Journal of Biomaterials and Nanobiotechnology
Vol.4 No.1(2013), Article ID:27008,10 pages DOI:10.4236/jbnb.2013.41009
Ultrastructure and Analytical Features of the Sinus Floor Augmentation with Osteograph® and PepGen P-15®
![]()
1Dental Faculty, University of Strasbourg, 1 Place de l’Hôpital, Strasbourg, France; 2National Institute for Health and Medical Research (INSERM) UMR 977, 11 Rue Humann, Strasbourg, France.
Email: *joseph.hemmerle@inserm.fr
Received October 15th, 2012; revised November 29th, 2012; accepted December 13th, 2012
Keywords: Sinus Floor Augmentation; Bone Grafting; Osteograph®; PepGen P-15®; Ultrastructure; Microanalysis
ABSTRACT
The aim of the present study was to investigate an inorganic bovine-derived hydroxyapatite bone substitute (Osteograph®) mixed with the same biomaterial coated with a synthetic peptide (P-15) analogue of collagen (PepGen P-15®). This blend of bone replacement materials was used for sinus floor augmentation. Assessments were carried out by using histology methods, transmission electron microscopy (TEM) and microanalysis (EDX). Ultrastructural and analytical features of the interfaces between the graft material and the peri-biomaterial tissues were evaluated six months after implantation. Our findings clearly show that newly-formed crystallites first develop at the surface of implanted crystals. Histological investigations revealed new bone tissue linking biomaterial particles together. TEM assessments pointed out that lamellar bone was generally separated from the graft material by a layer of woven bone measuring between 1 and 1.5 µm in thickness. Although calcified bone tissue was observed in direct contact with bone filling particles, the presence of mineralized granular material around implanted particles was also noticed. No characteristic periodic striation of mineralized collagen was evident within that mineralized structure. Chemical analyses (TEM-EDX) realized at different locations of newly formed mineralized granular substance along the interface revealed average Ca/P ratios ranging between 1.02 and 1.63. The different, concomitantly occurring, aforementioned structural features of the interfaces strongly suggested that the host responses to the used biomaterial blend resulted from dynamic osseointegration phenomena related to various interfacial mechanisms. Nevertheless, the biological response to the bone graft material appeared clinically and histologically satisfactory.
1. Introduction
The bone volume between the upper alveolar ridge and the sinus floor is often insufficient to place long lasting implants. This insufficiency can jeopardize a prosthetic rehabilitation with implants. As soon as in 1976, Tatum suggested to increase the bone volume with grafts to obtain a favourable bone support for implantation. In addition to autogenous bone, several materials were tested and then widely used [1,2]. The use of autogenous bone with its osteoinduction potential is considered as the “Gold standard”, particularly in augmentation surgery [3]. Autogenous bone has a remarquable capacity of regenerating and remodelling itself. This is due to the presence of osteoblasts and to a favourable environment that take part in the interactions with the cell matrix. It however carries several downsides as for example the bone products selection area, its availability and the removal constraints. The use of an only osteoconductive bone substitute, but with specific biomimetic properties initiating the first stage of bone production, could then also find its place as a sinus floor elevation material.
In the human body most cells are attached to collagen that shape cell differentiation and especially the osteoblasts [4,5]. It plays a key role in the regeneration processes. Each tightly coiled strand shows a specific sequence of 15 amino acids (766 - 780) able to fix primary cells in the α1(I) chain [6]. In 1996 the works of Qian and Bhatnagar showed an increase of cell attachment, migration and differentiation on hydroxyapatite in the presence of a synthetic peptide related to collagen, and more specifically in presence of a specific sequence of 15 amino acids designated as P-15 [7]. The adsorption of these peptides on an anorganic bone material (ABM) accelerates and facilitates the new bone formation by promoting cell migration on surrounding tissues. The peptide combined with its mineral substrate (ABM P-15) is thought to mimic the cellular receptors [7]. They also have shown that the binding mechanisms and the migration of several types of cells on the Type-I collagen are identical and use the same type of integrin receptors [8- 10]. P-15 is particularly responsible for osteogenous cell binding and triggers a cascade of events that results in new bone formation. In 2003 the same California team showed that the presence of ABM P-15 increased cell binding and osteoblastic activity [11]. These cells release, according of their metabolic needs, their own bone morphogenic proteins and growth factors. These molecules drive the cell differentiation which is the second step towards bone formation. According to Windhagen and Thorey and Valentin, bone regeneration seems to be proportional to the number of collagen cell-binding receptors [12,13]. Cells link to the ABM P-15 molecules the same way they would to autologous bone graft. ABM P-15 provides a biomimic environment analogue to autologous bone. It has been demonstrated that there is a correlation between the concentration of P-15 and the number of linked cells [8]. ABM P-15 is an augmentation graft material aimed to optimise, by mimic, the bone regeneration process. It is used in periodontics for treatment of bone defects and for bone grafting in implant dentistry. Clinical studies regarding the use of ABM P-15 in human infrabony defects in extraction sockets, in human periodontal osseous defects and in animal periodontal regeneration have been already published [14-23]. In pre-implant surgery ABM P-15 has also been used with cortical autologous bone in ridge augmentation [24,25]. It was also used for sinus elevation [26-33]. The findings of these different clinical studies demonstrate that the use of ABM P-15 seems to be favourable in all these applications.
In the past years, several publications increased the knowledge of the physical and biological properties of P-15. In 2004, Trasatti et al. showed a significant increase in Tgf-β1 production by rat osteoblasts in the presence of ABM P-15 in vitro [34]. Their results indicate that ABM-P15 stimulates osteoblasts to express Tgf-β1, which may accelerate bone repair. In 2005, several mechanical properties such as elasticity and rigidity of different graft materials including PepGen P-15® (comercial ABM P-15) have been studied and compared to original mandibular bone tissue [35]. Results of these micro-mechanical tests showed that PepGen P-15® gave the best biological response at the criteria requested from this type of material. Moreover, studies using atomic force microscopy and infra-red spectroscopy identified the type of link between the ABM and the P-15 [36]. Results showed an ionic interaction between a C-terminal of a peptide carboxyl group and a radical free hydroxyl of the surface of the apatite. This link is irreversible but also brings changes in the conformation of the peptide. In 2003, Turhani et al. showed in vitro that the growth of a osteoblast-like culture was more important on PepGen P-15® when compared to Algipore® and to Ostéograph®/ N700 [37].
The aim of the present study was to disclose by optical and transmission electron microscopy, the new bone formation obtained after six months of scarring in a sinus filled with a mix made of one third of PepGen P-15® and two thirds of Osteograph®. Our study will particularly focus on the interface between the filling material and the newly formed bone tissue with the goal to offer a better understanding of the interactions involved in this type of graft.
2. Materials and Methods
2.1. Bone Graft Materials
Ostéograph®/N700 was obtained from Dentsply Friadent (Mannheim, Germany). This inorganic bone mineral (ABM), already employed in sinus elevation, is in a particulate form with a particle size range of 420 - 900 µm [38].
PepGen P-15® also was obtained from Dentsply Friadent (Mannheim, Germany). This graft material is in a particulate form with a particle size range of 250 - 420 μm. PepGen P-15® (ABM-P15) is made up of two components: an organic part associated to an inorganic substrate.
The inorganic phase is naturally derived hydroxyapatite. It is also an inorganic bone mineral (Ostéograph®/ N300) with a particle size range of 250 - 420 μm. The inorganic bovine-derived bone mineral component provides calcium phosphate and a natural anatomical matrix aimed to favour cellular invasion. The inorganic bone mineral (Ostéograph®) is submitted to a high temperature treatment (1100˚C) that allows to eliminate all organic material, including prions. The manufacturer guarantees that there are no risks of immunizing reaction or disease transmission due to collagen residues [39,40].
The organic part is constituted by a sequence of 15 specific amino acids, and is designated under the name of P-15. This organic phase is aimed to favour cellular connection and supposed to initialize new bone formation. P-15 is of synthetic nature. Its composition and structure are identical to those of the natural sequence of 15 amino-acids of collagen type I: 766 G T P G P Q I A G Q R G V V 780 [7]. P-15 is a specific ligand for cells. Not only have its different connections with the cells been studied but the answer of the osteoblasts regarding the P-15 has also been studied in vitro [6,8,41]. They showed that expression of the different genes is modulated by the presence of P-15 in a MG-63 osteoblast like culture. The organic part is adsorbed by incubation, in normal physiological conditions in a stable manner, onto the inorganic bone mineral. P-15 is irreversibly bound to the surface of the inorganic phase [7,9]. The adsorption of P-15 on the naturally derived hydroxyapatite is performed at saturation: the quantity of petide associated to the inorganic substrate varies from 233 to 259 ng/g of inorganic bone mineral.
2.2. Surgery Protocol
The patient was a 39-year-old man, non smoker, with an absence of teeth 26 and 27. X-rays showed a residual bone thickness of 2 to 3 mm. A sinusien filling was programmed with 1/3 PepGen P-15® and 2/3 Osteograph®, the access being done by a vestibular shutter. This bone filling mixture with a few drops of physiological salt solution made a paste that was placed between the Schneider membrane and the sinus floor. The filled cavity was closed with a titanium membrane (Dentsply Friadent ) and nailed to avoid any externalization of the filling. The titanium membrane was removed during the second surgery. Stitches were done (Vicryl® braided 3/0; Ethicon GmbH & Co. KG, Nordersedt, Germany) and were removed ten days later. Six months after grafting, a bone core was trephined out of the augmented area and implants were put in place. All along these different rehabilitation phases, regular controls were done, the last one taking place two years after the filling. At no times have complications or swelling appeared. Alveolar retro radiographies were taken at each stage of the rehabilitation process.
2.3. Specimen Preparation
The biopsy, collected with informed consent from the donor, was immediately immersed in a glutaraldehydeparaformaldehyde solution at 2% in a 0.1 M sodium cacodylate buffer at pH 7.4. The specimen was cut in four equal-sized cylinders. The crestal cylinder was exclusively made of bone coming from the existing sinus floor. The apical cylinder that was in contact with the Schneider membrane was also the farthest from the existing bone zone, and therefore the least mineralized. These two cylinders corresponding to the ends of the biopsy were not used in this study. Only the two median cylinders were kept for the present evaluation. After post-fixation with osmic acid (OsO4) and dehydration with a series of increasing ethanol concentrations, samples were then included in an epoxy resin (Epon® 812).
2.4. Optical Microscopy
Sections were realized perpendicularly to the long axis of the core drilling. After a first trimming of the sample with a diamond mill (W3032-4, ESCIL, France), 2 μm thick sections were made using an ultramicrotom (Sorvall®, MT-2C, Porter-Blum). Sections were stained with toluidine blue. Observations were made with a Nikon Eclipse TE 200 microscope.
2.5. Transmission Electron Microscopy (TEM)
From the same blocs, ultrathin sections of about 100 nm thickness were prepared and deposited on 200 mesh cooper grids. They were stained with uranyl acetate and lead citrate. Conventional TEM observations were made with a Philips CM10 electron microscope operating at 100 kV. High resolution assessments and microanalyses were made with a TOPCON EM-002B electron microscope operating at 200 kV. For elemental analyses the microscope was coupled with a Kevex Delta Plus spectrometer working with a beam-sample incidence angle of 30˚.
2.6. XRD Analyse
X-ray diffraction was performed on the two raw biomaterials, prior to implantation, by means of a Kristalloflex D 5000 (Siemens, Karlsruhe, Germany) equipped with a primary beam monochromator (Cu-Kα; λCu-Kα = 0.154 nm). The collected data were compared with the JCPDS (Joint Committee of Powder Diffraction Standards) file number 9-0432 corresponding to hydroxyapatite.
3. Results
X-ray diffraction diagrams (data not shown) of Osteograph® and PepGen P-15® prior to implantation matched with JCPDS file number 9-0432. Thus both raw biomaterials corresponded to hydroxyapatite.
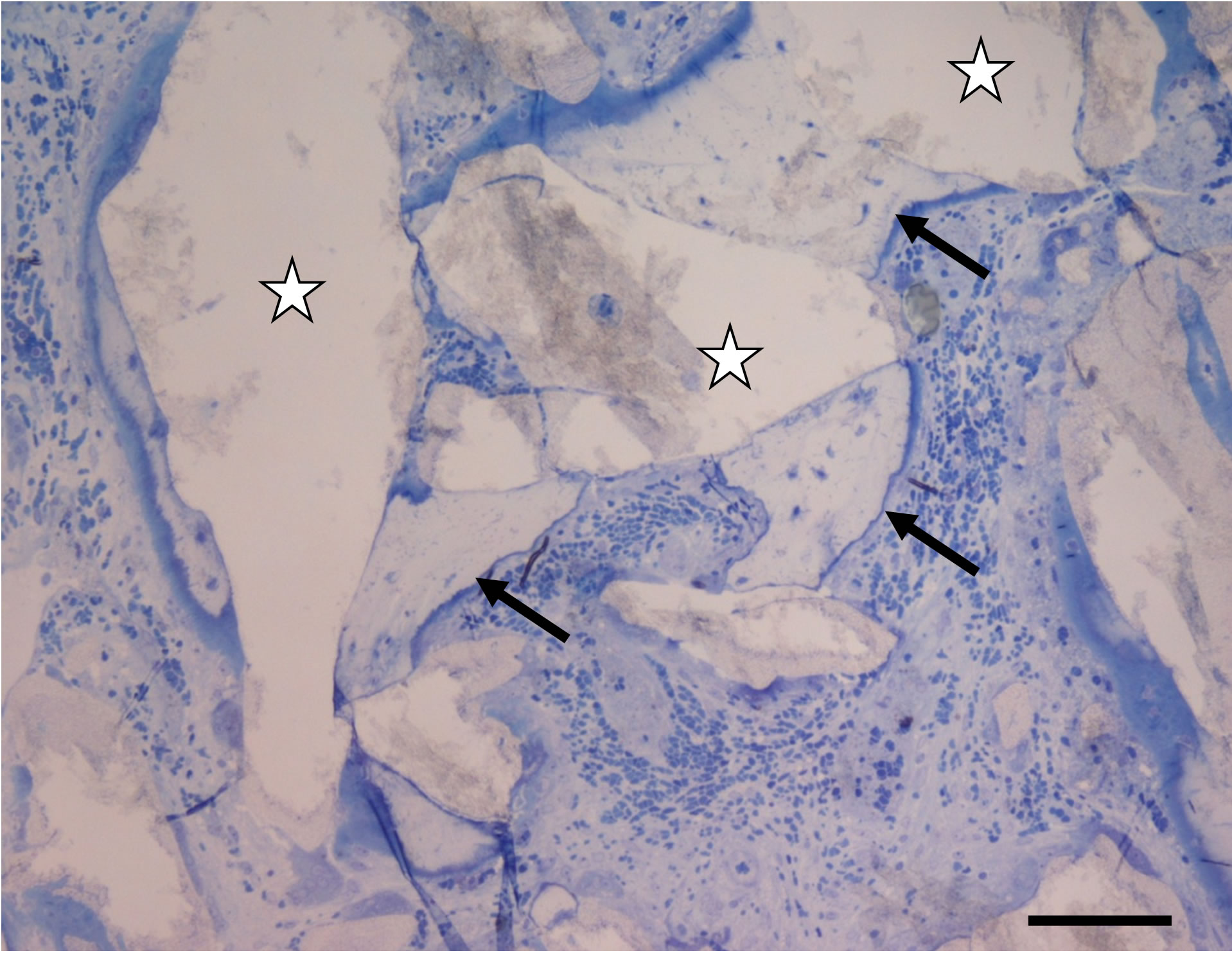
After six months of implantation, the biomaterial was found on almost all the sections of the selected samples. One could clearly see bone formation around the particles of Osteograph® and PepGen P-15® (Figure 1(a)). This newly formed bone was surrounded by osteoid tissue. We could note that most of the particles were in contact with at least one newly formed mineralized structure. At this scale, no space could be observed between the newly formed calcified tissue and the implanted particles. In many cases, we observed osseous bridging that linked the implanted particles. It was rather easy to distinguish the different biomaterial particles according to their respective sizes. Thanks to their porosity, the implanted mineral particles could be invested by blood vessels (Figures 1(a) and (b)). It was also evident that bone formations merging from different biomaterial particles joined and thus exhibited cement lines (Figure 1(b)). The sections studied by TEM were taken from the same samples than those used for the photonic microscopy assessments, (those in the median part of the cylindrical biopsy). From a qualitative point of view, the implanted particles were omnipresent on the sections.
In one part showing no or little ossification, one could find the intact product, seemingly having suffered no specific biological phenomenon. One could observe im-
 (a)
(a) (b)
(b)
Figure 1. (a) Optical micrograph showing Osteograph® and PepGen P-15® particles (stars) linked by bone bridges (arrows) exhibiting characteristic osteocyte lacunae. Toluidin blue staining. Bar = 100 µm; (b) Optical micrograph showing new bone formations interfaced by cement lines (arrows). Stars indicate the implanted particles. Six months after implantation. Bar = 100 µm.
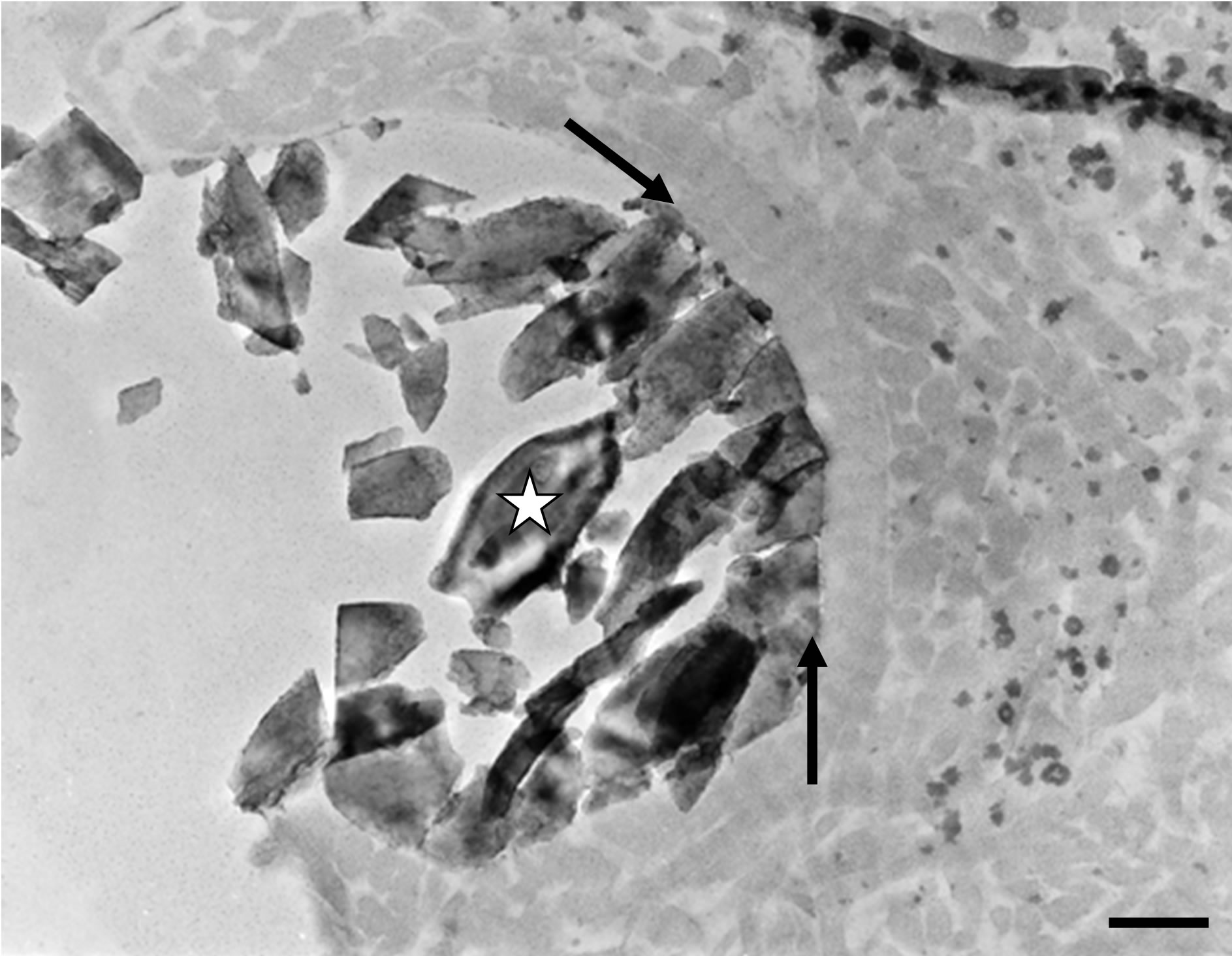
planted mineral particles surrounded by amorphous material without any mineralisation process at the interface (Figure 2(a)). The implanted biomaterial blend was also observed by high resolution transmission electronic microscopy (HRTEM). Thus it was possible to visualize the characteristic lattice planes of the mineral part of the bone filling material and, in some cases, the regular outlines of the implanted crystals (Figure 2(b)).
Nevertheless, several observations showed that a mineralization process took place at the periphery of the implanted particles of the filling material (Figure 3(a)). The spaces separating the biomaterial particles appeared to be filled with a non-mineralized collagen matrix (Figures 3(a) and (b)). At larger magnification, one could observe electron-dense structures, probably corresponding to new
 (a)
(a) (b)
(b)
Figure 2. (a) Transmission electron microscopy (TEM) micrograph showing amorphous material surrounding an implanted mineral particle (star). On can note that no mineralization process occurred at the interface (arrows). Bar = 300 nm; (b) High resolution transmission electron microscopy micrograph exhibiting lattice plans of the crystallized implanted material (star) from Figure 2(a). Note the absence of any newly formed mineralized structure at its surface (arrow). Six months after implantation. Bar = 5 nm.
crystal formations, at the surface of the bone filling mineral particles (Figure 3(b)). Moreover Figure 3(b) emphasized that the mineral response was definitely starting at the interface between the implanted crystals and the collagen matrix.
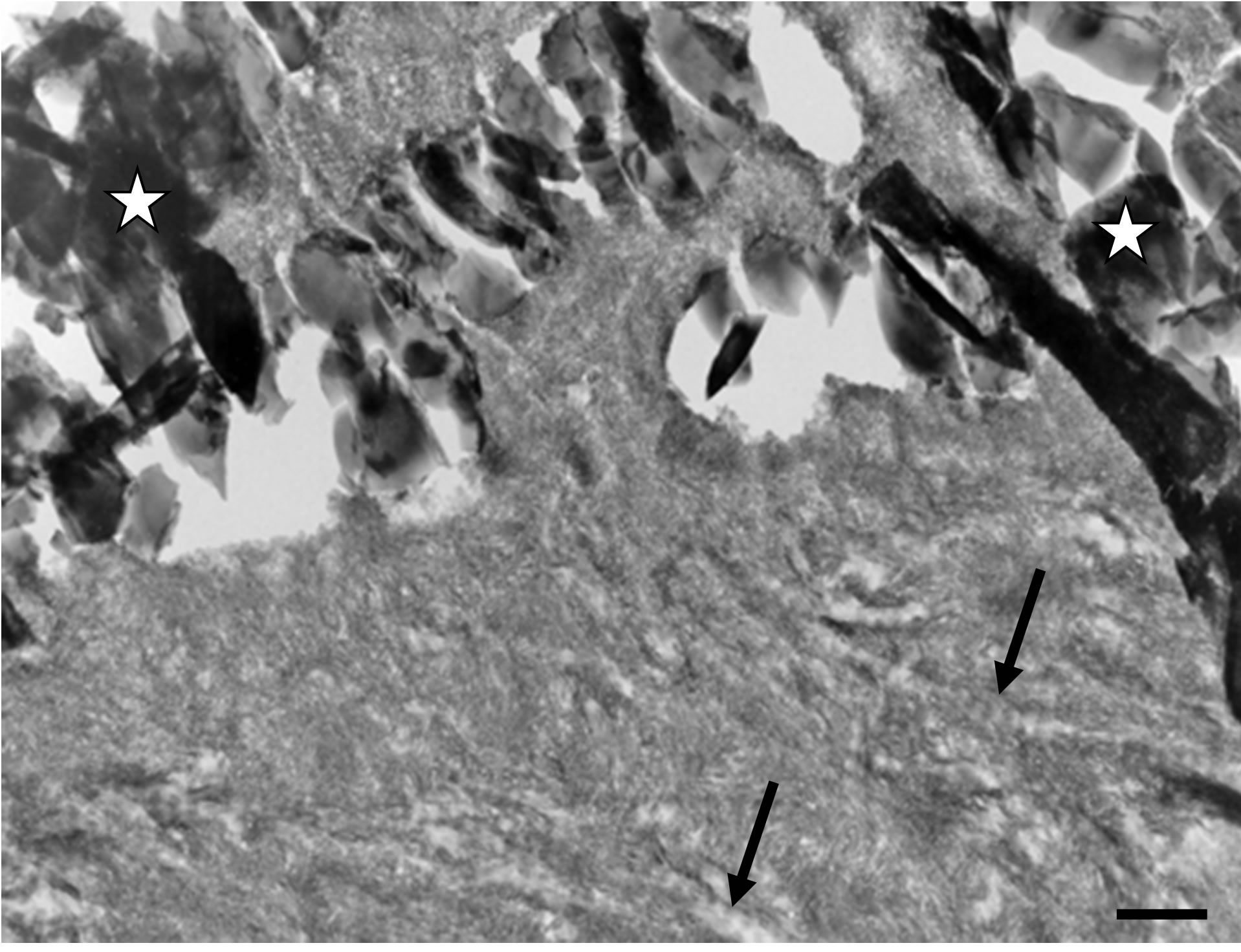
Many areas showed that newly formed mineralized tissue completely invested the implant material, thus embedding the individual mineral particles of the bone substitute Figure 4(a). These observations corresponded to a direct apposition of a calcified bone matrix around and within the implanted biomaterial, as mineralized collagen fibrils were evident (Figure 4(a)). However, it must be pointed out that in many cases the mineralized matrix being in direct contact with the implanted particles was reminiscent of woven bone (Figure 4(b)). Nev-
 (a)
(a) (b)
(b)
Figure 3. (a) TEM micrograph showing the first stages of a mineralization process (arrows) taking place around implanted particles. Note that the inter-crystalline spaces are invested by a collagenous matrix. Bar = 300 nm; (b) At higher magnification, one can distinguish newly formed crystallites (arrows) growing at the surface of the implanted crystals. Six months after implantation. TEM, Bar = 200 nm.
ertheless a more regularly ordered calcified collagen matrix was observed at distance of the implant-bone tissue interface. This inter-phase of seemingly woven bone, separating the biomaterial particles from the more or less parallel organized mineralized collagen fibrils reminiscent of lamellar bone, measured between 1 and 1.5 µm in thickness (Figure 4(b)).
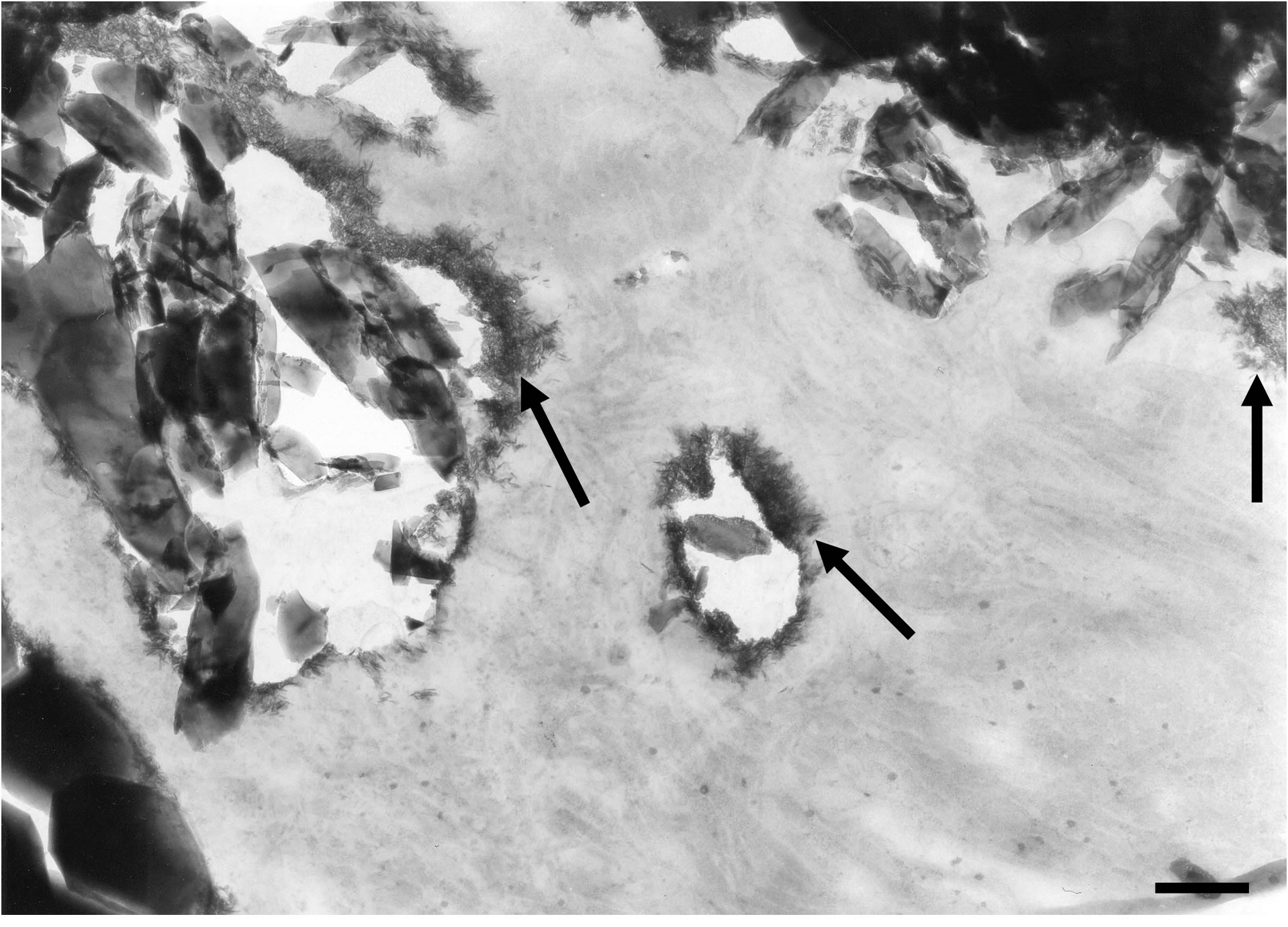
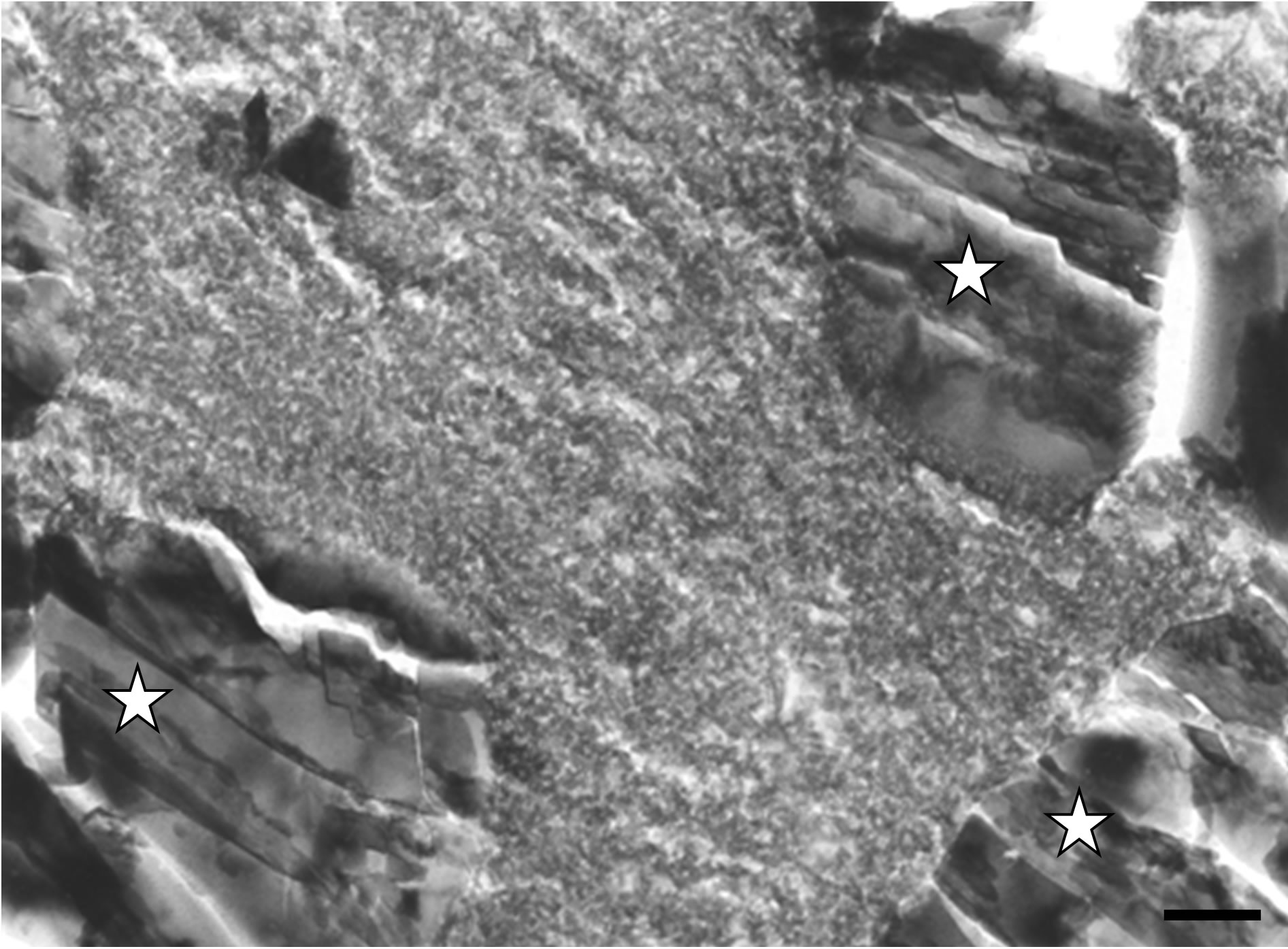
We could also observe inter-crystalline spaces within the filling material invested by an electron-dense substance exhibiting a granular aspect (Figure 5(a)). By filling spaces between implanted mineral particles, this granular electron-dense material bridged biomaterial crystals together. We observed a direct contact between the granular substance and the implanted particles (Figures 5(a) and (b)). However, the granular mineralization zones
 (a)
(a) (b)
(b)
Figure 4. (a) A calcified collagen matrix surrounds the implanted biomaterial and invests the inter-crystalline spaces of the bone substitute particles (stars). One can clearly identify periodic striation reminiscent of collagen fibres (arrows). TEM, Bar = 200 nm; (b) The calcified tissue immediately surrounding the implanted particles (stars) exhibit the characteristic features of woven bone. Arrows indicate a mineralized collagen matrix reminiscent of lamellar bone. Six months after implantation. TEM, Bar = 200 nm.
did not show the characteristic features of a calcified collagen matrix, i.e. no periodic striation corresponding to mineralized collagen was evident (Figure 5(a)).
On the other hand, some observations disclosed the concomitant presence of both aforementioned newly formed mineralized structures within the same interfacial area (Figure 5(b)). In that latter case, newly formed needle-like crystals developing in a collagen matrix were in direct contact with newly formed granular electrondense substance, both structures being in contact with biomaterial particles. At the analytical point of view, microanalysis measurements realized at different loca-
 (a)
(a) (b)
(b)
Figure 5. (a) Mineralized electron-dense granular material fills the spaces between crystals (stars) of the implanted bone filling blend. TEM, Bar = 100 nm; (b) TEM micrograph disclosing an interface exhibiting concomitantly needle-like crystals growing within a collagen matrix (at the top) and electron-dense granular materiel (at the bottom) filling gaps between implanted crystals (stars). Six months after implantation. Bar = 100 nm.
tions of newly formed mineralized granular substance along the interface revealed average Ca/P atomic-ratios ranging between 1.02 and 1.63.
4. Discussion
PepGen P-15® (ABM P-15) is considered as a tissueengineered bone substitute. In the “Tissue Engineering Triad” described by Ueda, P-15 is a signalling molecule, Osteograph® (ABM) the scaffold and the osteoblasts are the cells [42]. Following Qian and Bhatnagar’s researches other teams completed the study of ABM P-15, studying its effects in vitro, comparing it with other calcium phosphates, experimenting it on animals and at last using it in clinical tests [6,9]. Hanks and Atkinson compared in vitro the cellular behaviour and particularly the osteogenic cells present on ABM in the presence or not of P-15 [43]. The binding between the cell and the extra cellular matrix (ECM) is a key physiological element for the growth, the viability and the survival of the cell. The survival of a cell is determined by the signals from its environment: soluble factors present in the serum and ECM cells interactions through cellular integrines. They are these integrins that are used as mediators for the attachment to collagen. The aforementioned authors showed that P-15 associated with ABM, increases cellular binding and regulates apoptose. In 2004, Kubler studied in vitro, by electron and photonic microscopy the effects of the different bone substitutes (Bio-Base®, Algipore®, Ostéograph®, Bio-Oss® and PepGen P-15®) on the multiplication, the survival rate and the growth of osteoblasts [44]. After 6 and 9 months of implantation, PepGen P-15® gave the best differentiation rate and multiplication of osteoblasts, followed by Ostéograph®, Algipore®, Bio-Base® and Bio-Oss®. The nature of the bone substitute has an influence on the multiplication of osteoblasts. This latter study that focused on the first effects, taking place during the first days, of the different substitutes on the cellular multiplication, showed the ability of P-15 to accelerate the first phases of bone construction. PepGen P-15® was also studied in the filling of two experimental bone gaps on rabbits [45]. Histological and histomorphometrical results of these studies showed an increase and an acceleration of bone formation when compared to the control sites. This point was also revealed in the study of Thorwarth on the filling of bone cavities created in pig skulls [46]. The filling materials studied separately were the autogenous bone and the two biomaterials used for the present study, PepGen P-15® and Osteograph®N/700. For autogenous bone and PepGen P-15®, the first bone formation appeared on the 3rd day, whereas it took 7 days for Osteograph®N/700. After six months of implantation a normal and dense bone tissue completely filled the cavity previously occupied by autogenous bone and Ostéograph®N/700. On the other hand, a bone tissue of similar quality formed already after 12 weeks when PepGen P-15® was used.
The present in vivo findings show the presence of needle-like crystals at the surface of implanted particles. These newly formed crystallites were also described with other ABM of bovine origin in vitro and in vivo with Bio-Oss® [47,48]. These observations are in accordance with investigations carried out on the newly-formed crystal growth mechanisms [49]. In short, implanted calcium phosphate particles offer nucleation sites for the mineralization process of new crystallites of apatite nature.
Several interfaces showed the presence of a granular substance. No characteristic periodic striation of mineralized collagen was evident within that mineralized structure. This granular material could even invest all the spaces between the implanted mineral particles. Microanalysis of this newly formed material disclosed the calcium phosphate nature of the granular substance. The fact that calculations performed from X-ray microanalyses yielded various Ca/P ratios (between 1.02 and 1.63) in different areas of this newly formed material emphasizes the dynamic aspect of that post-implantation mineralization phenomenon. These observations might correspond to dissolution-re-precipitation phenomena described by Daculsi [50]. Anyhow, this mineral filling process of the grafting material’s porosity seems particularly interesting for the consolidation and solidity of the bone substitute.
On the other hand, we could clearly identify, by photonic microscopy, bone tissue linking implanted mineral particles together. Moreover, we were able to observe cement lines between new bone tissue formations bridging different implanted particles together. Such interfacial cement lines are thought to act as glue between different bone formations [51,52]. In a TEM study about Bio-Oss®, an amorphous zone of a width of 2 μm, located at the interface between the bovine-origin hydroxyapatite and the newly formed bone tissue was described [48]. Such an amorphous layer was not found in the present study, which favors the integration of the graft material. On the contrary, our observations by TEM on the interfaces showed a direct contact of the implanted particles with newly formed mineralized material; either granular mineral substance or calcified bone tissue. Actually, a calcified collagen matrix could be found in direct contact with bone filling particles. TEM assessments pointed out that lamellar bone was generally separated from the graft material by a layer of woven bone.
Krauser et al. compared Osteograph®/N300 versus Osteograph®/N300 combined with PepGen P-15® in bilateral sinus procedures. Photonic microscopy analysis showed enhanced bone formation and decreased healing times with the Osteograph®/N300-PepGen P-15® combination [26]. Results from a bilateral Osteograph®/N300 sinus grafting including PepGen P-15® on one side only were reported by Smiler [27]. Morphometric analysis at four months after grafting showed 45% bone regeneration in the PepGen P-15®-grafted sinus compared with 13% on the other side. In a wider study, Degidi et al. compared three combinations: autologous bone and BioOss®, Bio-Oss® and PepGen P-15®, autologous bone and PepGen P-15® [28]. The aim of that study was not to show the specific advantages of PepGen P-15®, but to demonstrate that the use of bone substitutes, without the addition of autologous bone, could be an alternative in sinus augmentation procedures. In a preliminary case report, Valentin and Weber simply described the principles and mechanisms of sinus floor elevation with Pep-Gen P-15® [29]. Philippart et al. reported important bone formations in 3 patients 6 months after sinus grafting, but this was obtained with a complex mixture including autologous bone and PepGen P-15® among others [31]. The specific role of PepGen P-15® in bone regeneration was difficult to underline in such conditions. Another study was based on 15 fillings with 3 types of combinations with PepGen P-15® [30]. Histologic analysis showed well-shaped trabecular bone in 12 patients 5 months after grafting. Yeung et al. used PepGen P-15 Flow® and showed histologicaly the ability to generate new bone growth in the sinus elevation procedure [33]. Recently, Degidi et al. (2005) assessed specimens retrieved 18 months after sinus lifting procedure using PepGen P-15®. They described all phases of bone formation in the newly formed bone around the biomaterial particles.
The present approach differs from the aforementioned studies as far as it is not focused on the amount of bone regeneration but rather addresses the interfacial processes and the mineralization mechanisms. Ultrastructural analyses of xenografted-hydroxyapatites are scarce [32,53, 54]. The direct implant-mineral response contact as well as the osseointegration level of the ABM/ABM P-15 are likely to offer a reliable basis for the stability of the graft. Nevertheless, our results show no major improvement over the use of apatite alone. Uniformly adsorbed P-15 cell-ligand on the ABM surface is expected to lead to homogenous formation of new bone tissue. But our findings rather showed focal bone formations as described when using other hydroxyapatites [38,47-49]. The variability of the observed mineral responses, i.e. either granular material or calcified bone tissue, might be explained by the hypothesis of Hole et al. which showed that P-15 attachment to ABM is not homogenous [36]. Nevertheless, in the main the present study suggests a satisfactory osseointegration process of the employed biomaterial.
5. Acknowledgements
Authors are indebted to Eric Mathieu for his invaluable technical assistance in electron microscopy.
REFERENCES
- O. H. Tatum, “Maxillary and Sinus Implant Reconstruction,” Dental Clinics of North America, Vol. 30, No. 2, 1986, pp. 207-229.
- O. H. Tatum, M. S. Lebowitz, C. A. Tatum and R. A. Borgner, “Sinus Augmentation. Rationale, Development, Long-Term Results,” New York State Dent Journal, Vol. 59, No. 5, 1993, pp. 43-48.
- E. Nkenke, V. Weisbach, E. Winckler, P. Kessler, S. Schultze-Mosgau, J. Wiltfang and F. W. Neukam, “Morbidity of Harvesting of Bone Grafts from the Iliac Crest for Preprosthetic Augmentation Procedures: A Prospective Study,” International Journal of Oral and Maxillofacial Surgery, Vol. 33, No. 2, 2004, pp. 157-163. doi:10.1054/ijom.2003.0465
- H. Kleinman, R. J. Klebe and G. R. Martin, “Role of Collagenous Matrices in Adhesion and Growth of Cells,” Journal of Cell Biology, Vol. 88, No. 3, 1981, pp. 473- 485. doi:10.1083/jcb.88.3.473
- L. Masi, A. Franchi, M. Santucci, D. Danielli, L. Arganini, V. Giannone, L. Formigli, S. Benvenuti, A. Tanini, F. Beghi, M. Mian and M. L. Brandi, “Adhesion, Growth, and Matrix Production by Osteoblasts on Collagen Substrata,” Calcified Tissue International, Vol. 51, No. 3, 1992, pp. 202-212. doi:10.1007/BF00334548
- R. S. Bhatnagar, J. J. Qian and C. A. Gough, “The Role in Cell Binding of a Beta-Bend within the Triple Helical Region in Collagen Alpha1 (I) Chain: Structural and Biological Evidence for Conformational Tautomerism on Fiber Surface,” Journal of Biomolecular Structure and Dynamics, Vol. 14, No. 5, 1997, pp. 547-560. doi:10.1080/07391102.1997.10508155
- J. J. Qian and R. S. Bhatnagar, “Enhanced Cell Attachment to Anorganic Bone Mineral in the Presence of a Synthetic Peptide Related to Collagen,” Journal of Biomedical Materials Research, Vol. 31, No. 4, 1996, pp. 545-554. doi:10.1002/(SICI)1097-4636(199608)31:4<545::AID-JBM15>3.0.CO;2-F
- R. S. Bhatnagar, J. J. Qian, A. Wedrychowska, M. Sadeghi, Y. M. Wu and N. Smith, “Design of Biomimetic Habitat for Tissue Engineering with P-15, a Synthetic Peptide Analogue of Collagen,” Tissue Engineering, Vol. 5, No. 1, 1999, pp. 53-65. doi:10.1089/ten.1999.5.53
- R. S. Bhatnagar, J. J. Qian, A. Wedrychowska, E. Dixon and N. Smith, “Biomimetic Habitat for Cells: Ordered Matrix Deposition and Differentiation in Gingival Fibroblasts Cultured on Hydroxyapatite Coated with a Collagen Analogue,” Cell Mater, Vol. 9, No. 2, 1999, pp. 93- 104.
- T. E. Lallier, R. Yukna, S. St Marie and R. Moses, “The Putative Collagen Binding Peptide Hastens Periodontal Ligament Cell Attachment to Bone Replacement Graft Materials,” Journal of Periodontology, Vol. 72, No. 8, 2001, pp. 990-997. doi:10.1902/jop.2001.72.8.990
- H. Nguyen, J. J. Qian, R. S. Bhatnagar and S. Li, “Enhanced Cell Attachment and Osteoblastic Activity by P- 15 Peptide Coated Matrix in Hydrogels,” Biochemical and Biophysical Research Communications, Vol. 311, No. 1, 2003, pp. 179-186. doi:10.1016/j.bbrc.2003.09.192
- H. Windhagen and F. Thorey, “Die Funktionelle Reaktion des Knochens auf Mechanische Reize,” Zahnärtzlische Implantolologie, Vol. 16, No. 3, 2000, pp. 139-145.
- A. H. Valentin, “Bone Regeneration through Biomimicry: The New PepGen P-15,” Zahnheilkunde, Vol. 18, No. 11, 2002, pp. 795-799.
- R. A. Yukna, J. T. Krauser, D. P. Callan, G. H. Evans, R. Cruz and M. Martin, “Multi-Center Clinical Comparison of Combination Anorganic Bovine-Derived Hydroxyapatite Matrix (ABM)/Cell Binding Peptide (P-15) and ABM in Human Periodontal Osseous Defects 6-Month Results,” Journal of Periodontology, Vol. 71, No. 11, 2000, pp. 1671-1679. doi:10.1902/jop.2000.71.11.1671
- R. A. Yukna, J. T. Krauser, D. P. Callan, G. H. Evans, R. Cruz and M. Martin, “Thirty-Six Months Follow-Ups of 25 Patients Treated with Combination Anorganic BovineDerived Hydroxyapatite Matrix (ABM)/Cell-Binding Peptide (P-15) Bone Replacement Grafts in Human Infrabony Defects. I. Clinical Findings,” Journal of Periodontology, Vol. 73, No. 1, 2002, pp. 123-128. doi:10.1902/jop.2002.73.1.123
- R. A. Yukna, T. J. Salinas and R. F. Carr, “Periodontal Regeneration Following Use of ABM/P-15: A Case Report,” International Journal of Periodontics and Restorative Dentistry, Vol. 22, 2002, pp. 146-155.
- S. Radhakrishnan and C. N. Anusuya, “Comparative Clinical Evaluation of Combination Anorganic Bovine-Derived Hydroxyapatitematrix (ABM)/Cell Binding Peptide (P-15) and Open Flap Debridement (DEBR) in Human Periodontal Osseous Defects: A 6-Month Pilot Study,” Journal of the International Academy of Periodontology, Vol. 6, No. 3, 2004, pp. 101-107.
- J. Hahn, M. D. Rohrer and A. J. Tofe, “Clinical, Radiographic, Histologic and Histomorphometric Comparison of PepGen P-15® Particulate and PepGen P-15 Flow® in Extraction Sockets. A Same-Mouth Case Study,” Implant Dentistry, Vol. 12, No. 2, 2003, pp. 170-174. doi:10.1097/01.ID.0000064812.39660.FF
- D. M. Thompson, M. D. Rohrer and H. S. Prasad, “Comparison of Bone Grafting Materials in Human Extraction Sockets: Clinical, Histologic and Histomorphometric Evaluations,” Implant Dentistry, Vol. 15, No. 1, 2006, pp. 89- 96. doi:10.1097/01.id.0000202426.62007.60
- R. A. Yukna, J. T. Krauser, D. P. Callan, G. H. Evans, R. Cruz and M. Martin, “Multi-Center Clinical Evaluation of Combination Anorganic Bovine-Derived Hydroxyapatite Matrix (ABM)/Cell Binding Peptide (P-15) as a Bone Replacement Graft Material in Human Periodontal Osseous Defects. 6-Month Results,” Journal of Periodontology, Vol. 69, No. 6, 1998, pp. 655-663. doi:10.1902/jop.1998.69.6.655
- E. P. Barboza, R. O. de Souza, A. L. Cauia, L. G. Neto, F. O. Cauia and M. E. Duarte, “Bone Regeneration of Localized Chronic Alveolar Defects Utilizing Cell Binding Peptide Associated with Anorganic Bovine-Derived Bone Mineral: A Clinical and Histological Study,” Journal of Periodontology, Vol. 73, No. 10, 2002, pp. 1153-1159. doi:10.1902/jop.2002.73.10.1153
- B. D. S. Tehemar, P. Hanes and M. Sharawy, “Enhancement of Osseointegration of Implant Placed into Extraction Sockets of Healthy and Periodontally Diseased Teeth by Using Graft Material and a PTFE Membrane, or Combination,” Clinical Implant Dentistry and Related Research, Vol. 5, No. 3, 2003, pp. 193-211. doi:10.1111/j.1708-8208.2003.tb00202.x
- S. Vastardis, R. A. Yukna, E. T. Mayer and B. L. Atkinson, “Periodontal Regeneration with Peptide-Enhanced Anorganic Bone Matrix in Particulate and Putty form in Dogs,” Journal of Periodontology, Vol. 76, No. 1, 2005, pp. 1690-1696. doi:10.1902/jop.2005.76.10.1690
- J. Hahn, “8-Year Onlay Bone Graft and Ridge Augmentation with PepGen P-15®: A Clinical and Radiographic Case Study,” Implant Dentistry, Vol. 13, No. 3, 2004, pp. 228-231. doi:10.1097/01.id.0000136916.28634.d3
- D. G. Smiler, “Advances in Endosseous Implant: The Sandwich Split Cortical Graft for Dental Implant Placement,” Dental Implantology Update, Vol. 11, No. 7, 2000, pp. 49-53.
- J. T. Krauser, M. D. Rohrer and S. S. Wallace, “Human Histologic and Histomorphometric Analysis Comparing Osteograf®/N with PepGen P-15® in the Maxillary Sinus Elevation Procedure: A Case Report,” Implant Dentistry, Vol. 9, No. 4, 2000, pp. 298-302. doi:10.1097/00008505-200009040-00004
- D. G. Smiler, “Comparison of Anorganic Bovine Mineral with and without Synthetic Peptide in a Sinus Elevation: A Case Study,” Implant Dentistry, Vol. 10, No. 2, 2001, pp. 139-142. doi:10.1097/00008505-200104000-00011
- M. Degidi, M. Piattelli, A. Scarano, G. Iezzi and A. Piattelli, “Maxillary Sinus Augmentation with a Synthetic Cell-Binding Peptide: Histological and Histomorphometrical Results in Humans,” Journal of Oral Implantology, Vol. 30, No. 6, 2004, pp. 376-383. doi:10.1563/0720.1
- A. H. Valentin and J. Weber, “Receptor Technology-Cell Binding to P-15: A New Method of Regenerating Bone Quickly and Safely-Preliminary Histomorphometrical and Mechanical Results in Sinus Floor Augmentations,” Keio Journal of Medicine, Vol. 53, No. 3, 2004, pp. 166-171. doi:10.2302/kjm.53.166
- M. Gelbart, R. Friedman, V. Burli, M. Rohmer and B. Atkinson, “Maxillary Sinus Augmentation Using a Peptide-Modified Graft Material in Three Mixtures: A Prospective Human Case Series of Histologic and Histomorphometric Results,” Implant Dentistry, Vol. 14, No. 2, 2005, pp. 185-193. doi:10.1097/01.id.0000165029.86196.27
- P. Philippart, V. Daubie and R. Pochet, “Sinus Grafting Using Recombinant Human Tissue Factor, Platelet-RichPlasma Gel, Autologous Bone and Anorganic Bovine Bone Mineral Xenograft: Histologic Analysis and Case Reports,” International Journal of Oral & Maxillofacial Implants, Vol. 20, No. 2, 2005, pp. 274-281.
- M. Degidi, A. Scarano, G. Iezzi, G. Orsini, V. Perrotti, R. Strocchi and A. Piattelli, “Maxillary Sinus Augmentation Using a Synthetic Cell-Binding Peptide: A Histologic and Transmission Electon Microscopy Case Study in Man,” Implant Dentistry, Vol. 14, No. 4, 2005, pp. 371-375.
- R. W. Yeung, L. J. Jin, M. Pang and E. Pow, “Human Histologic and Electronmicroscopic Analysis with Synthetic Peptide Enhanced Hydroyapatite in the Maxillary Sinus Elevation Procedure: A Case Report,” Implant Dentistry, Vol. 14, No. 3, 2005, pp. 237-241. doi:10.1097/01.id.0000173331.14116.7f
- C. Trasatti, R. Spears, J. L. Gutmann and L. A. Opperman, “Increased Tgf-Beta1 Production by Rat Osteoblasts in the Presence of PepGen P-15® in Vitro,” Journal of Endodon, Vol. 30, No. 4, 2004, pp. 213-221. doi:10.1097/00004770-200404000-00007
- T. Nomura, J. L. Katz, M. P. Power and C. Saito, “Evaluation of the Micromechanical Elastic Properties of Potential Bone-Grafting Materials,” Journal of Biomedical Materials Research Part B, Vol. 73, No. 1, 2005, pp. 29-34.
- B. B. Hole, J. A. Schwarz, J. L. Gilbert and B. L. Atkinson, “A Study of Biologically Active Peptide Sequences (P-15) on the Surface of an ABM Scaffold (PepGen P-15®) Using AFM and FTIR,” Journal of Biomedical Materials Research Part A, Vol. 74, No. 4, 2005, pp. 712- 721. doi:10.1002/jbm.a.30331
- D. Turhani, C. Item, D. Thurnher, D. Kapral, B. Cvikl, M. Weissenbock, K. Yerit, B. Erovic, D. Moser, F. Watzinger, R. Ewers and G. Lauer, “Evidence of Osteocalcin Expression in Osteoblast Cells of Mandibular Origin Growing on Biomaterials with RT-PCR and SDS-PAGE/ Western Blotting,” Mund-, Kieferund Gesichtschirurgie, Vol. 7, No. 5, 2003, pp. 294-330. doi:10.1007/s10006-003-0495-7
- S. J. Froum, D. P. Tarnow, S .S. Wallace, M. D. Rohrer and S. C. Cho, “Sinus Floor Elevation Using Anorganic Bovine Bone Matrix (Osteograph®/N) with and without Autogenous Bone: A Clinical, Histologic, Radiographic, and Histmorphometric Analysis,” International Journal of Periodontics & Restorative Dentistry, Vol. 18, No. 6, 1998, pp. 528-543.
- A. Sogal and A. J. Tofe, “Risk Assessment of Bovine Spongiform Encephalopathy Transmission through Bone Graft Material Derived from Bovine Bone Used for Dental Applications,” Journal of Periodontology, Vol. 70, No. 9, 1999, pp. 1053-1063. doi:10.1902/jop.1999.70.9.1053
- E. W. D. Huffman and R. L. Keil, “Determination of Trace Organic Carbon and Nitrogen in the Presence of Carbonates in Anorganic Bovine Bone Graft Materials,” Microchemical Journal, Vol. 74, No. 3, 2003, pp. 249- 256. doi:10.1016/S0026-265X(03)00031-6
- F. Carinci, F. Pezzetti, S. Volinia, G. Laino, D. Arcelli, E. Caramelli, M. Degidi and A. Piatelli, “P-15 Cell Binding Domain Derived from Collagen: Analysis of MG63 Osteoblastic-Cell Response by Means of a Microarray Technology,” Journal of Periodontology, Vol. 75, No. 1, 2004, pp. 66-83. doi:10.1902/jop.2004.75.1.66
- M. Ueda, I. Tohnai and H. Nakai, “Tissue Engineering Research in Oral Implant Surgery,” Artificial Organs, Vol. 25, No. 3, 2001, pp. 164-171. doi:10.1046/j.1525-1594.2001.025003164.x
- T. Hanks and B. L. Atkinson, “Comparison of Cell Viability on Anorganic Bone Matrix with or without P-15 Cell Binding Peptide,” Biomaterials, Vol. 25, No. 19, 2004, pp. 4831-4836. doi:10.1016/j.biomaterials.2003.12.007
- A. Kubler, J. Neugebauer, J. H. Oh, M. Scheer and J. E. Zoller, “Growth and Proliferation of Human Osteoblasts on Different Bone Graft Substitutes: An in Vitro Study,” Implant Dentistry, Vol. 13, No. 2, 2004, pp. 171-179. doi:10.1097/01.ID.0000127522.14067.11
- A. Scarano, G. Iezzi, G. Petrone, G. Orsini, M. Degidi, R. Strocchi and A. Piatelli, “Cortical Bone Regeneration with PepGen P-15®: A Histological and Histomorphometric Pilot Study in Rabbits,” Implant Dentistry, Vol. 12, No. 4, 2003, pp. 318-324. doi:10.1097/01.ID.0000095467.48241.68
- M. Thorwarth, S. Schultze-Mosgau, F. Wehrhan, P. Kessler, S. Srour, J. Wiltfang and K. A. Schlegel, “Bioactivation of an Anorganic Bone Matrix by P-15 Peptide for the Promotion of Early Bone Formation,” Biomaterials, Vol. 26, No. 28, 2005, pp. 5648-5657. doi:10.1016/j.biomaterials.2005.02.023
- S. Hofman, M. Sidqui, D. Abensur, P. Valentini and P. Missika, “Effects of Laddec on the Formation of Calcified Bone Matrix in Rat Calvariae Cells Culture,” Biomaterials, Vol. 20, No. 13, 1999, pp. 1155-1166. doi:10.1016/S0142-9612(97)00082-3
- G. Orsini, T. Traini, A. Scarano, M. Degidi, V. Perrotti, M. Piccirilli and A. Piattelli, “Maxillary Sinus Augmentation with Bio-Oss®: A Light, Scanning, and Transmission Electron Microscopy Study in Man,” Journal of Biomedical Materials Research Part B, Vol. 74, 2005, pp. 448- 457.
- J. Hemmerlé, F. J. Cuisinier, P. Schultz and J.-C. Voegel, “HRTEM Study of Biological Crystal Growth Mechanisms in the Vicinity of Implanted Synthetic Hydroxyapatite Crystals,” Journal of Dental Research, Vol. 76, No. 2, 1997, pp. 682-687. doi:10.1177/00220345970760020901
- G. Daculsi, R. Z. LeGeros, E. Nery, K. Lynch and B. Kerebel, “Transformation of Biphasic Calcium Phosphate Ceramics in Vivo: Ultrastructural and Physicochemical Characterization,” Journal of Biomedical Materials Researc, Vol. 23, No. 8, 1989, pp. 883-894. doi:10.1002/jbm.820230806
- M. D. McKee and A. Nanci, “Ultrastructural, Cytochemical and Immunocytochemical Studies on Bone and Its Interfaces,” Cell Mater, Vol. 3, 1993, pp. 219-243.
- M. McKee and A. Nanci, “Osteopontin at Mineralized Tissue Interfaces in Bone, Teeth and Osseointegrated Implants: Ultrastructural Distribution and Implications for Mineralized Tissue Formation, Turnover and Repair,” Microscopy Research and Technique, Vol. 33, No. 2, 1996, pp. 141-164. doi:10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W
- V. B. Rosen, L. W. Hobbs and M. Spector, “The Ultrastructure of Anorganic Bovine Bone and Selected Synthetic Hyroxyapatites Used as Bone Graft Substitute Materials,” Biomaterials, Vol. 23, No. 3, 2002, pp. 921-928. doi:10.1016/S0142-9612(01)00204-6
- F. I. Tapety, N. Amizuka, K. Uoshima, S. Nomura and T. Maeda, “A Histological Evaluation of the Involvement of Bio-Oss® in Osteoblastic Differentiation and Matrix Synthesis,” Clinical Oral Implants Research, Vol. 15, No. 3, 2004, pp. 315-324. doi:10.1111/j.1600-0501.2004.01012.x.
NOTES
*Corresponding author.

