Case Reports in Clinical Medicine
Vol.04 No.01(2015), Article ID:53427,4 pages
10.4236/crcm.2015.41009
Surgicel Induced Intraoperative Cardiovascular Collapse in a Child with Midbrain Glioma
Evgeni Brotfain1*, Akiva Korn2, Micky Gidon3, Alexander Zlotnik1, Moti Klein1, Israel Melamed3
1Department of Anesthesiology and Critical Care, General Intensive Care Unit, Soroka Medical Center, Ben-Gurion University of the Negev, Beer Sheva, Israel
2Surgical Monitoring Service Ltd., Jerusalem, Israel
3Department of Neurosurgery, Soroka Medical Center, Ben-Gurion University of the Negev, Beer Sheva, Israel
Email: *bem1975@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 December 2014; accepted 14 January 2015; published 21 January 2015
ABSTRACT
Introduction: Intraoperative cardiovascular complications were previously demonstrated in relation to high sympathetic nervous system outflow or stimulation of the vagal nerve nucleus during theneurosurgical procedures on brain tumors. Main Symptoms and Important Clinical Findings: We are presenting clinical case of 13-year-old girl who suffered from midbrain glioma and developed life threatening cardiovascular events during neurosurgical procedure after surgicel hemostatic agent application. Therapeutic Interventions and Outcomes: Cardiovascular stability has been restored after immediate removal of surgicel from the operating field. We believe that it might be related to direct application of the surgicel hemostatic agent.
Keywords:
Thalamic Glioma, Pediatric Neurosurgery, Cardiovascular Collapse, Surgicel-Induced Hypotension, Extracellular Acidosis

1. Introduction
Wide spectrum of intraoperative cardiovascular compromise has been previously described [1] during neurosurgical procedures of different types of brain stem lesions (glioma, cavernoma, ventricular tumor etc.). Clinically, the phenomenon has been associated with elevated sympathetic activity or vagal stimulation (arterial hypo- or hypertension, heart rate variations from brady- to tachyarrhythmia) related to elevated intracranial pressure, brain stem manipulation, intraoperative re-bleeding and others [1] [2] . Intraoperative bleeding control by the surgical hemostatic agent surgicel (oxidized regenerated cellulose) is an integral part of any neurosurgical procedure [3] . In some cases it might be followed by different adverse reactions [4] . Progressive swelling associated with surgicel might cause compressive effects on spinal cord, optic nerve [5] , and, even, risk of granuloma development due to formation of a tumor-like space-occupying lesion [6] . In this paper, we presented a case of intraoperative life threatening hypotension with tachyarrhythmia after surgicel application in a child with midbrain glioma.
2. Case Report
A 13-year-old healthy, girl was admitted to emergency room (ER) with a severe frontal headache accompanied by vomiting. On admission to the neurosurgical department she was fully alert and did not display significant neurological deficit. She had no history of elevated temperature or any seizure episodes in the past. Significant bilateral papilledema was found on ophthalmologic exam. Both computer tomography (CT) and magnetic resonance imaging (MRI), revealed remarkable focal brain lesion embracing right thalamic and midbrain area that led to compression of the aqueduct and developing of acute hydrocephalus (see Figure 1).
The patient underwent an urgent endoscopic third ventriculostomy (ETV) continued by a right temporal craniotomy. An excisional biopsy of the lesion was done via a subtemporal transtentorial approach. Following the biopsy and, partial resection of the tumor hemostasis was carried out with small pieces of surgicel (Figure 2). This application of surgicel prompted sudden-onset narrow-complex tachycardia (170 - 180 beats/min) follow- ed by remarkable hypotension (50/30 mmHg) (Graph 1).
Figure 1. MRI images of large midbrain lesion with acute brain edema of 13-year- old female (immediately after admission to neurosurgical department).

Figure 2. Intraoperative images: (a) Prominent midbrain surface before opening; (b) Tumor bad after partial resection; (c) Application of surgicel for hemostasis.
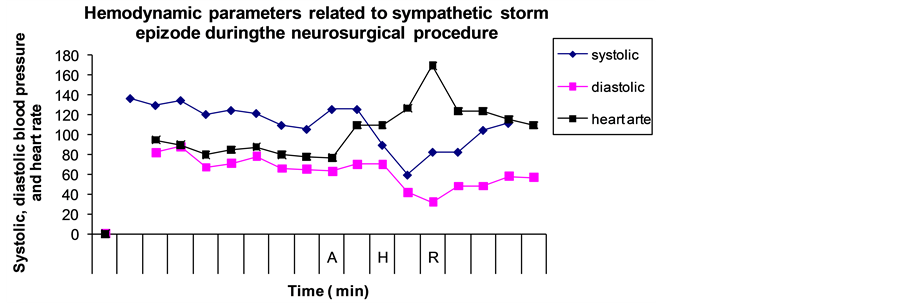
Graph 1. Hemodynamic changes during an intraoperative surgicel application episode. Note: “A”―the application of hemostatic surgical by the surgeon; “H”―the hypotension and tachycardia beginning; “R”―the removing of surgical followed by complete cardiovascular recovery.
Anesthetic management during or before the tachycardia remained unaltered. Surgical manipulation was immediately halted and surgicel was removed from the wound. A bolus of 500 ml normal saline was administered. Both the tachycardia and hypotension were resolved over next two minutes. The oxygen saturation remained at 100% during hypotensive episode. Examination of sample of the fluid obtained from tumor cavity after withdrawal of surgicel revealed pH at level 6.8.
After the surgery, the patient was transferred to pediatric intensive care unit (PICU), and was fully conscious and extubated without incident 72 hours after the procedure. The postoperative MRI images was subsequently done and showed full and extensive tumor resection with no signs of rebreeding or postoperative edema. No further episodes of heart rate or blood pressure aberrations were observed, and no seizure episodes occurred during hospitalization.
3. Discussion
Increased sympathetic or parasympathetic system outflow has been reported as a clinical phenomenon associated with tumors of different localization―midbrain, thalamus, hypothalamus, brain stem [2] [4] [7] . Typical presentation might include heart rate variations, respiratory rate, hyperthermic reaction, shivering, lacrimation, and elevated or decreased blood pressure, and diaphoresis and epileptiformic activity. Our patient presented with acute hydrocephalus and midbrain tumor. Both of components, elevated intracranial pressure and surgical tissue manipulationin operating field, might contribute to development of sympathetic hyperactivity [4] . However, the event began just after the application of surgicel to tumor bed. No other surgical manipulation was preceded at the same time. We believe the application of surgicel on the operating field is a major trigger for the development of tachyarrhythmia and hypotension in the present case. According to pathogenesis of cardiovascular event, we suggest that remarkable hypotension was related to “low cardiac output state” as result of extensive tachyarrhythmia. The stroke volume of the left ventricle is described to be unproductive in correlation with highly elevated heart rate (usually more than 150 beats/min) [8] . Such a decline in effective stroke volume results in decreased in total cardiac output and consequently in systemic blood pressure. Importantly, the hypotensive episode completely resolved immediately after removal of the surgicel.
The surgicel is an absorbal hemostat; oxidized regenerated cellulose product used in the neurosurgical procedures since 1956 [9] [10] . A major component of surgicel hemostatic effect is based on low pH mechanism [11] . In previously published data surgicel was found to cause significant decreasing pH of blood, plasma [11] and surrounding tissues [12] . Borenson et al. [11] demonstrated in animal model decrease of pH values of heap- rinized blood and plasma to less than 6.5 - 6.0 after the surgicel presentation. Some patients notice irritation and report of “burning” sensation related to low pH of surgicel after use it as packing in epistaxis, and other rhinological procedures [3] . Moreover, paralysis and nerve damage (spinal cord, optic nerve) have been reported as result of application of the surgicel during neurosurgical procedures [7] [13] [14] . These complications were explained by mechanical compression of neurological structures caused by oxidize cellulose (surgicel) [7] [13] [14] . Most of those adverse effects [7] [13] [14] have been described as later findings after several hours of direct operating field compression by swollen surgicel mass. In our case we used small pieces of surgicel (marks) applied only to the wall of cavity remained after the resection of the tumor. The cavity space was not filled by surgicel and was not mass effect on the surrounding tissue.
Thus, in present case we believe that the low pH in operating field (6.8) after surgicel application supposed to be the major contributing factor of direct chemical triggering and development of the cardiovascular instability.
Neurogenic cells are highly sensitive to extracellular acidosis [15] .
Acidosis-mediated activation of calcium channels plays a critical role in elevation intracellular calcium levels and further neuronal cell impairment [16] . Such acidification might follow by strong local inflammatory reaction of the surgical site [11] [17] . Moreover, Nagamatsu et al. [18] and Alkan et al. [19] found development of significant neuropathy followed by reduction in nerve conduction velocity caused by acidity of the oxidized cellulose (surgicel).
We suggest that every case of intraoperative surgicel use for the neurosurgical procedure near to brainstem or cranial nervesneeds especial attention in risk of development local reaction on direct chemical triggering by low pH in the surgical site.
4. Conclusion
We strongly suggested that urgent intraoperative hemodynamic instability in our case might be associated with surgicel chemical triggering and could be life-threatening problem without an appropriate treatment. We believe that every case involving a midbrain tumor has to be associated with the possibility of sudden cardiovascular instability and acute blood pressure disturbances.
Conflict of Interest Statement
There are no any financial or other potential conflicts of interest between authors.
This research has not been funded please state the following:
This research did not receive any specific grant from any funding agency in the public, commercial or not-for- profit sector.
References
- Ali, Z., Prabhakar, H. and Bithal, P.K. (2009) Dash HHA Review of Perioperative Complications during Frameless Stereotactic Surgery: Our Institutional Experience. Journal of Anesthesia, 23, 358-362. http://dx.doi.org/10.1007/s00540-009-0759-y
- Engle, G.L. and Aring, C.D. (1945) Hypothalamic Attacks with Thalamic Lesion. I. Physiologic and Psychologic Considerations. II. Anatomic Considerations. Archives of Neurology and Psychiatry, 54, 37-44. http://dx.doi.org/10.1001/archneurpsyc.1945.02300070047004
- Schonauer, C., Tessitore, E. and Barbagallo, G. (2004) The Use of Local Agents: Bone Wax, Gelatin, Collagen, Oxidized Cellulose. European Spine Journal, 13, S89-S96. http://dx.doi.org/10.1007/s00586-004-0727-z
- Venkatraghavan, L., Manninen, P., Mark, P., Lukitto, K., Hodaie, M. and Lozano, A. (1995) Anesthesia for Functional Neurosurgery Review of Complications. Journal of Neurosurgical Anesthesiology, 7, 100-108.
- Brodbelt, A.R., Miles, J.B., Foy, P.M. and Broome, J.C. (2002) Intraspinal Oxidised Cellulose (Surgicel®) Causing Delayed Paraplegia after Thoracotomy―A Report of Three Cases. Annals of The Royal College of Surgeons of England, 84, 97-99.
- Lin, B., Yang, H.F., Cui, M.Z., Li, Y. and Yu, J.L. (2014) SurgicelTM Application in Intracranialhemorrhage Surgery Contributed to Giant-Cell Granuloma in a Patient with Hypertension: Case Report and Review of the Literature. World Journal of Surgical Oncology, 12, 101. http://dx.doi.org/10.1186/1477-7819-12-101
- Ethicon Biosurgery (2011) The Most Comprehensive Portfolio of Hemostasis Solutions. SurgicelTM Family of Absorbal Hemostats. Ethicon, Inc.
- Lima, J.A., Weiss, J.L., Guzman, P.A., Weisfeldt, M.L., Reid, P.R. and Traill, T.A. (1983) Incomplete Filling and Incoordinate Contraction as Mechanisms of Hypotension during Ventricular Tachycardia in Man. Circulation, 68, 928- 938. http://dx.doi.org/10.1161/01.CIR.68.5.928
- McLean, A.J. (1934) Autonomic Epilepsy: Report of a Case with Observation at Necropsy. Archives of Neurology and Psychiatry, 32, 189. http://dx.doi.org/10.1001/archneurpsyc.1934.02250070195012
- Luks, S. (1956) Root and Amalgam Technique in the Practice of Endodontics. The Journal of the American Dental Association, 53, 424-428.
- Bjorenson, J.E., Grove, H.F., List, M.G., Haasch, G.C. and Austin, B.P. (1986) Effects of Hemostatic Agents on the pH of Body Fluids. Journal of Endodontics, 12, 289-292. http://dx.doi.org/10.1016/S0099-2399(86)80110-8
- Wang, H. and Chen, P. (2013) Surgicel (Oxidized Regenerated Cellulose) Granuloma Mimicking Local Recurrent Gastrointestinal Tumor: A Case Report. Oncology Letters, 5, 1497-1500.
- Otenasek, F.J. and Otenasek, R.J. (1986) Dangers of Oxidizedcellulose in Chiasmal Surgery. Journal of Neurosurgery, 29, 209-210. http://dx.doi.org/10.3171/jns.1968.29.2.0209
- Menovsky, T., Plazier, M., Rasschaert, R., Maas, A.I.R., Parizel, P.M. and Verbeke, S. (2011) Massive Swelling of Surgicel Fibrillar Hemostat after Spinal Surgery. Minimally Invasive Neurosurgery, 54, 257-259. http://dx.doi.org/10.1055/s-0031-1284394
- Wang, Y.-Z. and Xu, T.-L. (2011) Acidosis, Acid-Sensing Ion Channels and Neuronal Cell Death. Molecular Neurobiology, 44, 350-358. http://dx.doi.org/10.1007/s12035-011-8204-2
- Xiong, Z.G., Chu, X.P. and Simon, R.P. (2006) Ca2+-Permeable Acid-Sensing Ion Channels and Ischemic Brain Injury. The Journal of Membrane Biology, 209, 59-68. http://dx.doi.org/10.1007/s00232-005-0840-x
- Tomizawa, Y. (2005) Clinical Benefits and Risk Analysis of Topical Hemostats: A Review. Journal of Artificial Organs, 8, 137-142. http://dx.doi.org/10.1007/s10047-005-0296-x
- Nagamatsu, M., Podratz, J., Windebank, A.J. and Low, P.A. (1997) Acidity Is Involved in the Development of Neuropathy Caused by Oxidized Cellulose. Journal of the Neurological Science, 146, 97-102. http://dx.doi.org/10.1016/S0022-510X(96)00295-X
- Alkan, A., Inal, S., Yildirim, M., Bas, B. and Agar, E. (2007) The Effects of Hemostatic Agents on Peripheral Nerve Function: An Experimental Study. Journal of Oral and Maxillofacial Surgery, 65, 630-634. http://dx.doi.org/10.1016/j.joms.2005.12.076
NOTES

*Corresponding author.





