Food and Nutrition Sciences
Vol.05 No.22(2014), Article ID:52088,9 pages
10.4236/fns.2014.522231
Green Tea (Camellia sinensis): Hypocholesterolemic Effects in Humans and Anti-Inflammatory Effects in Animals
Márcia Reto, Cristina Almeida, João Rocha, Bruno Sepodes, Maria-Eduardo Figueira*
Research Institute for Medicines (iMed. ULisboa), Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal
Email: *efigueira@ff.ulisboa.pt
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 19 September 2014; revised 16 October 2014; accepted 2 November 2014
ABSTRACT
In its essence, tea is an infusion of Camellia sinensis leaves and it is the most widely consumed beverage in the world, aside water. Animal and epidemiological studies have associated green tea consumption with several health benefits, which include hypocholesterolemic effect and anti-in- flammatory activity. In this study catechins levels of green tea and its effect on the lipid profile of humans were evaluated as well as the protective effects against H2O2-mediated damage in human fibroblasts and anti-inflammatory activity of (−)-epigallocatechingallate (EGCG). The daily con- sumption of 1200 mL of green tea for 30 days by 15 human volunteers promoted the decrease of cholesterol and LDL levels after 15 days, but this effect was not persistent after 30 days. No changes were observed in lipid profile after the consumption of green tea capsules. EGCG, a major green tea catechin, demonstrated an anti-inflammatory action in rats and an antioxidant effect in cultured human fibroblasts.
Keywords:
Green Tea, Infusions, Hypocholesterolemic Effect, Anti-Inflammatory Activity, Antioxidant Activity

1. Introduction
Green tea became a very popular beverage in the western countries and almost everyday new functional beverages with green tea extract as an ingredient are developed. The increased popularity of these beverages is clearly related to the health benefits attributed to its consumption. Several human and animal studies suggest that green tea has antioxidant effect, antibacterial and antiviral activity, cancer chemo preventive properties, contributes to a reduction in the risk of cardiovascular disease, enhances weight loss, amongst other effects [1] .
The chemical composition of green tea is similar to that of fresh tea leaves containing several compounds with interest for human health such as polyphenols, fluoride, vitamin K, caffeine, minerals (sodium, potassium, calcium) and trace elements like, aluminium, chromium, selenium, manganese and iron. We have previously determined and studied these compounds [2] -[4] . Catechins [(−)-epigallocatechingallate (EGCG) and (−)-epi- gallocatechin (EGC) followed by (−)-epicatechin 3-gallate (ECG), catechin (C) and (−)-epicatechin (EC)] are the most biologically active group of tea compounds [1] .
Cardiovascular disease (CVD) is a leading cause of death around the world [5] . Atherosclerosis is the principal cause of CVD including myocardial infarction, heart failure and stroke [6] . Hypercholesterolemia, low levels of serum HDL cholesterol and high levels of serum LDL cholesterol are on the genesis of atherosclerotic lesions and are strongly associated with the risk of coronary heart disease [7] [8] .
Atherosclerosis is an inflammatory disease of the wall of arteries with passive accumulation of lipids and other factors. The complications of atherosclerosis may be caused by a response to the oxidative components of modified low-density lipoprotein (LDL) [9] . Inflammatory processes can be associated to risk factors in atherosclerosis and, consequently, the development of cardiovascular diseases [10] .
Similarly, oxidative stress shows a key role on the development of the pathology, namely, because ROS are responsible for the oxidation of LDL cholesterol and consequently the induction of vascular lesions with subsequent atherosclerosis [11] .
The control of some risk factors can reduce significantly the morbidity and mortality associated with atherosclerosis and cardiovascular disease.
Evidence from animal studies suggests that catechins inhibit cholesterol absorption and lower plasma cholesterol [8] .
Epidemiological studies indicated a significant inverse relationship between drinking green tea and the plasma levels of cholesterol [12] [13] . There are some human studies about the effect of green tea and its extract on total cholesterol and LDL and HDL cholesterol. Although some of them have shown reduction on total and LDL cholesterol serum levels, no changes were observed in other studies; hence the existence of controversy requiring further clarification is justified by the controversial results found in the literature [14] [15] .
Anti-inflammatory activities are also attributed to tea flavonoids. Das et al. observed that green tea extract (black and green) presents anti-inflammatory activity in rats [16] and attenuates inflammation in mice [17] . The anti-inflammatory effect of green tea is mediated, in part, through the regulation of the expression of tumour necrosis alfa (TNF-for inhibition of the activation of nuclear factor kappa B (NF-B) [17] [18] . Green and black tea catechins and theaflavins can inhibit the lipopolysaccharide-induced iNOS gene expression and the activity of iNOS in macrophages [19] -[22] .
The antioxidant activity of tea catechins has been evaluated by several methods. EGCG, the major catechin in green tea, is more effective antioxidant than vitamins C and E [1] . Data from animal studies and ex vivo studies also confirm that tea polyphenols act as antioxidants [23] .
As previously mentioned, controlling certain risk factors will decrease the premature development of atherosclerosis and cardiovascular diseases in humans. Tea, especially green tea, is rich in biologically active phenolic compounds as already demonstrated, so it seemed interesting to study the effects of green tea on the lipid profile in human volunteers, and of its major catechin, EGCG, in a model of acute inflammation in rat, along with its antioxidant effect in an in vitro model.
As a consequence, in the present study, two experiments were performed to examine the effect of green tea and green tea capsules consumption, during 30 days, on the lipid profile of healthy Portuguese volunteers. The anti-inflammatory activity was also assessed in rats in order to evaluate the role of EGCG in the prevention of inflammation related diseases. In addition, the protective effects of this catechin against H2O2-mediated damage in human fibroblasts were investigated.
In order to evaluate the hypocholesterolemic and anti-inflammatory activity of green tea in our study, it is detrimental to determine the levels of catechins levels in this beverage. Since green tea is consumed essential as infusion, the chemical analysis was made in the infusion and not in the leaves. The High Performance Liquid Chromatography with Ultra Violet Detection (HPLC-UV) method for the analysis of catechins in green tea infusions was previously optimised [2] .
2. Experimental Methods
2.1. Catechins Characterization
2.1.1. Tea Samples
For catechins and caffeine analysis eight green tea samples from different brands were used. These samples were purchased from several shops in Lisbon (markets and herbalists). Five of them are sold in tea bags and the other three as loose-leaf tea. The different brands of green tea were represented by the acronyms G, S, T, TN, TV, F, B, M and T. The TN and TV green tea are the same brand but from different lots. Tea infusions preparations were described elsewhere [2] . TV tea was later chosen for presenting the best relation catechins/caffeine, hence, lower levels of caffeine.
Green tea capsules were obtained in a popular herbalist in Lisbon region. For catechin analysis the content of the capsules were dissolve in ultra-pure water.
Each tea was analysed in triplicate using separate infusions.
2.1.2. Methods
The HPLC/UV method was optimised for the simultaneous determination of catechins and caffeine in the tea infusions [2] . A high-performance liquid chromatograph system equipped with a binary LC-6A pump controlled by a SCL-6B controller, a ultra violet detector model SPD-M10A DAD and a LC workstation (class LC-10 versão1) integrator, all from Shimadzu (Shimadzu Europa GmbH, Duisburg, Germany) and a Lichrosper 100 RP-18 (5 µm), 250 mm × 4 mm column (Merck KGaA, Germany), was used.
2.1.3. Reagents and Standard Solutions
The standards of (+)-catechin hydrate, (−)-epicatechingallate, (−)-epigallocatechin from green tea and (−)-epi- gallocatechingallate from green tea were supplied by Sigma (Sigma-Aldrich, St. Louis, MO, USA). The (−)- epicatechin was from Fluka (Buchs SG, Switzerland). All the aqueous solutions and serial dilutions were prepared by dilution with ultra-pure water (Mili Q System, Millipore, Billerica, MA, USA).
2.2. Clinical Trial
Subjects
Healthy, normal weight, aged ≥18 years male and female volunteers were recruited from our Faculty and from a public Institute in Lisbon. All subjects were submitted to routine haematological and biochemical analyses. All participants in the studies gave their informed consent. No screening criteria about smoking and diet habits were made. All participants were requested to maintain their habitual lifestyles during the entire experiment.
On the first study 15 volunteers drank 6 cups of green tea (TV brand) a day in 200 mL amounts during a period of 30 days. After that time they were not allowed to consume this beverage for another 30 days. Participants were instructed to follow a standardised protocol in tea preparation in which each bag was left in 200 mL of boiling water for 10 minutes. From these 15 subjects, 11 were females and 4 were males. Age range was 24 to 55 years old. Mean total cholesterol values were 234 mg/dL and for LDL mean values were 136 mg/dL.
For the second study men and women were selected based on the following criteria: mildly elevated total cholesterol, no major health problems and not taking medications known to affect serum lipids. In this study the subjects took six capsules of green tea per day (two at each meal―breakfast, lunch and dinner) during 30 days. From these 15 subjects, 10 were females and 5 were males. Age range was 37 to 60 years old. Mean total cholesterol values were above 240 mg/dL and for LDL mean values were above 160 mg/dL.
Thirty adults (men and women) were selected to participate in the two studies. In both studies blood samples were obtained, at a fasting state, before (0 day), during (15 days and 30 days) and after (60 days) the daily consumption of green tea or ingestion of extract capsules. Concentrations of total cholesterol, triglycerides, LDL and HDL cholesterol were assayed enzymatically using commercial kits (Roche Diagnostics, GmbH, Mannheim, Germany) and an auto-analyser Hitachi mod 717 (Hitachi-Roche, Tokyo, Japan). Results are presented as mean ± SEM.
2.3. Animal Studies
Animals
Sixteen male Wistar rats (4 - 5 weeks old, 125 - 132 g) were purchased from Harvard Ibérica. Animals were maintained under standard conditions of temperature (25˚) and humidity with an alternating 12 hours light/dark cycles. Experiments were conducted according to the Home Office Guidance in the Operation of Animals (Scien- tific Procedures) Act 1986, published by Her Majesty’s Stationary Office, London, UK and the Institutional Animal Research Committee Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85 - 23, revised 1996), as well as to the currently adopted EC regulations. Finally, the studies are in compliance with the ARRIVE Guidelines for Reporting Animal Research’ summarized at www.nc3rs.org.uk. Hence, the Ethics Committee of the Research Institute endorsed the animal study protocol, considering also that authors Sepodes and Rocha, are licensed by the Portuguese General Directorate of Veterinary to coordinate and conduct independent animal research.
The carrageenan-induced paw edema of the rat hind-paw, is a suitable model to study acute local inflammation and widely considered to be one of the most useful models in the evaluation of anti-inflammatory activity of investigational compounds [24] [25] . Paw edema was induced by a single subplantar injection into the rat left hind paw of 0.1 ml of a 1% λ-carrageenan sterile saline solution. Paw volume was measured by means of a volume displacement method using a plethysmometer (Digital Plethysmometer LE7500-Letica Scientific Instruments). Paw volume was measured before, immediately after injection of carrageenan (V0 or basal volume) and 6 hours later (V6h). Paw edema was expressed as percentage of increase in paw volume 6 hours after carrageenan injection relative to the basal values according to the equation: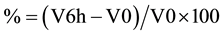 . Animals were divided in two groups. The control group was fed ad libitum on a standard diet (IPM-R20 from Letica). The other group was fed with the same diet supplemented with EGCG (5 mg/kg) for a period of 30 days. Results are presented as mean ± SEM of paw volume observed.
. Animals were divided in two groups. The control group was fed ad libitum on a standard diet (IPM-R20 from Letica). The other group was fed with the same diet supplemented with EGCG (5 mg/kg) for a period of 30 days. Results are presented as mean ± SEM of paw volume observed.
2.4. Cells Studies
Isolated human fibroblasts embryonic human lung fibroblasts (HPF-1), purchased from Pharmacological Institute of Medicine Faculty of University of Lisbon, were grown in Eagle’s minimal essential medium (EM) with 10% fetal bovine serum, 1% glutamine and 1% penicillin-streptomycin at 37˚C under 5% CO2. The protective effect of EGCG against cellular injury H2O2-induced was investigated using the MTT method [26] . The cells were plated in 96 wells at a density of 25,000 cells/well. To study the effect of EGCG the cells were incubated for 4 hours with H2O2 (3 mM) and with different concentrations of EGCG (0 - 0.3 mM).
2.5. Statistical Analysis
Results are expressed by mean ± SEM and all the comparisons were done by ANOVA method and considered different when p < 0.05.
3. Results
The changes of the lipid profile of the volunteers who drank green tea are presented in Table 1. Some of the volunteers presented high values of cholesterol (>240 mg/dL) at the beginning of this study meaning that they have twice the risk of coronary heart disease than the subjects that presented desirable values of cholesterol (<190 mg/L) [27] . The levels of LDL cholesterol, HDL cholesterol and triglycerides of the volunteers were in the borderline-high category.
Tea consumption for 15 and 30 days reduced triglycerides level about 25%, but the differences were not significant. No statistically significant changes in HDL cholesterol were observed. Additionally, the LDL levels decreased significantly (p < 0.05) with the daily intake of 6 cups of green tea for 15 days. However, this effect disappeared at the 30 day of treatment. The levels of total cholesterol had significantly decreased (p < 0.05) from 0 day to 15 day of treatment. The biggest differences were observed in the individuals with higher levels (287 mg/dL and 300 mg/dL). However, at day 30 the statistical differences were not significant but a reduction of 7% is nevertheless observed.
From the fifteen individuals, seven had total cholesterol levels equal or above 240 mg/dL. Since the highest differences were detected in individuals with higher levels of total cholesterol we decided to analyse those data separately (Table 2). For the hypercholesterolemic volunteers a significant reduction of total cholesterol levels was observed after 15 days of tea consumption (about 50%). At day 30 for some volunteers the total cholesterol levels were normal (<200 mg/dL). For these individuals the hypocholesterolemic effect of green tea was longer
Table 1. Effect of treatment with green tea during 30 days on the lipid profile of 15 volunteers.
*p < 0.05 versus 0 day.
Table 2. Effect of the treatment with green tea during 30 days on the lipid profile of hypercholesterolemic volunteers.
than for those with acceptable values of cholesterol. The LDL levels decreased after 15 days of treatment. However after 30 days its levels were similar to the one observed at the beginning of the study. No statistically significant differences in HDL levels, triglycerides and total lipids were observed.
On the second study all the volunteers showed at the beginning of the study high values of total cholesterol, medium levels of HDL (corresponding to a moderate risk) and values of LDL corresponding to a high risk (>160 mg/dL) (Table 3); here the inclusion criteria was based on high levels of total cholesterol and LDL since we had previously observed that the effect of catechins was more pronounced in these two parameters. Based on the findings of the first study it was expected that a daily intake of tea extract capsules would lower serum cholesterol concentrations in the mildly hypercholesterolemic subjects but no significant alterations in total cholesterol, HDL and LDL and triglycerides levels were observed.
On another research pathway of our study, the carrageenan-induced oedema test was used to verify the in vivo anti-inflammatory effect of EGCG. Results are presented in Figure 1. The animals pre-treated with EGCG showed a decrease in the acute inflammation response to carrageenan, characterized by a decrease on the volume of the paw compared with the control group (p < 0.05).
Also at the pre-clinical level, the effect of EGCG at different concentrations on the cytotoxicity induced by H2O2 in isolated human fibroblasts is presented in Figure 2. The results showed that the protection against cellular injury H2O2-induced in human fibroblasts was dose-dependent. EGCG had the significantly protective effect on H2O2-damaged cells (p < 0.05 or p < 0.01). At the concentration of 0.3 mM EGCG showed stronger protective effects.
4. Discussion
Results obtained in our study suggest that green tea have a hypocholesterolemic effect. This finding corroborates the data from some epidemiological studies that reported an inverse correlation between total cholesterol levels and tea consumption [28] -[30] . In a human study green tea and green tea extract consumption was associated with the decrease of LDL and total cholesterol levels but all the volunteers were hypercholesterolemic and they all were on a low fat diet [31] . Not all human studies have shown similar results, some failed to prove an association between tea consumption with a reduction on plasma lipids.
Green tea ingestion did not affect the HDL levels, triglycerides and total lipids that are in accordance with the described by Maron et al. and van het Hof [31] [32] .
No changes on total cholesterol, HDL and LDL and triglycerides levels were observed after green tea capsules consumption. These results are similar to those obtained by Princen et al. and van het Hof et al. that reported no effects related to green tea consumption on plasma lipids and LDL oxidation [31] -[33] .
The differences observed between the two studies can be related with the amount of catechins ingested by the volunteers (Table 4). The levels of catechins consumed by the participants in the study with the green tea were approximately 970 mg per day. The capsules had about 61 mg/g of catechins and since the volunteers consumed 6 capsules per day, the daily consumption was in this case approximately 366 mg. Thus, the amount of catechins
Table 3. Effect of the treatment with green tea capsules during 30 days on the lipid profile of 15 volunteers.
Table 4. Concentrations of catechins and caffeine in green tea infusions and capsules by HPLC-UV (the compounds were ordinate by their retention time) (2) (SD-Standard deviation).
Figure 1. Effect of ECGC (5 mg/kg. p.o.) on the paw volume 6 hours after carrageenan injection. **p < 0.05 vs Control group.
ingested corresponds to about a third of the existing in 1.2 L of green tea infusion.
The ratio total cholesterol/HDL (atherogenic index) is a useful factor to predict myocardial infarction risk. An atherogenic index >5 indicate a high probability of cardiac stroke. In the two studies carried out in this work the atherogenic index for the majority of the individuals were near 5. The consumption of green tea during 15 and 30 days did not originate any alteration in this value, although a reduction of total and LDL cholesterol was observed in the study with green tea infusion.
Many potential mechanisms for the hypocholesterolemic effect have been proposed: reduction of micelarsolubilization of lipids and intestinal absorption of cholesterol, increase in the fecal excretion of lipids and cholesterol, reduction of the hepatic cholesterol and regulation of LDL receptors in liver cells [34] -[37] . These mechanisms have been established mainly in animal and in vitro studies.
Figure 2. Protective effect of EGCG on the cytotoxicity induced by H2O2 (3 mM) in isolated human fibroblasts. *p < 0.05 and **p < 0.01 vs control.
Our work suggests that supplementation with green tea may reduce the risk of coronary disease because a trend for decrease in the total cholesterol level (of 7%) was observed in the individuals that participated in this study, and a decrease in LDL level was also observed. However the results suggest that green tea had only acute beneficial effects in individuals with mild hypercholesterolemia since the effects were only observed by a period of 15 days, and although our experimental design cannot explain the absence of the same positive results after 30 days in these subjects, it may be hypothesised that the observed effects in the biomarkers assessed have an acute nature that might not be sustainable in time and that chronic beneficial effects associated to the consumption of green tea might be related to other biochemical pathways.
Capsules ingestion may be advantageous for individuals that do not appreciate the green tea flavour or did not like to consume high volumes of liquids. EGCG doses up to 1 g per day have a favourable safety profile, so further studies with higher concentrations of tea extract should be made in order to evaluate the correlation between tea catechins and plasma lipid levels in hypercholesterolemic individuals.
EGCG showed a significant anti-inflammatory effect in rats. These data are in accordance with the results obtained by Das et al. and Varilek et al. [16] [17] . The inflammation is a complex process in which several cellular and mediating types participate. In the initial phase, the mediators of the inflammatory, stored in mast cell and basophil granules, are histamine and serotonin or 5-hydroxytryptamine. To the early phase mediators also belong cytokines such as IL-1, IL-6, and TNF-a. Another important mediator is nitric oxide (NO). The later vascular events are mediated by arachidonic acid metabolites, produced and by neutrophils and other cells. Some studies have demonstrated that EGCG affects several signalling mechanisms in inflammation. EGCG inhibits in vivo neutrophil recruitment [38] . Green and black tea polyphenols can affect cyclooxygenase (COX)-2 and lipoxygenase (LOX)-dependent arachidonic acid metabolism in normal human colon mucosa and colon cancers [39] . Ingestion of green tea decreased rectal mucosal concentrations of prostaglandin E2 in humans [40] . Tea and tea components are scavengers of NO and peroxynitrite and also inhibit the activity of iNOS and the induction of iNOS by LPS [41] . In rats subjected to myocardial ischemia and reperfusion an inhibition the NF- kB and AP-1 pathway were observed [42] . According to Cao et al. the molecular mechanisms of anti-inflam- matory effects of green tea may be related to the increase of mRNA levels of anti-inflammatory factors and the decrease of pro-inflammatory factors [43] .
Inflammatory diseases are currently treated with steroidal and non-steroidal drugs, as standard of care. Despite its generalized use, these medicines are many times associated with adverse effect, being the most common, gastrointestinal bleeding and renal failure. For this reason, it is necessary to find safer drugs. Future clinical trials could further elucidate the anti-inflammatory effect of EGCG, however based on the results of this study this catechin could be an interesting pharmacological tool to modulate inflammation. EGCG showed a protective effect against H2O2-mediated damage in human fibroblasts. But this data must be analysed with careful. The in vitro results are dependent of the type of assay employed. Bioavailability and metabolism are very important factors to be taken into account when extrapolating to humans [1] . Numerous studies have demonstrated that tea catechins have a protective effect against damage caused by physiologically relevant reactive oxygen and nitrogen species in vitro, including superoxide, peroxyl radicals, singlet oxygen, peroxynitrite, and hypochlorous acid. This antioxidant activity appears to be related to its molecular structure [23] .
Saffari et al. have shown that EGCG is a powerful antioxidant capable of protecting erythrocyte membrane- bound ATPases against oxidative stress [44] . In whole blood lymphocytes EGCG significantly suppressed the DNA strand breakage in a concentration-dependent manner. However, this catechin was genotoxic at high concentration [45] . The biological activity of green tea catechins can also be influenced by the pH of the surrounding medium or tissues [46] . The potential mechanisms described for the antioxidant capacity of tea are: 1) scavenging of highly reactive oxygen (ROS) and nitrogen species (RNS), 2) chelation of transition metal ions, 3) inhibition of redox-sensitive transcription factors such as nuclear factor κB and activator protein AP-1, and/or 4) induction of phase II and “antioxidant” enzymes [45] . In vitro evidence and numerous animal studies strongly suggests that antioxidant properties of green tea are also responsible for reducing the risk of heart disease [13] .
Our results suggest that consumption of green tea may be associated to a favourable lipid profile in humans. EGCG, a major green tea catechin component, seems to be, at least in part, responsible for some of the beneficial effects described. In fact, in the rat, EGCG had no significant effect on the lipid profile but showed a marked anti-inflammatory action, as well as an antioxidant effect in cultured human fibroblasts, hence supporting the rational that the anti-inflammatory effects are relevant for the pharmacological actions attributed to green tea.
5. Conclusion
In conclusion, green tea consumption can be a useful pharmacological tool with modulatory actions important in controlling some of the risk factors for the development of atherogenesis and, therefore, cardiovascular disease.
Acknowledgements
We thank all the volunteers who so kindly accepted to participate in this study.
Conflicts of Interest
There were no conflicts of interest.
References
- MacKay, D.L. and Blumberg, J.B. (2000) The Role of Tea in Human Health: An Update. Journal of the American College of Nutrition, 21, 1-13. http://dx.doi.org/10.1080/07315724.2002.10719187
- Reto, M., Figueira, M.E., Filipe, H.M. and Almeida, C.M.M. (2007) Chemical Composition of Green Tea (Camellia sinensis) Infusions Commercialized in Portugal. Plant Foods for Human Nutrition, 62, 139-144. http://dx.doi.org/10.1007/s11130-007-0054-8
- Reto, M., Figueira, M.E., Filipe, H.M. and Almeida, C.M.M. (2007) Analysis of Vitamin K in Green Tea Leafs and Infusions by SPME-GC-FID. Food Chemistry, 100, 405-411. http://dx.doi.org/10.1016/j.foodchem.2005.09.016
- Reto, M., Figueira, M.E., Filipe, H.M. and Almeida, C.M.M. (2008) Teor de Fluoretos em Infusões de Chá Verde (Camellia sinensis). Química Nova, 31, 317-320. http://dx.doi.org/10.1590/S0100-40422008000200024
- WHO (2007) Cardiovascular Diseases. Fact Sheet, No. 317. http://www.who.int/cardiovascular_diseases/en/index.html
- Frostegard, J. (2013) Immunity, Atherosclerosis and Cardiovascular Disease. BMC Medicine, 11, 117. http://dx.doi.org/10.1186/1741-7015-11-117
- Orekhov, A.N. (2014) Direct Anti-Atherosclerotic Therapy; Development of Natural Anti-Atherosclerotic Drugs Preventing Cellular Cholesterol Retention. Current Pharmaceutical Design, 19, 5909-5928.
- Bursill, C.A., Abbey, M. and Roach, P.D. (2007) A Green Tea Extract Lowers Plasma Cholesterol by Inhibiting Cholesterol Synthesis and Upregulating the LDL Receptor in the Cholesterol-Fed Rabbit. Atherosclerosis, 193, 86-93. http://dx.doi.org/10.1016/j.atherosclerosis.2006.08.033
- Paoletti, R., Gotto A.M., DPhil, M.D. and Hajjar, D. (2004) Inflammation in Atherosclerosis and Implications for Therapy. Circulation, 109, 20-26. http://dx.doi.org/10.1161/01.CIR.0000131514.71167.2e
- Sherer, Y. and Shoenfeld, Y. (2006) Mechanisms of Disease: Atherosclerosis in Autoimmune Diseases. Nature Clinical Practice Rheumatology, 2, 99-106. http://dx.doi.org/10.1038/ncprheum0092
- Kolluru, G.K., Bir, S.C. and Kevil, C.G. (2012) Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing. International Journal of Vascular Medicine, 2012, Article ID: 918267, 30 p. http://dx.doi.org/10.1155/2012/918267
- Peters, U., Poole, C. and Arab, L. (2001) Does Tea Affect Cardiovascular Disease? A Meta-Analysis. American Journal of Epidemiology, 154, 495-503. http://dx.doi.org/10.1093/aje/154.6.495
- Bacquer, D.D., Clays, E., Delanghe, J. and Backer, G.D. (2006) Epidemiological Evidence for an Association between Habitual Tea Consumption and Markers of Chronic Inflammation. Atherosclerosis, 189, 428-435. http://dx.doi.org/10.1016/j.atherosclerosis.2005.12.028
- Zheng, X.X., Xu, Y.L., Li, S.H., Liu, X.X., Hui, R. and Huang, X.H. (2011) Green Tea Intake Lowers Fasting Serum Total and LDL Cholesterol in Adults: A Meta-Analysis of 14 Randomized Controlled Trials. American Journal of Clinical Nutrition, 94, 601-610. http://dx.doi.org/10.3945/ajcn.110.010926
- Davies, M.J., Judd, J.T., Baer, D.J., Clevidence, B.A., Paul, D.R., Edwards, A.J., Wiseman, S.A., Muesing, R.A. and Chen, S.C. (2003) Black Tea Consumption Reduces Total and LDL Cholesterol in Mildly Hypercholesterolemic Adults. Journal of Nutrition, 133, 3298S-3302S.
- Das, M., Sur, P., Gomes, A., Vedasiromoni, J.R. and Ganguly, D.K. (2002) Inhibition of Tumour Growth and Inflam- mation by Consumption of Tea. Phytotherapy Research, 16, 40-44. http://dx.doi.org/10.1002/ptr.797
- Varilek, G.W., Yang, F., Lee, E.Y., de Villiers, J.S., Zhong, J., Oz, H.S., Westberry, K.F. and McClain, C.J. (2001) Green Tea Polyphenol Extract Attenuates Inflammation Interleukin-2-Deficient Mice, a Model of Autoimmunity. Journal of Nutrition, 131, 2034-2039.
- Yang, F., de Villiers, J.S., McClain, C.J. and Varilek, G.W. (1998) Green Tea Polyphenols Block Endotoxin-Induced Tumor Necrosis Factor-Production and Lethality in a Murine Model. Journal of Nutrition, 128, 2334-2340.
- Chan, M.M., Fong, D., Ho, C.T. and Huang, H.I. (1997) Inhibition of Inducible Nitric Oxide Synthase Gene Expression and Enzyme Activity by Epigallocatechin Gallate, a Natural Product from Green Tea. Biochemical Pharmacology, 54, 1281-1286. http://dx.doi.org/10.1016/S0006-2952(97)00504-2
- Lin, Y.L. and Lin, J.K. (1997) (−)-Epigallocatechin-3-gallate Induction of Nitric Oxide Synthase by Down-Regulating Lipopolysacharide-Induced Activity of Transcription Factor Nuclear Factor-κB. Molecular Pharmacology, 52, 465- 472.
- Lin, Y.L., Tsai, S.H., Lin-Shiau, S.Y., Ho, C.T. and Lin, J.K. (1999) Theaflavin-3,3’-digallate from Black Tea Blocks the Nitric Oxide Synthase by Down-Regulating the Activation of NF-κB in Macrophages. European Journal of Pharmacology, 367, 379-388. http://dx.doi.org/10.1016/S0014-2999(98)00953-4
- Lin, Y.S., Wu, S.S. and Lin, J.K. (2003) Determination of Tea Polyphenols and Caffeine in Tea Flowers (Camellia sinensis) and Their Hydroxyl Radical Scavenging and Nitric Oxide Suppressing Effects. Journal of Agricultural and Food Chemistry, 51, 975-980. http://dx.doi.org/10.1021/jf020870v
- Frei, B. and Higdon, J.V. (2003) Antioxidant Activity of Tea Polyphenols in Vivo: Evidence from Animal Studies. Journal of Nutrition, 133, 3275S-3284S.
- Vogel, H.G. (2002) Drug Discovery and Evaluation: Pharmacological Assays. Springer-Verlag Berlin and Heidelberg GmbH & Co., Berlin, Heidelberg, 751-772.
- Bignotto, L., Rocha, J., Sepodes, B., Eduardo-Figueira, M., Pinto, R., Chaud, M., et al. (2009) Anti-Inflammatory Effect of Lycopene on Carrageenan-Induced Paw Oedema and Hepatic Ischaemia-Reperfusion in the Rat. British Journal of Nutrition, 102, 126-133. http://dx.doi.org/10.1017/S0007114508137886
- Cole, S.P. (1986) Rapid Chemosensitivity Testing of Human Lung Tumor Cells Using the MTT Assay. Cancer Chemotherapy and Pharmacology, 17, 259-263. http://dx.doi.org/10.1007/BF00256695
- Sousa, J.C. and Pinto, D.S. (2003) Valores Laboratoriais do estudo lipídico e risco vascular. Revista Faculdade de Medicina de Lisboa Série III, 8, 75-81.
- Kono, S., Shinchi, K., Ikeda, N., Yanai, F. and Imanishi, K. (1992) Green Tea Consumption and Serum Lipid Profiles: A Cross-Sectional Study in Northern Kyushu, Japan. Preventive Medicine, 21, 526-531. http://dx.doi.org/10.1016/0091-7435(92)90060-U
- Imai, K. and Nakachi, K. (1995) Cross Sectional Study of Effects of Drinking Green Tea on Cardiovascular and Liver Diseases. British Medical Journal, 310, 693-696. http://dx.doi.org/10.1136/bmj.310.6981.693
- Tokunaga, S., White, I.R., Frost, C., Tanaka, K., Kono, S., Tokudome, S., Akamatsu, T., Moriyama, T. and Zakouji, H.A. (2002) Green Tea Consumption and Serum Lipids and Lipoproteins in a Population of Healthy Workers in Japan. Annals of Epidemiology, 12, 157-165. http://dx.doi.org/10.1016/S1047-2797(01)00307-6
- Maron, D.J., Lu, G.P., Ca, N.S., Wu, Z.G., Li, Y.H., Chen, H., Zhu, J.Q., Jin, X.J., Wouters, B.C. and Zhao, J. (2003) Cholesterol-Lowering Effect of a Theaflavin-Enriched Green Tea Extract: A Randomized Controlled Trial. JAMA Internal Medicine, 163, 1448-1453. http://dx.doi.org/10.1001/archinte.163.12.1448
- van het Hof, K.H., de Boer, H.S., Wiseman, S.A., Lien, N., Westrate, J.A. and Tijburg, L.B. (1997) Consumption of Green or Black Tea Does Not Increase Resistance of Low-Density Lipoprotein to Oxidation in Humans. American Journal of Clinical Nutrition, 66, 1125-1132.
- Princen, H.M., van Duyvenvoorde, W., Buytenhek, R., Blonk, C., Tijburg, L.B., Langius, J.A., Meinders, A.E. and Pijl, H. (1998) No Effect of Consumption of Green and Black Tea on Plasma Lipid and Antioxidant Levels and on LDL Oxidation in Smokers. Arteriosclerosis, Thrombosis, and Vascular Biology, 18, 833-841. http://dx.doi.org/10.1161/01.ATV.18.5.833
- Yang, T.T. and Koo, M.W. (1999) Chinese Green Tea Lowers Cholesterol Level trough an Increase in Fecal Lipid Excretion. Life Sciences, 66, 411-423. http://dx.doi.org/10.1016/S0024-3205(99)00607-4
- Bursill, C., Roach, P.D., Bottema, C.D. and Pal, S. (2001) Green Tea Upregulates the Low-Density Lipoprotein Receptor through the Sterol-Regulated Element Binding Protein in HepG2 Liver Cells. Journal of Agricultural and Food Chemistry, 49, 5639-5645. http://dx.doi.org/10.1021/jf010275d
- Raederstorff, D.G., Schlachter, M.F., Elste, V. and Weber, P. (2003) Effect of EGCG on Lipid Absorption and Plasma Lipid Levels in Rats. Journal of Nutritional Biochemistry, 14, 326-332. http://dx.doi.org/10.1016/S0955-2863(03)00054-8
- Koo, S.I. and Noh, S.K. (2007) Green Tea as Inhibitor of the Intestinal Absorption of Lipids: Potential Mechanism for Its Lipid-Lowering Effect. Journal of Nutritional Biochemistry, 18, 179-183. http://dx.doi.org/10.1016/j.jnutbio.2006.12.005
- Donà, M., Dell’Aica, I., Calabrese, F., Benelli, R., Morini, M., Albini, A. and Garbisa, S. (2003) Neutrophil Restraint by Green Tea: Inhibition of Inflammation, Associated Angiogenesis, and Pulmonary Fibrosis. Journal of Immunology, 170, 4335-4341. http://dx.doi.org/10.4049/jimmunol.170.8.4335
- Hong, J., Smith, T.J., Ho, C.T., August, D.A. and Yang, C.S. (2001) Effects of Purified Green and Black Tea Polyphenols on Cyclooxygenase- and Lipoxygenase-Dependent Metabolism of Arachidonic Acid in Human Colon Mucosa and Colon Tumor Tissues. Biochemical Pharmacology, 62, 1175-1183. http://dx.doi.org/10.1016/S0006-2952(01)00767-5
- August, D.A., Landau, J., Caputo, D., Hong, J., Lee, M.J. and Yang, C.S. (1999) Ingestion of Green Tea Rapidly Decreases Prostaglandin E2 Levels in Rectal Mucosa in Humans. Cancer Epidemiology, Biomarkers & Prevention, 8, 709-713.
- Paquay, J.B., Haenen, G.R., Stender, G., Wiseman, S.A., Tijburg, L.B. and Bast, A. (2000) Protection against Nitric Oxide Toxicity by Tea. Journal of Agricultural and Food Chemistry, 48, 5768-5772. http://dx.doi.org/10.1021/jf981316h
- Aneja, R., Hake, P.W., Burroughs, T.J., Denenberg, A.G., Wong, H.R. and Zingarelli, B. (2004) Epigallocatechin, a Green Tea Polyphenol, Attenuates Myocardial Ischemia Reperfusion Injury in Rats. Molecular Medicine, 10, 55-62.
- Cao, H., Kelly, M.A., Kari, F., Dawson, H.D., Urban, J.F., Coves, S., Roussel, A.M. and Anderson, R.A. (2007) Green Tea Increases Anti-Inflammatory Tristetraprolin and Decreases Pro-Inflammatory Tumor Necrosis Factor mRNA Levels in Rats. Journal of Inflammation, 4, 1-12. http://dx.doi.org/10.1186/1476-9255-4-1
- Saffari, Y. and Sadrzadeh, S.M. (2004) Green Tea Metabolite EGCG Protects Membranes against Oxidative Damage in Vitro. Life Sciences, 74, 1513-1518. http://dx.doi.org/10.1016/j.lfs.2003.08.019
- Kanadzua, M., Lua, Y. and Morimoto, K. (2006) Dual Function of (−)-epigallocatechingallate (EGCG) in Healthy Human Lymphocytes. Cancer Letters, 241, 250-255. http://dx.doi.org/10.1016/j.canlet.2005.10.021
- Muzolf, M., Szymusiak, H., Gliszczy?ska-Swigło, A., Rietjens, I.M. and Tyrakowska, B. (2008) pH-Dependent Radical Scavenging Capacity of Green Tea Catechins. Journal of Agricultural and Food Chemistry, 56, 816-823. http://dx.doi.org/10.1021/jf0712189
NOTES
*Corresponding author.





