Advances in Bioscience and Biotechnology
Vol.2 No.6(2011), Article ID:8901,7 pages DOI:10.4236/abb.2011.26065
Modulation of biological activities produced by an endophytic fungus under different culture conditions
![]()
Departamento de Ciências Farmacêuticas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil.
Email: *henpramos@yahoo.com.br
Received 17 September 2011; revised 29 October 2011; accepted 25 November 2011.
Keywords: Arthrinium state of Apiospora montagnei Sacc.; Antibacterial; Antifungal; Antitumoral; Antiparasitic
ABSTRACT
The effect of culture conditions on the production of bioactive secondary metabolites by the endophytic fungus Arthrinium state of Apiospora montagnei Sacc. was investigated. Culture broths were partitioned with ethyl acetate and the resulting extracts were evaluated for antibacterial, antifungal, cytotoxic, and antiparasitic activities. The highest levels of activities were arisen from cultures cultivated at 30˚C in modified Czapek medium. The best antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa (MIC 90 μg/ml), and against Aspergillus fumigatus (MIC 130 μg/ml) were found in extracts from cultures grown in medium containing 3.1% (w/v) sucrose and 0.1% (w/v) sodium nitrate at pH 4.0 after 9 days of incubation. Cultures grown in medium without modification also showed antiparasitic and antitumoral activities after 9 days at pH 4.0, antifungal activity against Candida albicans at pH 4.0 - 7.0 (MIC 140 μg/ml), but against A. fumigatus just after 27 days of incubation. A specific bioactive compound may have its production improved if the culture conditions parameters that affect or influence the production were known, which in turn makes the purification process easier.
1. INTRODUCTION
Endophytic fungi have been considered as possible useful sources of natural products in the search for new and innovative biologically active compounds [1-3]. They produce a wide variety of chemical substances, many of which show antibacterial, antifungal, cytotoxicity and anticancer, anti-inflammatory, antioxidant, antiviral, antiinsect, antiparasitic, antidiabetic and immunosuppressive activities [4,5]. Most of the bioprospecting programs on detection and isolation of bioactive compounds are based on single culture condition to screening biological activities of many fungi. Nevertheless, microorganisms are able to synthesize a great variety of secondary metabolites according to their environment and the available nutritional resources [6]. Studies using one fungus cultivated under different culture conditions are an interesting strategy to discover its potentiality to synthesize different bioactive secondary metabolites. Herein, the bioactive secondary metabolites production was evaluated, when the endophytic fungus Arthrinium state of Apiospora montagnei was cultivated, varying parameters like nutrients and nutrient concentrations, pH, temperature, and incubation periods.
2. MATERIALS AND METHODS
The endophytic fungus Arthrinium state of Apiospora montagnei Sacc. (Arthrinium arundinis) collected from thin roots of Smallanthus sonchifolius was isolated, identified and maintained as described by Ramos et al. [7].
2.1. Production of Secondary Metabolites
Secondary metabolites were produced by two-step cultures. A suspension of spores and fungal mycelium were inoculated in Erlenmeyer flask containing 200 ml of prefermentative liquid medium [8] and incubated at 120 rpm for 2 days at 30˚C. Resulting mycelium was harvested, rinsed with sterile distilled water, and transferred to 400 ml of Czapek liquid medium [9]. At this second step, the effects of culture conditions (cultivation time, carbon and nitrogen source, pH, temperature, and carbon/nitrogen ratio) on the secondary metabolites with bioactivity were examined. Parameters of culture conditions were changed one by one. The mycelial mass obtained were separated from the broth by filtration, the culture broths were extracted with ethyl acetate (3 × 200 ml) and dried in a rotary evaporator. The extracts were examined for antibacterial, antifungal and cytotoxic activities as well as inhibition of enzymes from parasites.
2.2. Antimicrobial Assay
MIC of ethyl acetate extracts were determined by microbroth dilution assay on 96-well plates according to Clinical and Laboratory Standards Institute (CLSI) documents M7-A6 [10], M27-A2 [11] and M38-A [12]. Extracts were dissolved in DMSO at 0.1% (v/v) and each well contained 50 µl or 100 µl of extracts diluted two-fold serially in Mueller Hinton Broth or RPMI 1640. In each well, 50 µl of the diluted suspension of Staphylococcus aureus ATCC 25923, Kocuria rhizophila ATCC 9341, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922 was inoculated at final concentrations of 5 × 105 CFU/ml or Candida albicans ATCC 1023 at 2.5 × 103 CFU/ml. To Aspergillus fumigatus assays, 100 µl of crude extract plus 100 µl of fungus suspension containing 5 × 104 CFU/ml was used. After incubation at 35˚C for 24 h (bacteria and C. albicans) and 48 h (A. fumigatus) the MIC was determined. DMSO at 0.1% (v/v) was used to negative control of solvent activity. Penicillin G was used for positive control to S. aureus (MIC 0.046 µg/ml) and K. rhizophila (MIC 0.011 µg/ml), streptomycin sulfate was used to E. coli (MIC 0.023 µg/ml) and P. aeruginosa (MIC 0.023 µg/ml), miconazole was used to C. albicans (MIC 0.36 µg/ml) and A. fumigatus (MIC 0.73 µg/ml).
2.3. Morphological Alteration Assay
Morphological alterations on A. fumigatus vegetative hyphae grown on PDB medium and incubated at 30˚C were analyzed. A spore suspension (1.3 × 102 CFU/ml) was inoculated into each well of 96-well plates containing 100 µl of extracts two-fold and serially diluted. Plates were incubated for 6, 12, 18, or 24 h, after which the cultures were observed under a Zeiss stereomicroscope.
2.4. Antiparasitic Assays
Antiparasitic activity was analyzed by the enzymatic inhibition of gGAPDH of Trypanosoma cruzi and APRT of Leishmania tarentolae. Both enzymes were recombinants obtained in an E. coli expression [13,14]. The gGAPDH assay was performed according to Barbosa and Nakano [15]. Briefly, the reaction mixture contained 50 mmol/l Tris-HCl pH 8.6, buffer with 1 mmol/l EDTA and 1 mmol/l β-mercapto-ethanol, 30 mmol/l Na2HAsO4, 2.5 mmol/l NAD+, 0.3 mmol/l glyceraldehyde-3-phosphate and 1.5 μg protein, in a total volume of 1 ml. The reaction was initiated by the addition of enzyme, NADH that was formed was evaluated at 340 nm at 30 s intervals. APRT activity was determined by spectro-photometric measurements of AMP resultant at 259 nm after 60 s [16]. The reaction mixture contained 100 mmol/l Tris-HCl, pH 7.4, 5 mmol/l MgCl2, 100 mmol/l adenine, 560 mmol/l phosphoribosyl pyrophosphate, and 7.5 μg APRT, in a total volume of 1 ml. Extracts were evaluated at a concentration of 100 µg/ml (gGAPDH) or 50 µg/ml (APRT). All the measurements were carried out in triplicate. Negative controls with 1% DMSO were used.
2.5. Cytotoxicity Assay
The cytotoxicity of the fungal extracts was performed using the MTT assay [17]. Briefly, 3 tumor cell lines were used, HCT-8 (colon), MDA-MB-435 (breast), and SF295 (brain) cells (1 × 105 cells/well or 0.5 × 105 cells/well) were incubated in 96-well plates during 24 h and treated with 200 µg/ml of extracts for 72 h. Then, the medium was removed, and 100 µl of MTT reagent (0.5 mg/ml) was added. After another 3 h, MTT was removed, and 150 µl of DMSO was added. Absorbance was measured using a plate reader (Spectra Count, Packard, Ont., Canada), drug effect was quantified as the percentage of control absorbance of reduced dye at 595 nm.
3. RESULTS
3.1. Effect of Different Culture Conditions on the Production of Bioactive Substances
3.1.1. Incubation Time
The effect of incubation time on bioactivity synthesis is shown in Tables 1 and 2. Antibacterial activity, especially against E. coli (MIC 110 μg/ml) was detected in the extract from fungus cultivated for 9 days. On the other hand, antifungal activity against C. albicans (MIC 240 μg/ml) was present in the fungal extracts after 15th and 18th days of incubation. After 21st, 24th, and 27th days of incubation, the antifungal activity was just against A. fumigatus. The extract obtained on 27th day of incubation presented strong cytotoxic activity against the tumor cell line HCT-8 and moderate activity against SF295. The 18-day extract, which showed low level of antimicrobial activity, was strongly active against ever tumor cell line tested, and moderately active against APRT.
3.1.2. Carbon and Nitrogen Sources
Extracts obtained from cultures supplemented with sucrose provided the best levels of antimicrobial activity, enzymatic inhibition of gGAPDH and APRT, and cytotoxic activity, except for HCT-8 cell line, whose extracts from maltose cultures showed more cytotoxic activity than with sucrose (Tables 3 and 4). No activities were detected in extracts from cultures supplemented with sucrose for 9 days of incubation and supplemented with different nitrogen sources, (NH4)2SO4, NH4NO3, proline, or casamino acids (data not shown). Bioactivities were presented only in cultures supplemented with sodium nitrate. The carbon and nitrogen ratio was also evaluated, after the establishment of the ideal pH and temperature.
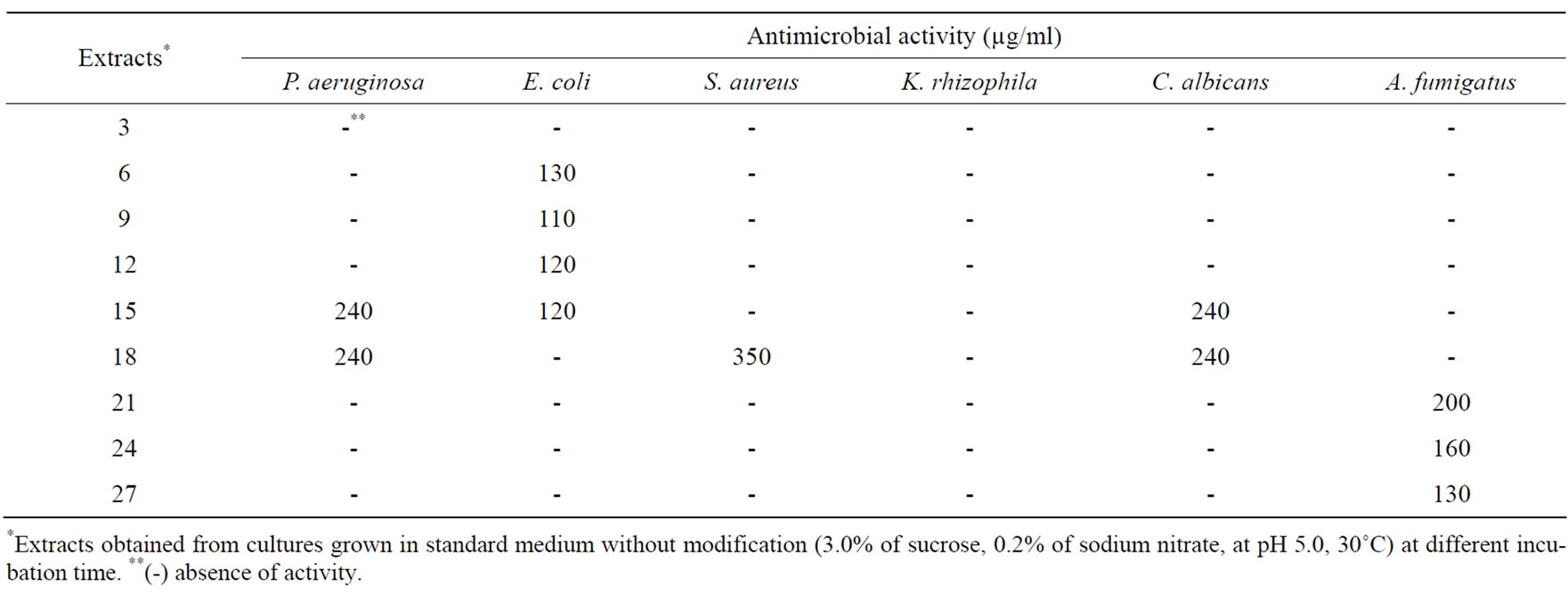
Table 1. Effect of incubation periods on antimicrobial metabolites production.
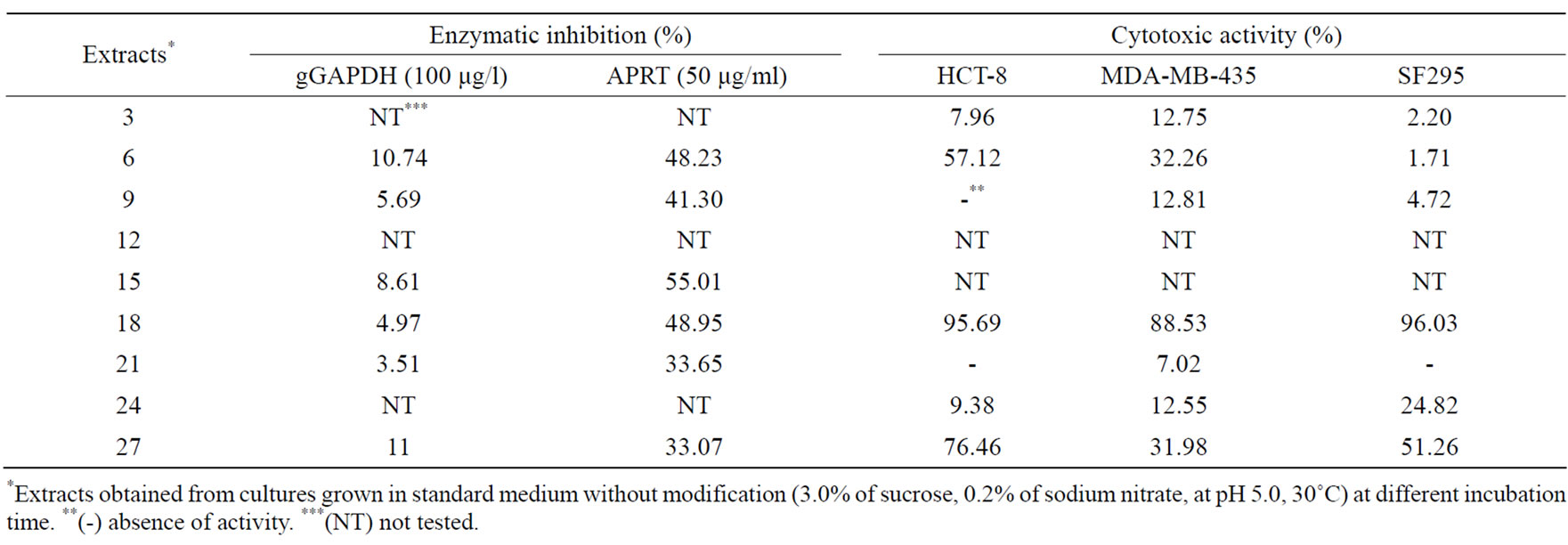
Table 2. Effect of incubation periods on cytotoxic and antiparasitic metabolites production.
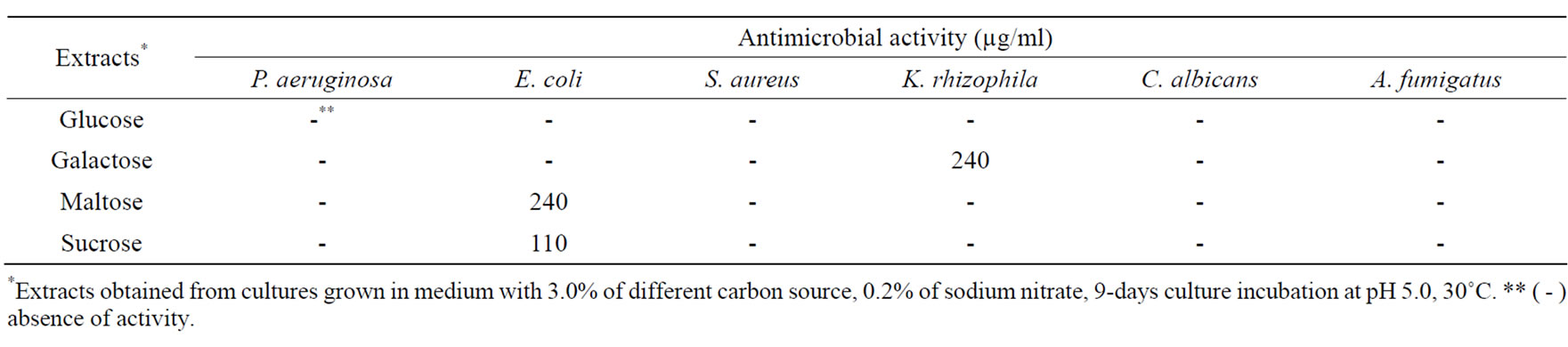
Table 3. Effect of carbon source on antimicrobial metabolites production.
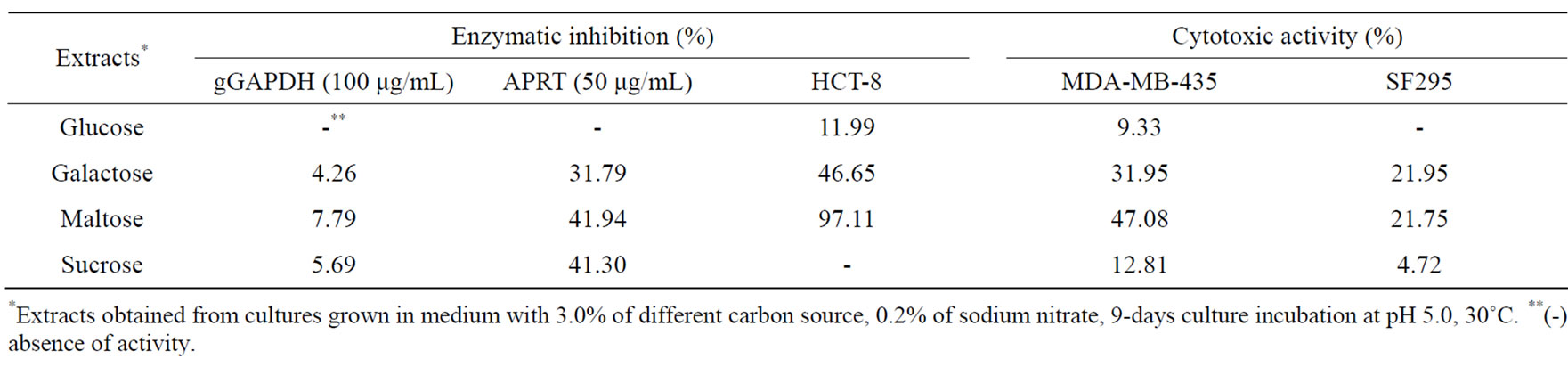
Table 4. Effect of carbon source on cytotoxic and antiparasitic metabolites production.
Table 5 shows that while antimicrobial activity against E. coli (MIC 90 μg/ml), P. aeruginosa (MIC 90 μg/ml), and A. fumigatus (MIC 130 μg/ml) increased when the fungus was incubated in culture medium containing 3.1% of sucrose and 0.1% of sodium nitrate, antifungal activity against C. albicans (MIC 250 μg/ml) and enzymatic inhibition of APRT activity (45.67% of inhibition) decreased. Fungus cultures grown in medium without nitrogen source and high levels of carbon source, as well as low levels of carbon source and high levels of nitrogen source, showed no activities detected in their extracts.
3.1.3. pH
When the fungus was incubated at pH 4.0, the level of antibacterial activity was increased (Table 6). No antibacterial activity was found in extracts obtained from cultures at pH 3.0 and pH 7.0. Antifungal activity as well as enzymatic inhibition of APRT, and cytotoxic activity against tumor cell lines was found in extracts of every pH value evaluated, except for pH 5.0 (Tables 6 and 7).
3.1.4. Temperature
Antimicrobial activity was just obtained when the fungus was incubated at 30˚C, except for antifungal activity against C. albicans (Table 8). Enzymatic inhibition of APRT activity was not affected by incubation temperature. Strong cytotoxic activity against tumor cell lines was found in extracts of cultures incubated at 25˚C and 30˚C (Table 9).

Table 5. Effect of carbon/nitrogen ratio on bioactivities metabolites production.
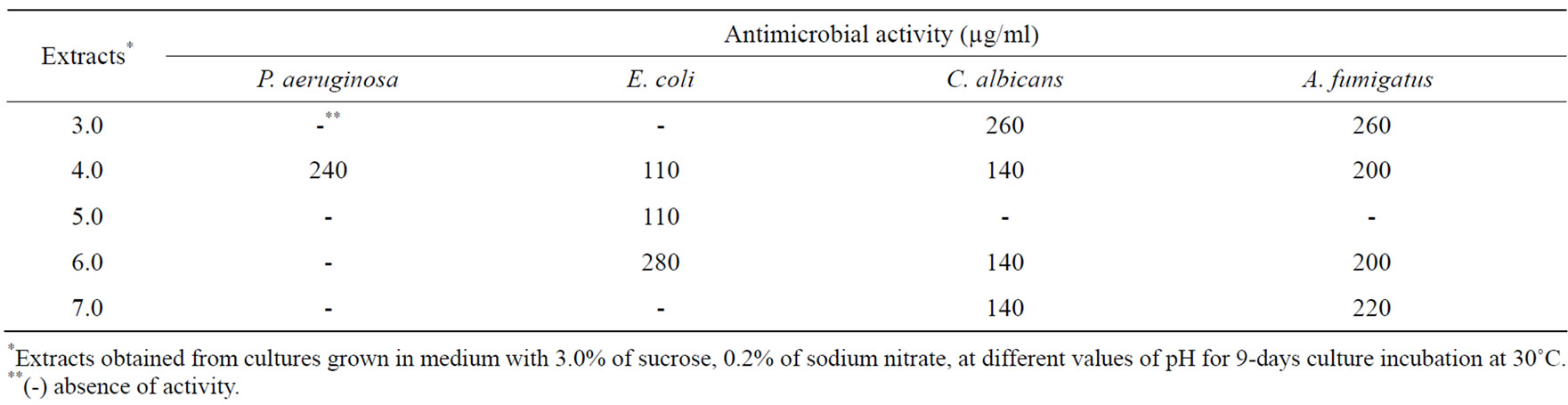
Table 6. Effect of pH on antimicrobial metabolites production.
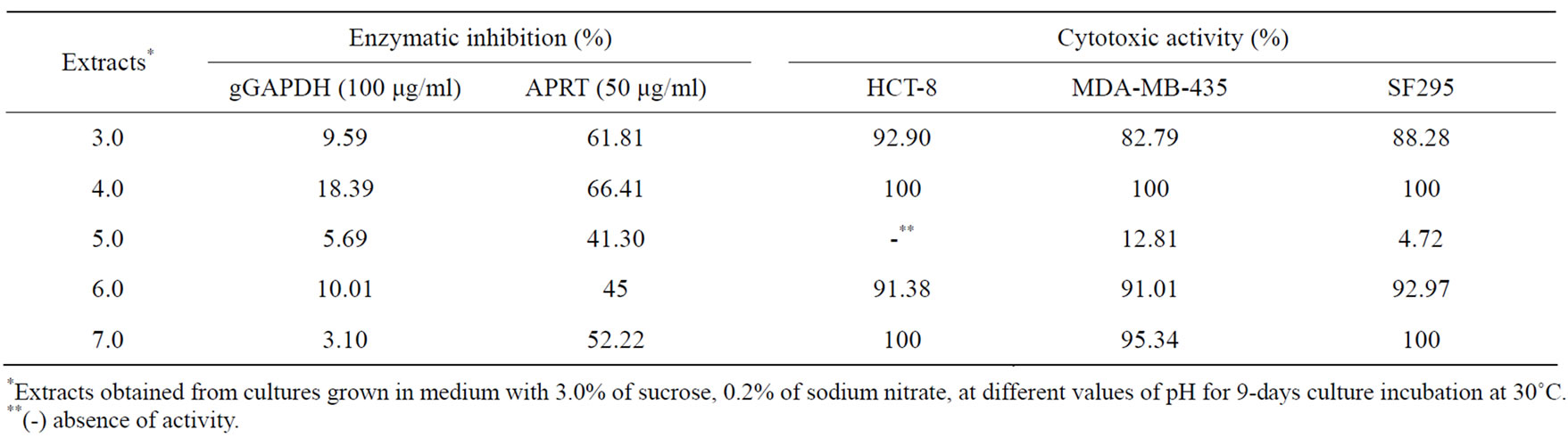
Table 7. Effect of pH on cytotoxic and antiparasitic metabolites production.
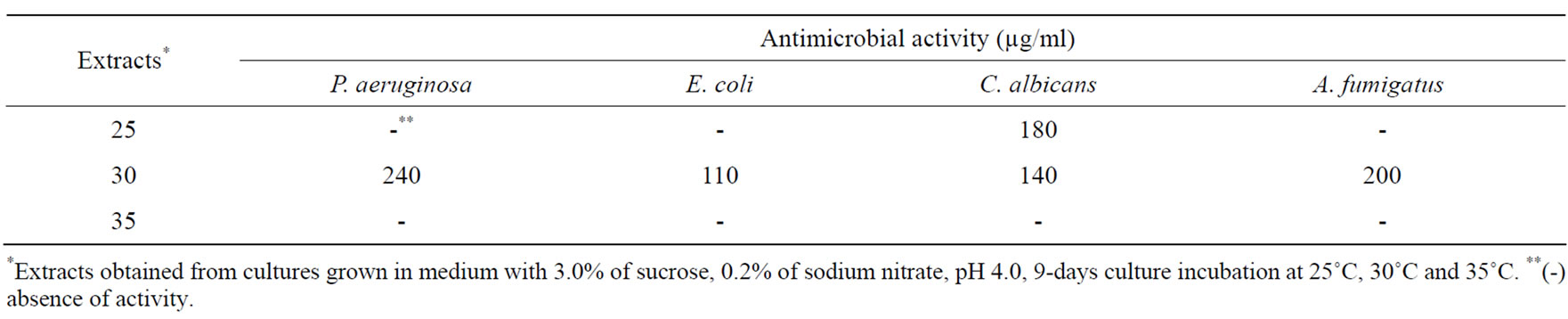
Table 8. Effect of incubation temperature on antimicrobial metabolites production.
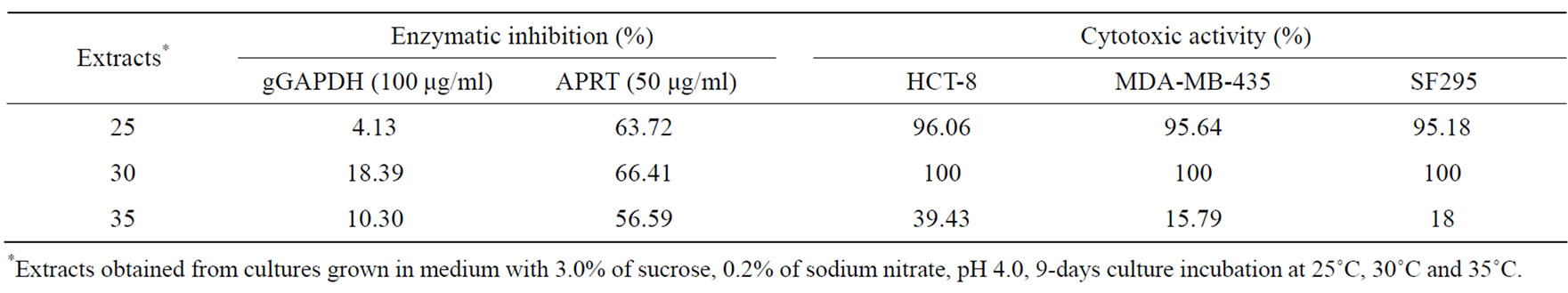
Table 9. Effect of incubation temperature on cytotoxic and antiparasitic metabolites production.
3.2. Morphological Alteration
None of the extracts that presented antifungal activity showed capacity to produce morphological alterations on A. fumigatus (data not shown).
4. DISCUSSION
According to Cabello et al. [18], Arthrinium state of Apiospora montagnei Sacc. produces arundifungin, an antifungal of glucan synthesis inhibitor. This class of antifungals is known to alter the morphology of filamentous fungi. This compound was not produced by the endophytic strain isolated from S. sonchifolius when cultivated at the described conditions. Many bioactivities are being found in extracts of Arthrinium state of Apiospora montagnei Sacc. as cytotoxic [19], proteasome inhibitors [20] and antimicrobial [21]. Up to the present, no data on antiparasitic activity produced by Arthrinium state of Apiospora montagnei Sacc. has been reported. Although, crude extracts of endophytic Arthrinium strains isolated from leaves of bioactive Brazilian plant species Palicourea tetraphylla, Piptadenia adiantoides and Trixis vauthieri, showed cytotoxic against human cancer cells, trypanocidal and leishmanicidal activities [22].
In fungi, the biosynthesis of secondary metabolites is regulated in response to nutrient availability, or as a result of changes in the environment, and its stage of development [23]. Many carbon and nitrogen substrates can inhibit secondary metabolite production. High concentrations of glucose, phosphate, and ammonium are repressors of secondary metabolism, and the carbon/ nitrogen ratio can affect the type and yield of metabolite synthesis [24]. Here, the period of incubation, pH values, and temperature were the parameters, which mostly affected the production of bioactive secondary metabolites. The effect of incubation time on antibacterial, antiparasitic and antitumoral activities indicated a sequential synthesis of secondary metabolites. Antifungal and antitumoral activities were detected at different pH values. It is known that secondary metabolites production is controlled by global transcription factors encoded by the genes responsive to environmental cues. Miao et al. [24] suggested that the two optimal pH levels (4.5 and 7.5) on bioactive metabolites production by Arthrinium c.f. saccharicola, might be due to different compound synthesis at these pH. Here, this factor might be responsible for the results detected for antifungal and antitumoral activities but not at the pH 5.0, probably because this is a value of pH more propitious to fungal grow than to synthesize secondary metabolites.
Different population of fungi species, genetically and metabolically diverse, from different habitats results in variation among secondary metabolites production [25, 26]. Thus, bioactivities reported here may be due to other substances produced by the endophytic fungus Arthrinium state of Apiospora montagnei Sacc. than those reported before. Studies using one single fungus cultivated under different culture conditions are not only suitable to produce different compounds, but also provide conditions to guide the production of a specific compound and become easier for the purification process.
5. ACKNOWLEDGEMENTS
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-Proc n: 04/07935-6), HP Ramos received a masters fellowship from FAPESP (Proc n: 05/58427-3). We are grateful to all the staff of Laboratório de Cristalografia de Proteínas e Biologia Estrurural-Instituto de Física de São Carlos—USP and Departamento de Fisiologia e Farmacologia da Universidade Federal do Ceará, Fortaleza, CE - Brazil for antiparasitic and cytotoxicity assays, respectively.
REFERENCES
- Borges, W.S., Borges, K.B., Bonato, P.S., Said, S. and Pupo, M.T. (2009) Endophytic fungi: Natural products, enzymes and biotransformation reactions. Current Organic Chemistry, 13, 1137-1163. doi:10.2174/138527209788921783
- Newman, D.J. and Cragg, G.M. (2007) Natural products as sources of new drugs over the last 25 years. Journal of Natural Products, 70, 461-477. doi:10.1021/np068054v
- Strobel, G.A., Daisy, B., Castillo, U. and Harper, J. (2004) Natural products from endophytic microorganisms. Journal of Natural Products, 67, 257-268. doi:10.1021/np030397v
- Demain, A.L. (1999) Pharmaceutically active secondary metabolites of microorganisms. Applied Microbiology and Biotechnology, 52, 455-463. doi:10.1007/s002530051546
- Firáková, S., Sturdíková, M. and Múcková, M. (2007) Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia (Bratislava), 62, 251-257. doi:10.2478/s11756-007-0044-1
- Bode, H.B., Bethe, B., Höfs, R. and Zeeck, A. (2002) Big effects from small changes: Possible ways to explore nature’s chemical diversity. Biochemistry (Chemical Biology), 3, 619-627. doi:10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9
- Ramos, H.P., Braun, G.H., Pupo, M.T. and Said S. (2010) Antimicrobial activity from Endophytic fungi Arthrinium state of Apiospora montagnei Sacc. and Papulaspora immersa. Brazilian Archives of Biology and Technology, 53, 629-632. doi:10.1590/S1516-89132010000300017
- Jackson, M., Karwowski, J.P., Humphrey, P.E., Kohl, W.L., Barlow, G.J. and Tanaka, S.K. (1993) Calbistrins, novel antifungal agents produced by Penicillium restrictum. I. Production, taxonomy of the producing organism and biological activity. Journal of Antibiotics, 46, 34-38.
- Alviano, C.S., Farbiarz, S.R., Travassos, L.R., Angluster, J. and Souza, W. (1992) Effect of environmental factors on Fonsecaea pedrosoi morphogenesis with emphasis on sclerotic cells induced by propanol. Mycopathologia, 119, 17-23.
- National Committee for Clinical Laboratory Standards. (2003) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard NCCLS document M7-A6. NCCLS, Wayne.
- National Committee for Clinical Laboratory Standards. (2002) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast; Approved Standard NCCLS document M27-A2. NCCLS, Wayne.
- National Committee for Clinical Laboratory Standards. (2002) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard NCCLS document M38-A. NCCLS, Wayne.
- Souza, D.H.F., Garratt, R.C., Araújo, A.P.U., Guimarães, B.G., Jesus, W.D.P., Michels, P.A.M., Hannaert, V. and Oliva, G. (1998) Trypanosoma cruzi glycosomal glyceralehyde-3-phosphate dehydrogenase: Structure, catalytic mechanism and targeted inhibitor design. FEBS Letters, 424, 131-135. doi:10.1016/S0014-5793(98)00154-9
- Thiemann, O.H., Alfonzo, J.D. and Simpson L. (1998) Cloning and characterization of Leishmania tarentolae adenine phosphoribosyltransferase. Molecular and Biochemical Parasitology, 95, 141-146. doi:10.1016/S0166-6851(98)00089-9
- Barbosa, V. and Nakano, M. (1987) Muscle D-glyceraldehyde-3-phosphate dehydrogenase from Anas sp.—1. Purification and properties of the enzyme. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 88, 563-568. doi:10.1016/0305-0491(87)90345-2
- Tuttle, J.V. and Krenitsky T.A. (1980) Purine Phosphoribosyltransferases from Leishmania donovani. Journal of Biological Chemistry, 255, 909-916.
- Oliveira, J.S., Bezerra, D.P., Freitas, C.D.T., MarinhoFilho, J.D.B., Moraes, M.O., Pessoa C., Costa-Lotufo, L.V. and Ramos, M.V. (2007) In vitro cytotoxicity against different human cancer cell lines of laticifer proteins of Calotropis procera (Ait.) R. Br. Toxicology in Vitro, 21, 1563-1573. doi:10.1016/j.tiv.2007.05.007
- Cabello, M.A., Platas, G., Collado, J., Díez, M.T., Martín, I., Vicente, F., Meinz, M., Onishi, J.C., Douglas, C., Thompson, J., et al. (2001) Arundifungin, a novel antifungal compound produced by fungi: Biological activity and taxonomy of the producing organisms. International Microbiology, 4, 93-102.
- Klemke, C., Kehraus, S., Wright, A.D. and König, G.M. (2004) New Secondary Metabolites from the Marine Endophytic Fungus Apiospora montagnei. Journal of Natural Products, 67, 1058-1063. doi:10.1021/np034061x
- Kohno, J., Koguchi, Y., Nishio, M., Nakao, K., Kuroda, M., Shimizu, R., Ohnuki, T. and Komatsubara, S. (2000) Structures of TMC-95A-D: Novel Proteasome Inhibitors from Apiospora montagnei Sacc. TC 1093. Journal of Organic Chemistry, 65, 990-995. doi:10.1021/jo991375
- Alfatafta, A.A., Gloer, J.B., Scott, J.A. and Malloch, D. (1994) Apiosporamide, a new antifungal agent from the coprophilus fungus Apiospora montagnei. Journal of Natural Products, 57, 1696-1702. doi:10.1021/np50114a012
- Rosa, L.H., Gonçalves, V.N., Caligiorne, R.B., Alves, T.M.A., Rabello, A., Sales, P.A., Romanha A.J., Sobral, M.E.G., Rosa, C.A. and Zani, C.L. (2010) Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Brazilian Journal of Microbiology, 41, 420-430. doi:10.1590/S1517-83822010000200024
- Sanchez, S. and Demain, A.L. (2002) Metabolic regulation of fermentation processes. Enzyme and Microbial Technology, 31, 895-906. doi:10.1016/S0141-0229(02)00172-2
- Miao, L., Kwong, T.F.N. and Qian, P.Y. (2006) Effect of culture conditions on mycelial growth, antibacterial activity, and metabolite profiles of the marine-derived fungus Arthrinium c.f. saccharicola. Applied Microbiology and Biotechnology, 72, 1063-1073. doi:10.1007/s00253-006-0376-8
- Freitas, T.P.S., Furtado, N.A.J.C., Bastos, J.K. and Said, S. (2002) Active substances against trypomastigote forms of Trypanosoma cruzi and microorganisms are produced in sequence by Talaromyces flavus. Microbiological Research, 157, 201-206. doi:10.1078/0944-5013-00148
- Seymour, A.F., Cresswell, J.E., Fisher, P.J., Lappin-Scott, H.M., Haag, H. and Talbot, N.J. (2004) The influence of genotypic variation on metabolite diversity in populations of two endophytic fungal species. Fungal Genetics and Biology, 41, 721-734. doi:10.1016/j.fgb.2004.02.007

