Green and Sustainable Chemistry
Vol.1 No.3(2011), Article ID:7076,5 pages DOI:10.4236/gsc.2011.13016
Preparation of BiVO4@Fiber Composites and the Photocatalytic Property for Degradation of Organic Dyes under Visible-Light
Key Laboratory of New Fiber Materials and Modern Textile, Qingdao University, Qingdao, China
School of Chemistry, Chemical and Environmental Engineering, Qingdao University, Qingdao, China
E-mail: *jianqyu@qdu.edu.cn
Received April 9, 2011; revised May 18, 2011; accepted May 25, 2011
Keywords: BiVO4@Fibers Composite Photocatalyst, Red FN-3G Decomposition, Photocatalysis
Abstract
In this article, a novel BiVO4@fibers composite photocatalyst was prepared by a process that monoclinic scheelite BiVO4 nano/micro particles were in situ formated onto fiber materials. The structure, morphology and photophysical properties of the composite materials were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and UV-Vis diffuse reflectance spectroscopy, respectively. The immobilization of BiVO4 photocatalyst on fibers reduced the particle size of the photoactive phase, and a few visible-light absorption abilities. The decomposition of a non-biodegradable dye Red FN-3G was selected to examine the photocatalytic activity of the composite photocatalyst. It was found that the formation of composite materials of BiVO4 with fibers didn’t decrease the photocatalytic activity with comparison to that of pure BiVO4. Moreover, it demonstrated that when adjusting the dye solution into about pH = 3, the highest efficiency of dye degradation over the fiber composite material can be obtained.
1. Introduction
It is a dream for mankind to convert sustainable solar energy to electric and chemical energies. People has been pursuing this dream for hundreds of years until the photoelectrochemical splitting of water to produce H2 and O2 under light irradiation (the Honda-Fujishima effect) was discovered in 1972 [1]. At present, great progresses had been made on this area, and to date, the design and development of visible-light responsive photocatalysts as well as the improvement of photocatalytic reactivity and efficiency became the major goals that most of the researches focused on [2,3].
For the first goal, it is utmost important to develop photocatalyst with narrow band-gap provide that we could know how to engineer smaller band-gap materials with more suitable band edge positions. One of the efforts consists of creating an electron donor level between the valence band and conduction band of TiO2 by doping with metallic or nonmetallic elements such as V and Cr or N, S, and C. However, although the doping of foreign elements extends the absorption to visible-light ranges, it increases the defects of semiconductor photocatalysts, which therefore, a part of the ultraviolet light-responsive performance that the titanium oxide originally possessed was occasionally ruined. This situation led to fewer breakthroughs for direct utilization of solar energy. Another effort is the exploring of complex compounds that containing Bi3+, In3+, Sn2+ (s2 configuration) or Ag+ (d10 configuration) ions in an oxide system. Thus it is able to elevate the valence band by means of the hybridization of their respective orbitals with the O2p orbital, and also narrowing the band gap of the semiconductor [4].
Of various visible-light responsive metal-oxide photocatalysts studied thus far, BiVO4 is one of the typical complex oxides with narrow band-gap that showed not only excellent visible-light photocatalytic properties but also high photo-to-current conversion efficiency [5-9]. However, at the present stage, BiVO4 is still far away the practical applications due to a number of devices and material parameters have yet to be optimized, e.g. the morphology, particle size, et al., which leading to the efficient dissociation of all generated excitons, and subsequent transport of charge carriers out of the particles. Moreover, because the semiconductor particles are slight and hard to settle down, powder photocatalyst can be easily drained away in waste water treatment and is therefore go against to the regeneration and recycle, causing waste and secondary pollution [10]. However, this problem might be solved by dispersing the photocatalysts onto a support. Nonwoven fiber material is a green material. It provided with flexile, soft, easy to be decomposed, nontoxic, nonirritating, inexpensive and recyclable characters, which could be used not only as absorbing material, but also be functionalized to new material by means of physics, chemistry methods. These nonwoven could be used as the material of loading catalyst.
Several approaches have been reported for the preparation of BiVO4, such as solid-state reaction, coprecipitation, hydrothermal treatment and metalorganic decomposition [11-14]. However, there is almost no report about catalyst loading on nonwoven fibers. In this article, the loading of BiVO4 particles on fiber materials to form BiVO4@fibers photocatalyst by coprecipitation process were reported. An active and nonbiodegradable dye Red FN-3G was used to estimate the photocatalytic properties. The pH condition was also adjusted to get a better activity.
2. Experiments
2.1. Preparation of BiVO4@Fibers Photocatalyst
All chemicals (Shanghai Chemical Reagents Company, China) were of analytic grade and used directly without any further purification, and solutions were prepared using deionized water. BiVO4@fibers composite photocatalyst was synthesized by an in-situ co-precipitation process. First, the required amount of Bi(NO3)3·5H2O and NH4VO3 were separately dissolved in 2.0 mol/L of nitric acid solution. 5 g of urea was added into the solution of Bi(NO3)3 and NH4VO3, then pretreated fibers were added. The solution was then stirred at 353 - 363 K for 12 h. At last, the mixture was washed, and dried. The BiVO4@fibers photocatalyst was received. As comparison, pure BiVO4 was also prepared in the same procedure except without fibers were added.
2.2. Characterizations
X-ray powder diffraction (XRD) patterns were recorded in the range of 2q (5 - 75) with CuK radiation (λ = 0.1541 nm) using a Bruker AXS, D8 Advance X-ray diffractometer. The morphologies and microstructures were determined by a scanning electron microscopy (SEM, JEOL JSM-6390LV). Diffuse reflectance UV-vis spectrophotometer (DRS, Peking Puxi TU-1901) was used to obtain the absorption spectra of samples in the region of 200 - 800 nm using BaSO4 (IS19-1) as a reference.
2.3. Photocatalytic Performances
Photocatalytic activities of the BiVO4@fibers were evaluated by the degradation of red FN-3G dye in a glass reactor with a circulating water system. The light source was a 300 W Xe illuminator (Peking Changtuo, PLS- 3XE300) and about 7 cm apart from the liquid level. The 420 nm cutoff filter was placed between the Xe-illuminator and the reactor to ensure the reaction occurred under visible-light condition. About 0.2 g catalyst laden fiber composite was added into 150 ml of 15 mg/l red FN-3G solution. Before illumination, the dye solution were stirred in the dark for 30 minutes to reach a adsorption/desorption equilibrium for the dye and fiber composite, then the light was switched on, and the solution were kept stirring in the process of illumination. At a certain intervals, a few amount of solution were collected, measured by UV-vis spectrophotometer (Shanghai Longnike, UNIC7200) at the absorbance at 490 nm. We also observed the photocatalytic effects under different pH.
3. Results and Discussions
3.1. The Crystalline Structure of BiVO4
Figure 1 shows the X-ray diffraction patterns of the nonwoven fibers, pure BiVO4 and the synthesized composite material. It is observed that all the peaks of BiVO4 and the composite material are match well to the JCPDS No.14-0688 standard card, confirming that the crystal formatted on the fibers is similar to pure phased powder and showed a monoclinic scheelite BiVO4 with high crystallinity. At BiVO4@fibers composite, there is no peaks of any other phases or impurities can be detected in the composite sample, but have a little lower diffraction intensities than the pure BiVO4.
As we have known, BiVO4 exists naturally in three different phases, monoclinic sheelite, tetragonal zircon and tetragonal sheelite. The photocatalytic properties of BiVO4 are strongly related to its crystal phase, and only monoclinic scheelite structure showed high photocatalytic activity under visible-light irradiation [15,16]. So the target for synthesis of BiVO4 is to form more amount of monoclinic scheelite structured material. The above XRD results suggested that the fibers gave a tiny effect on the crystalline structure of BiVO4.
3.2. The Morphologies of BiVO4
The SEM images of different catalysts are shown in Figure 2. The primary particles of pure BiVO4 presented a
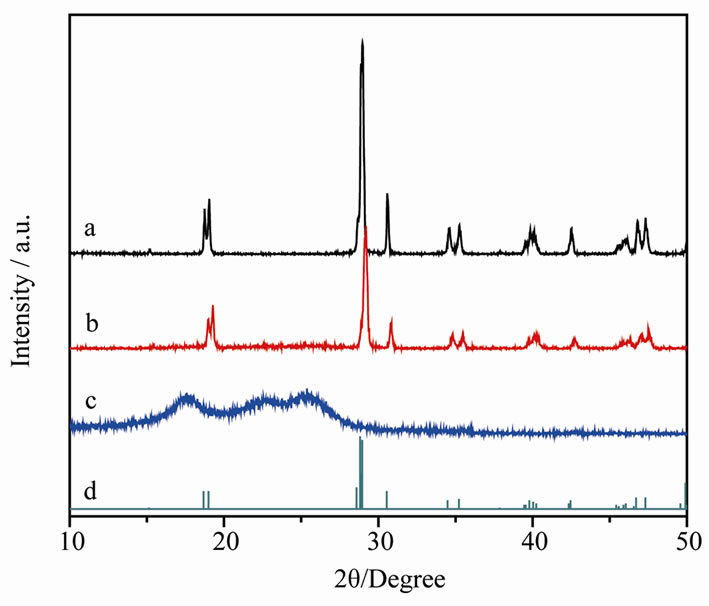
Figure 1. XRD patterns of (a) BiVO4 powder, (b) BiVO4@ fibers composite, (c) Blank fiber, (d) JCPDS No.14-0688 standard card.
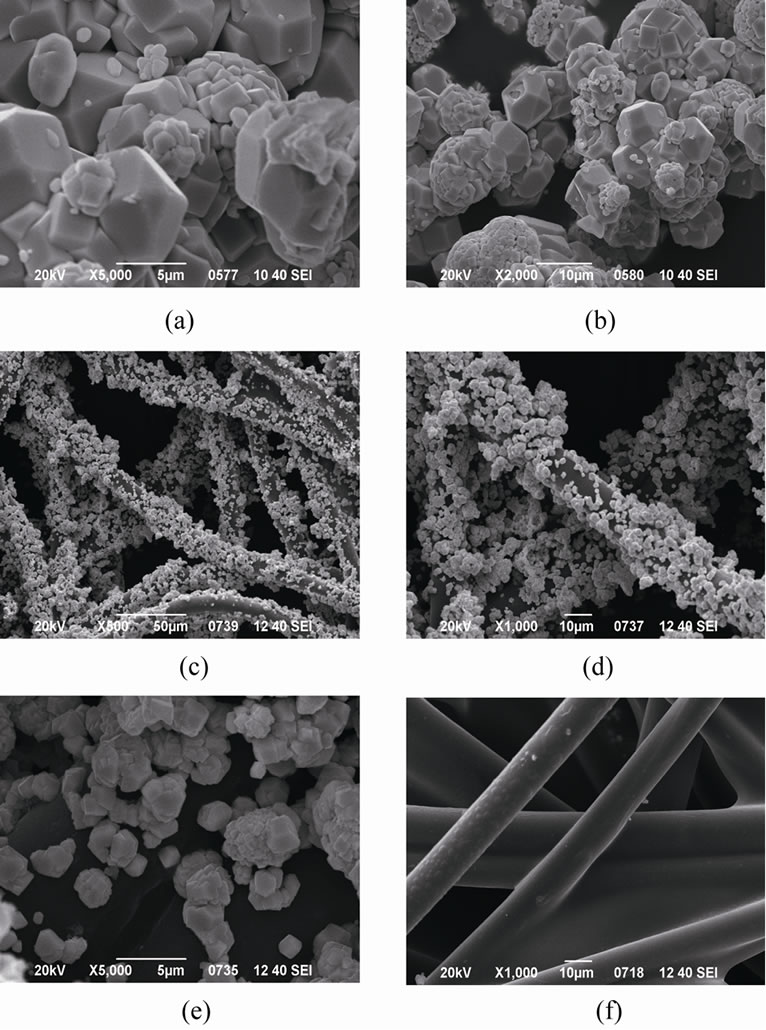
Figure 2. SEM micrographs of (a) (b) BiVO4 powders, (c) (d) (e) BiVO4@fibers composite, (f) Blank fiber.
little large size and aggregated with each other strongly to be a cluster as shown in Figures 2(a) and (b). From Figures 2(c)-(e), with comparison to Figure 2(f), it could be seen that the BiVO4 particles are attached tightly on the surface of the fiber materials, by which the BiVO4 particles cannot be removed during the practical applications. Moreover, it can be observed clearly from Figures 2(a) and (e) that when BiVO4 was loaded on the surface of nonwoven fiber, the particle size appeared to be much smaller than that of BiVO4 powder. This result suggested that the confine effect of fibers is beneficial for the formation of nano/micro particles of BiVO4.
3.3. UV-Vis Diffusion Reflectance Spectra
The UV-Vis diffuse reflectance spectra of the pure BiVO4, the synthesized composite material and the nonwoven fibers, are depicted in Figure 3. The nonwoven fibers gave a strong absorption in the UV regin of 200 - 340 nm. It can also absorb some portion of light with wavelength higher than 350 nm. When the spectra of the synthesized composite material and pure BiVO4 are compared, it can be found that the absorption edges have almost no difference, only a small difference in the absorption of wavelength higher than 550 nm was observed. The pure BiVO4 has almost no absorption in the region of 550 - 800 nm, but BiVO4@fibers have some parts of absorption in this region. This result may be attributed to the absorption of fibers, indicating that the fiber materials gave a certain influence on the light absorption of BiVO4@fibers composites. This absorption might redound to the improvement of photocatalytic activity.
3.4. The Photocatalytic Performances
The photocatalytic performance of BiVO4@fibers composites were evaluated in terms of the degradation of Red FN-3G in aqueous solution under visible-light irradiation. Red FN-3G is an active dye which is hard to be decomposed by biological process. Figure 4 shows the results of Red FN-3G photodecomposition over BiVO4@fibers and pure BiVO4 sample. As a comparison, the photodecomposition of Red FN-3G was also performed without adding photocatalyst. The result without photocatalyst indicates that the decomposition of Red FN-3G could be ignored after 10 h of irradiation. However, in 10 hours irradiation, 45% of Red FN-3G can be decomposted by pure BiVO4, and about 50% of dyes can be photodecomposed over BiVO4@fibers composites. The BiVO4@ fibers photocatalyst gave a similar activity to pure BiVO4. The particles size of BiVO4 formatted on the composite photocatalyst was smaller than that of pure BiVO4, which may be beneficial for the improvement of photocatalytic activity. However, the available surface was fewer due to part of the surface was adhered to fiber and the amount of photoactive BiVO4 was much lower than that of pure BiVO4 powder. Thereby, these two reverse
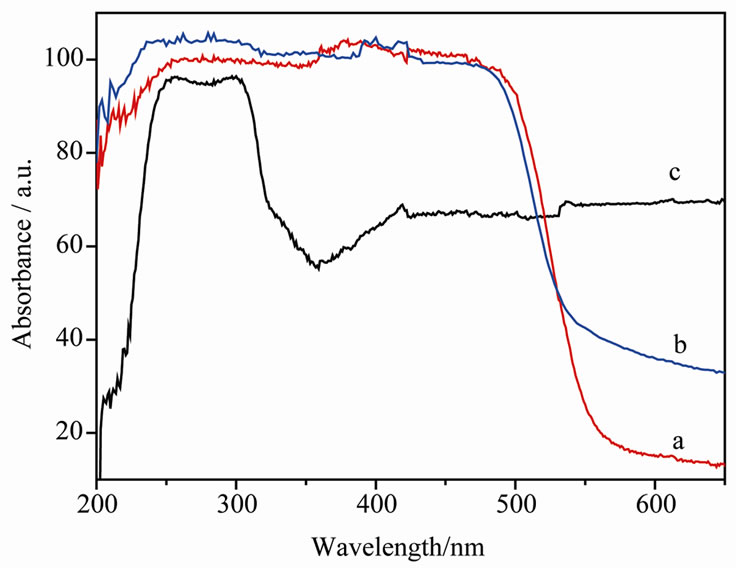
Figure 3. Diffuse-reflectance spectra of (a) BiVO4 powder, (b) BiVO4@fibers composite, (c) Blank fiber.

Figure 4. Comparison of degradation red FN-3G efficiency over (a) Blank solution, (b)BiVO4 powder, (c)BiVO4@fibers composite.
effects make a similar photocatalytic degradation efficiency over composite photocatalyst with pure BiVO4. Furthermore, the reactor used in our experiment may have influence on the light absorption, which resulted in low fraction of light are irradiated. The BiVO4@fibers is soft and sometimes was folded during the experiment, the received illumination upon the active BiVO4 may be decreased, by which the photocatalytic activity was ruined.
In order to improve the activity of BiVO4@fibers, we tried to change the pH to observe the photocatalytic property. In this experiment, NaOH and HCl were used to adjust the pH. From Figure 5, we could see that the photocatalytic activity is the best when pH is 3. That is to say, BiVO4 get better catalytic effect under acidic condition, and then followed neutral, alkalinity. And with a higher acidity of the dye solution give a better decomposition efficiency. This observation may be attributed to
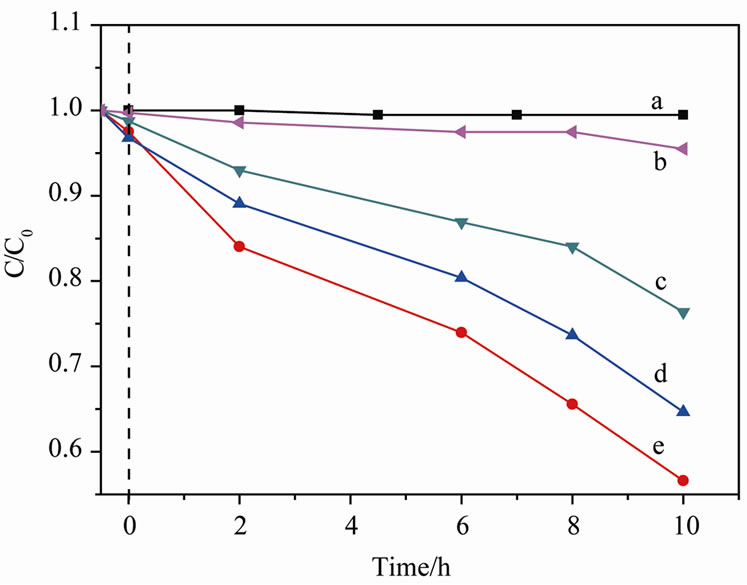
Figure 5. Comparison of degradation red FN-3G efficiency at different pH (a) Blank solution (b) PH = 9, (c) PH = 7, (d) PH = 5, (e) PH = 3.
the charge character formed on the surface of BiVO4. Because BiVO4 is an n-type semiconductor, the isoelectric point of BiVO4 is arrived at alkaline addition. When the pH was adjusted to 9, almost no net charges on the surface of BiVO4 are left, the solution will be tend to balance. However, in acidic condition, the H+ in the acid solution promote the formation of B acid site on the surface of BiVO4, which is favourable for the photogenerated electrons on BiVO4’s conduction band move to surface, leading to the effective separation of photonactivated electrons and hole, thus enhance the phtotcatalytic efficiency.
4. Conclusions
BiVO4 with a small particle-size has been immobilized on the surface of fiber materials. The synthesized fiber composites exhibited the typical monoclinic scheelite BiVO4 structure. The BiVO4@fibers showed the absorption in the visible region between 550 and 800 nm, though presented a little blue shift compared with pure BiVO4. It was also found that a better photocatalytic activity over BiVO4 is in acidic condition. The photocatalytic activity of BiVO4@fibers was similar to that of pure BiVO4. The results improved the industrial applications of this advanced material.
5. Acknowledgements
The financial supports from the National Basic Research Program of China (973 Program, No. 2009CB220000), the National Foundation of Natural Sciences (No. 20973097, No. 50878107), and the Key Technology R&D Program of Qingdao Municipal Science and Technology Commission (No. 08-2-1-17-nsh) are gratefully acknowledged. The authors also thank the support from Taishan Scholars Program of Shandong Province.
6. References
[1] A. Fujishima and K. Honda, “Electrochemical Photolysis of Water at a Semiconductor Electrode,” Nature, Vol. 238, 1972, pp. 37-38. doi:10.1038/238037a0
[2] M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, “Environmental Applications of Semiconductor Photocatalysis,” Chemical Reviews, Vol. 95, No. 1, 1995, pp. 69-96. doi:10.1021/cr00033a004
[3] A. Mills and S. L. Hunte, “An Overview of Semiconductor Photocatalysis,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 108, No. 1, 1997, pp. 1-35. doi:10.1016/S1010-6030(97)00118-4
[4] P. M. Ajayan, O. Stephan, Ph. Redlich and C. Colliex, “Carbon Nanotubes as Removable Templates for Metal Oxide Nanocomposites and Nanostructures,” Nature, Vol. 375, 1995, pp. 564-567. doi:10.1038/375564a0
[5] S. Kohtani, M. Koshiko, A. Kudo, K. Tokumura, Y. Ishigaki, A. Toriba, K. Hayakawa and R. Nakagaki, “Photodegradation of 4-Alkylphenols Using BiVO4 Photocatalyst under Irradiation with Visible Light from a Solar Simulator ,” Applied Catalysis B: Environmental, Vol. 46, No. 3, 2003, pp. 573-586. doi:10.1016/S0926-3373(03)00320-5
[6] J. Yu and A. Kudo, “Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4,” Advanced Functional Materials, Vol. 16, No. 16, 2006, pp. 2163-2169. doi:10.1002/adfm.200500799
[7] S. Kohtani, J. Hiro, N. Yamamoto, A. Kudo, K. Tokumura and R. Nakagaki, “Adsorptive and Photocatalytic Properties of Ag-Loaded BiVO4 on the Degradation of 4-Nalkylphenols under Visible Light Irradiation,” MaterialsCatalysis Communications, Vol. 6, No. 3, 2005, pp. 185-189. doi:10.1016/j.catcom.2004.12.006
[8] L. Zhou, W. Z. Wang, S. W. Liu, L. S. Zhang, H. L. Xu and W. Zhu, “A Sonochemical Route to Visible-LightDriven High-Activity BiVO4 Photocatalyst,” Journal of Molecular Catalysis A: Chemical, Vol. 252, No. 1-2, 1995, pp. 120-124. doi:10.1016/j.molcata.2006.01.052
[9] F. Wang, M. Shao, L. Cheng, J. Hua and X. Wei, “The Synthesis of Monoclinic Bismuth Vanadate Nanoribbons and Studies of Photoconductive, Photoresponse and Photocatalytic properties,” Materials Research Bulletin, Vol. 44, No. 8, 2009, pp. 1687- 1691.
[10] Y. Z. Wang and C. Hu, “A Study on Fixed Technology in the Reaction of Heterogeneous Photocatalytic Degradation of Organics,” Chinese Environmental Sciences, Vol. 19, No. 7, 1998, pp. 40-42.
[11] H.-Q. Jiang, H. Endo, H. Natori, M. Nagai and K. Kobayashi, “Fabrication and Photoactivities of SphericalShaped BiVO4 Photocatalysts through Solution Combustion Synthesis Method,” Journal of the European Ceramic Society, Vol. 28, No. 15, 2008, pp. 2955-2962. doi:10.1016/j.jeurceramsoc.2008.05.002
[12] H. Liu, R. Nakamura and Y. Nakato, “Promoted PhotoOxidation Reactivity of Particulate BiVO4 Photocatalyst Prepared by a Photoassisted Sol-Gel Method,” Journal of the Electrochemicl Society, Vol. 152, No. 11, 2005, pp. G856-G861. doi:10.1149/1.2051868
[13] M. Gotić, S. Musić, M. Ivanda, M. Šoufek and S. Popović, “Synthesis and Characterisation of Bismuth(III) Vanadate,” Journal of Molecular Structure, Vol. 744-747, No. 3, 2005. pp. 535-540.
[14] X. Chen, Z. Zhang and S. W. Lee, “Selective SolutionPhase Synthesis of BiOCl, BiVO4 and δ-Bi2O3 Nanocrystals in the Reaction System of BiCl3-NH4VO3-NaOH,” Journal of Solid State Chemistry, Vol. 181, No. 1, 2008, pp. 166-174. doi:10.1016/j.jssc.2007.10.031
[15] A. Kudo, K. Omori and H. Kato, “A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties,” Journal of the American Chemical Society, Vol. 121, No. 49, 1999, pp. 11459-11469. doi:10.1021/ja992541y
[16] S. Tokunaga, H. Kato and A. Kudo, “Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties,” Chemistry of Materials, Vol. 13, No. 12, 2001, pp. 4624-4628. doi:10.1021/cm0103390

