
Table 5. Effect of co-culture duration on number of blue spot per explants and number of explants with blue spot.
Current investigation developed a protocol to increase the regeneration and transformation efficiency in spinach. Due to obtain results 15 mg/L NAA increased regeneration and 3 days co-cultivation lead to the best result of transformation.
![]()
![]()
REFERENCES
- Bao, J.H., Chin, D.P., Fukami, M., Ugaki, M., Nomura, M. and Mii, M. (2009) Agrobacterium-mediated transformation of spinach (Spinacia oleracea) with Bacillus thuringiensis cry1Ac gene for resistance against two common vegetable pests. Plant Biotechnology, 26, 249-254. doi:10.5511/plantbiotechnology.26.249
- Geekiyanage, S., Takase, T., Watanabe, S., Fukai, S. and Kiyosue, T. (2006) The combined effect of photoperiod, light intensity and GA3 on adventitious shoot regeneration from cotyledons of spinach (Spinacia oleracea L.) Plant Biotechnology, 23, 431-435. doi:10.5511/plantbiotechnology.23.431
- Neskovic, M. and Radojevic, L. (1973) The growth of and morphogenesis in tissue cultures of Spinacia oleracea L. Bulletin De Linstitute Et Du Jardin Botaniques De Luniversite De Beograd, 8, 35-37.
- Al-Khayri, J.M., Huang, F.H., Morelock, T.E., Busharar, T.A. and Gbur, E.E. (1991) Genotype dependent response of spinach cultivars to in vitro callus induction and plant regeneration. Plant Science, 78, 121-127. doi:10.1016/0168-9452(91)90168-8
- Al-Khayri, J.M., Huang, F.H., Morelock, T.E. and Busharar, T.A. (1992) Stimulation of shoot regeneration in spinach callus by gibberellic acid. Horticultural Science, 27, 1046.
- Komai, F., Okuse, I. and Harada, T. (1996) Somatic embryogenesis and plant regeneration in culture of root segments of spinach (Spinacia oleracea L.). Plant Science, 113, 203-208. doi:10.1016/0168-9452(95)04285-7
- Komai, F., Okuse, I. and Harada, T. (1996) Effective combinations of plant growth regulators for somatic embryogenesis from spinach root segments. Journal of the Japanese Society for Horticultural Science, 65, 559-564. doi:10.2503/jjshs.65.559
- Knoll, K.A., Short, K.C., Curtis, I.S., Power, J.B. and Davey, M.R. (1997) Shoot regeneration from cultured root explants of spinach (Spinacia oleracea L.): A system for Agrobacterium transformation. Plant Cell Reports, 17, 96- 101. doi:10.1007/s002990050359
- Zhang, H.X. and Zeevaart, J.A.D. (1999) An efficient Agrobacterium tumefaciens-mediate transformation and regeneration system for cotyledons of spinach (Spinacia oleracea L.). Plant Cell Reports, 18, 640-645. doi:10.1007/s002990050635
- Ishizaki, T., Komai, F. and Masuda, K. (2001) Screening for strongly regenerative genotypes of spinach in tissue culture using subcultured root explants. Plant Cell, Tissue and Organ Culture, 67, 251-255. doi:10.1023/A:1012791611632
- Molvig, L. and Rose, R.J. (1994) A regeneration protocol for Spinacia oleracea using gibberellic acid. Australian Journal of Botany, 42, 763-769. doi:10.1071/BT9940763
- Goto, T., Miyazaki, M. and Oku, M. (1998) Varietal variations in plant regenerative potential from protoplasts in spinach (Spinacia oleracea L.). Journal of the Japanese Society for Horticultural Science, 67, 503-506.
- Leguillon, S., Charles, G. and Branchard, M. (2003) Plant regeneration from thin cell layers in Spinacia oleracea. Plant Cell, Tissue and Organ Culture, 74, 257-265. doi:10.1023/A:1024042522940
- Mii, M., Nakano, M., Okuda. K. and Iizuka, M. (1992) Shoot regeneration from spinach hypocotyls segments by short term treatment with 5,6-dichloro-indole-3-acetic acid. Plant Cell Reports, 11, 58-61. doi:10.1007/BF00235253
- Ishizaki, T., Megumi, C., Komai, F., Masuda, K. and Oosawa, K. (2002) Accumulation of a 31-kDa glycoprotein in association with the expression of embryogenic potential by spinach callus in culture. Plant Physiology, 114, 109- 115. doi:10.1034/j.1399-3054.2002.1140115.x
- Davey, M.T., Rech, E.L. and Mulligan, B.J. (1989) Direct DNA transfer to plant cells. Plant Molecular Biology, 13, 273-285. doi:10.1007/BF00025315
- Cervera, M., Pina, J.A., Juarez, J., Navarro, L. and Pena, L. (1998) Agrobacterium mediated transformation of citrange: Factors affecting transformation and regeneration. Plant Cell Reports, 18, 271-278. doi:10.1007/s002990050570
- Cao, X., Liu, Q., Rawland, L.J. and Hammerschlag, F.R. (1998) GUS expression in Blueberry (Vaccinium spp.) factors influencing Agrobacterium mediated gene transfer efficiency. Plant Cell Reports, 18, 266-270. doi:10.1007/s002990050569
- Shuangxia, J., Xianlong, Z., Shaoguang, L., Yichun, N., Xiaoping, G. and Chao, H. (2005) Factors affecting transformation efficiency of embryogenic callus of upland cotton (Gossypium hirsutum) with Agrobacterium tumefaciens. Plant Cell, Tissue and Organ Culture, 81, 229-237. doi:10.1007/s11240-004-5209-9
- Moralejo, M., Rochange, F., Boudet, A.M. and Teulieres, C. (1998) Generation of transgenic Eucalyptus globulus plant lets through Agrobacterium tumefaciens-mediated transformation. Australian Journal of Plant Physiology, 25, 207-212. doi:10.1071/PP97041
- Geeta, N., Venkatachalam, P. and Lakshmi, S. (1999) Agrobacterium mediated genetic transformation of pigeon pea (Cajanus cajan L.) and development of transgenic plants via direct organogenesis. Plant Biotechnology, 16, 213- 218. doi:10.5511/plantbiotechnology.16.213
- Lawrence, P.K. and Koundal, K.R. (2001) Agrobacterium tumefaciens mediated transformation of pigeon pea (Cajanus cajan L. Millsp) and molecular analysis of regenerated plants. Current Science, 80, 1428-1432.
- Jefferson, R.A. (1987) Assaying chimeric genes in plantThe GUS gene fusion system. Plant Molecular Biology Reporter, 4, 387-405.
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Plant Physiology, 15, 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
NOTES
*Corresponding author.

