Food and Nutrition Sciences
Vol. 4 No. 9 (2013) , Article ID: 36067 , 10 pages DOI:10.4236/fns.2013.49122
Evaluation of Sensory and in Vitro Cardio Protective Properties of Sardine (Sardina pilchardus): The Effect of Grilling and Brining*
![]()
1Laboratory of Food Chemistry, Department of Chemistry, School of Sciences, National and Kapodistrian University of Athens, Athens, Greece; 2Laboratory of Biochemistry, Department of Chemistry, School of Sciences, National and Kapodistrian University of Athens, Athens, Greece.
Email: #cnasopoulou@chem.uoa.gr, evapsa8@gmail.com, esioriki@gmail.com, demopoulos@chem.uoa.gr, izabet@chem.uoa.gr
Copyright © 2013 Constantina Nasopoulou et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 6th, 2013; revised July 6th, 2013; accepted July 14th, 2013
Keywords: Sardina pilchardus; Cardio-Protection; Sensory Evaluation; Grilling; Brining
ABSTRACT
The aim of this study was to investigate the effect of grilling and brining on the sensory properties, the fillet fatty acid composition and the cardio-protective activity of sardine (Sardina pilchardus), studying the in vitro activity against Platelet-Activating-Factor (PAF) induced platelet aggregation. Sensory evaluation of grilled and brined sardine showed that grilled sardine had higher scores for the attributes: grilled fish, marine and fresh fish whereas brined sardine had higher scores for the attributes: salty, iodine, oily and bitter. Grilled sardine exhibited significantly increased fillet fatty acid content while the brined fish sample significantly decreased fatty acid levels. Polar lipids of all specimens (raw, grilled and brined) showed strong inhibitory activity against PAF action indicating that grilling and brining have not diminished the cardio-protective properties of sardine.
1. Introduction
The benefits of a diet rich in fish on human health have been linked to ω-3 fatty acids’ content, especially eicosapentaenoic acid (EPA; 20:5ω-3) and docosahexaenoic acid (DHA; 22:6ω-3) [1]. These fatty acids are reputed to have beneficial properties against cardiovascular diseases (CVDs) [2] by lowering blood pressure and serum triglycerides and improving endothelial function and plaque stability [3]. Apart from these ω-3 fatty acids it has been reported the presence of polar lipid compounds in raw and fried fish that posses in vitro antithrombotic properties [4] and in vivo anti-atherogenic activity [5].
Atherosclerosis is a chronic inflammatory condition with clinical end points of heart attack and stroke. PlateletActivating-Factor (PAF) [6] is a potent inflammatory phospholipid mediator, implicated in the mechanism of atherogenesis [7]. The protective role, against CVDs, of compounds that act as PAF agonists/inhibitors has been reported [8,9].
Fish is rarely eaten raw but is usually cooked in different ways or brined before consumption. During cooking, chemical and physical reactions take place that improve or impair the food nutritional value (e.g. digestibility is increased due to protein denaturation in food but the content of thermolabile compounds, fat-soluble vitamins or polyunsaturated fatty acids is often reduced) [10]. Cooking induces water loss in the food, that in turn increases its lipid content in most cases and only some fat is lost in the case of the fish richer in fat content.
The aim of the current study was to investigate the effect of grilling and brining of sardine (Sardina pilchardus) on the sensory properties, the fish fillet fatty acid composition and the in vitro biological activities of fish polar lipids to induce platelet aggregation or/and inhibit the PAF-induced platelet aggregation.
2. Materials and Methods
2.1. Reagents and Instruments
All reagents and solvents were of analytical grade purchased from Merck (Darmstadt, Germany). Fatty acid methyl ester standards bought individually, were of GCquality and supplied by Sigma-Aldrich (St. Louis, MO, USA), as well as bovine serum albumin (BSA) and PAF.
Chromatographic material used for Thin Layer Chroma-tography (TLC) was silica gel G-60 supplied be Merck (Darmstadt, Germany) and polar lipid standards used for TLC was a mix standard of hen egg yolk supplied by Sigma-Aldrich (St. Louis, MO, USA). Platelet aggregation was measured in a Chrono-Log (Havertown, PA, USA) aggregometer (model 400-VS) coupled to a Chrono-Log recorder (Havertown, PA, USA).
2.2. Fish Sampling
Five kilograms (5 Kg) (one batch) of raw Greek sardines (Sardina pilchardus) were purchased from a local market and transported to the laboratory in ice. Individual weight fish was 21 ± 3.0 g. Raw fish were washed and filleted after fish head, scales, viscera, backbone, skin and tail were removed.
300 g of raw fish fillets-of the original sample of 5 kg were pooled together and that was the raw fish sample. Similarly 300 g of raw fish fillets of the original sample of 5 kg were pooled together and grilled-according to the following grilling procedure-and that was the grilled fish sample, while 300 g of raw fish fillets of the original sample of 5 kg were pooled together and immersed in brine solution according to the following brining procedure and that was the brined fish sample.
2.3. Grilling Procedure
Grilled fish fillets were prepared in a Black and Decker contact griller with its thermostat set at 217˚C. After the set temperature was attained the fish fillets were grilled for three minutes on each side.
2.4. Brining Procedure
Brine concentration was 26.5% (w/w) sodium chloride in distilled water. Brining performed at 20˚C for 3 h. This temperature is usually used in traditional salting of fish and the period of 3 h was used for salting at 20˚C to avoid fillet spoilage.
Raw fish fillets were covered by aluminum paper and were placed vertically-free lower edge surface in contact with brining solution-in order to allow unidirectional migrations of salt and moisture within the fillets. During the first two hours, the brine concentration was adjusted through renewing the brine solution each 30 min and then each hour in order to limit the external resistance to mass transfer [11].
2.5. Isolation of Fish Total Lipids, Total Polar and Total Neutral Lipids
Total lipids (TL) of raw, grilled and brined fish samples were extracted according to the Bligh-Dyer method [12] to obtain the aforementioned lipid fraction of each fish sample (raw, grilled or brined). For each extraction, a sample of 50 g of fish fillets was obtained by combining seven different fish fillets from each fish sample (raw, grilled or brined) and this sampling procedure was carried out in triplicates. One tenth of the TL was stored in sealed vials at −20˚C until Gas Chromatographic analysis. The rest was further separated into total polar lipids (TPL) and total neutral lipids (TNL) using the counter-current distribution method [13]. The TPL fractions (containing glycolipids and phospholipids) were assayed for PAF activities (i.e. for their ability to either induce washed platelet aggregation or inhibit the PAFinduced platelet aggregation).
2.6. Gas Chromatography Analysis
Fatty acid methyl esters of TL of raw, grilled and brined fish fillets were prepared using a solution 0.5N KOH in CH3OH 90% and extracted with n-hexane. The fatty acid analysis was carried out using the internal standard method as described extensively by Nasopoulou [14]. The gas chromatographer used was a Shimadzu CLASSVP (GC-17A) (Kyoto, Japan) equipped with a split/ splitless injector and flame ionisation detector.
Separation of fatty acid methyl esters was achieved on an Agilent J&W DB-23 fused silica capillary column (60 mx 0.251 mm i.d., 0.25 μm; Agilent, Santa Clara California, USA). The oven temperature program was: 120˚C for 5 min, raised to 180˚C at 10˚C∙min−1, then to 220˚C at 20˚C∙min−1 and finally isothermal at 220˚C for 30 min. The injector and detector temperatures were maintained at 220˚C and 225˚C, respectively. The carrier gas was high purity helium with a linear flow rate of 1 ml∙min−1 and split ratio 1:50. Fatty acid methyl esters were identified using fatty acid methyl esters standards by matching retention times of the relative peaks.
2.7. Fractionation of TPL by TLC
The fractionation of TPL took place by TLC as described extensively by Nasopoulou [15]. Approximately 60.0 mg of TPL of each sample were applied to the TLC plates. A developing system consisting of chloroform:methanol: water 65:35:6 (v/v/v) was utilized for the separation of TPL. The obtained TPL were weighed, redissolved in 1 ml chloroform:methanol 1:1 (v/v) and stored at −20˚C.
2.8. Biological Assay
TPL and purified polar lipid fractions of raw, grilled and brined sardine obtained by the above TLC separations were tested for their biological activity according to the washed rabbit platelet aggregation assay [6]. 0.2 ml of each TPL sample corresponding to 7.12 mg TPL of raw sardine, 61.2 mg TPL of grilled sardine and 29.5 mg TPL brined sardine were redissolved in a final volume of 200 μL BSA (2.5 mg of bovine serum albumin, BSA, mL−1 of saline). The three solutions were further diluted in a volume of BSA by a factor of 10 and 4 μL of each diluted solution used in the biological assays. The amounts of TPL of raw, grilled and brined sardine that were present in the 4 μL aliquots were 1.42 μg, 12.3 μg and 5.89 μg, respectively. In a further experiment, approximately 60.0 mg of TPL of raw, grilled and brined sardine were fractionated by TLC and each TLC polar lipid fraction obtained was dissolved in 200 μL BSA; these aliquots were biologically assayed and the quantity (μg) of the each TLC polar lipid fraction used in the biological assay is given.
The aggregatory biological activity of each lipid fraction to induce platelet aggregation was expressed as height (cm) of the aggregation curve. The inhibitory activity was expressed as % inhibition of platelet aggregation caused by PAF at a final concentration of 10−9 M.
2.9. Sensory Evaluation
An untrained panel of 10 assessors (six females, four males), aged 21 - 42 years, was recruited from the University of Athens. They were subjected to training in order to be able to identify taste, aftertaste, odour and give scores for each attribute. The tests for training and monitoring the assessors as well as for the right selection were duo-trio [16], triangle [17] and ranking tests [18]. Solutions for identification of the basic senses of tastesweet, salty, bitter and sour-were used. Concentrations of the solutions were varied in proportion to the threshold of each ingredient [19]. Five products, enclosed in dark bottles (seaweed, shells, sea salt, fish oil and tincture of iodine) were used for training the subjects to different odours. The basic training programme was carried out during 10 sessions of 45 min each. During the seven succeeded sessions the lexicon was created, using four samples of grilled and brined sardines different from the samples that were used for the purpose of the experiment. These samples were used as a frame of reference in order to develop terminology based on the attribute differences. The final list was determined so that all sensory descriptors were comprehensive and understood by all assessors. This list comprised of seventeen attributes (Table 1). During the final step of training (five sessions), panelists practiced the use of descriptors, using four samples of grilled and brined sardines different from the samples that were used for the purpose of the experiment, as explained above.
The fish fillets were grilled just before the sensory analysis for three minutes on each side in a Black and Decker contact griller as described above. Fish brining performed as described above and the excess of brine was washed off before evaluation. Portions of approximately 30 g of grilled and brined fish samples were served to each panelist on plastic plates. Grilled and brined sardine samples were sequential monadic, random allocated, coded with three-digit random numbers [19] and assessed twice over a 2 week period in different orders, balanced for order presentation in the session and for interference effect of the previous sample [20]. Water and plain biscuits were supplied to all panelists to wash
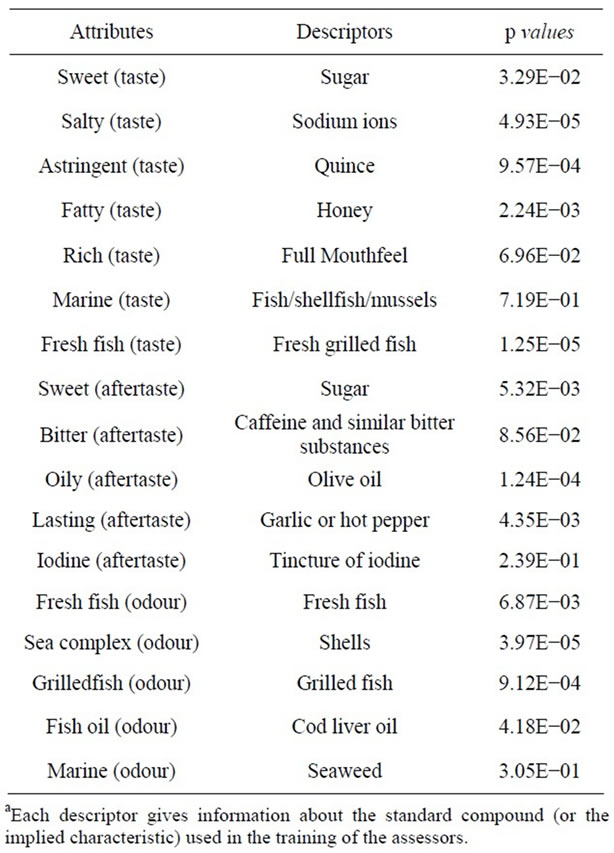
Table 1. Attributes (taste, aftertaste, odour) and their descriptorsa used during sensory analysis for testing of the grilled and brined sardine samples based on the lexicon developed by assessors; p values produced from ANOVA analysis, represent the variance of the assessors’ responses for each attribute given specific descriptors.
out their mouth during assessment of samples. The assessors marked each sample of grilled and brined fish for all the seventeen attributes on a paper based continuous scale from 0 (lowest) to 10 (highest). All attributes were scored at ambient temperature and the rooms had natural light.
2.10. Statistical Analysis
All experimental analysis was carried out in triplicates and all results were expressed as mean value ± SD. Oneway analysis of variance (ANOVA) was used in order to find the significant differences between raw and grilled samples.
Differences were considered to be statistically significant when p value was less than 0.05. The data were analysed using a statistical software package (PASW 18 for Windows, SPSS Inc., Chicago, IL, 17 USA).
3. Results and Discussion
3.1. TL, TPL and TNL Content of Raw, Grilled and Brined Sardine Samples
TL of the three fish samples (i.e. raw, grilled and brined) were extracted and separated into TNL and TPL. The obtained amounts of TL, TPL and TNL expressed as g (100 g fish tissue)‑1 are given in Table 2. Grilled and brined sardine samples exhibited statistically elevated TL levels in comparison to raw sardine TL levels (Table 2), data that is in accordance with the literature [21,22]. This is probably due to the fish tissue water loss during cooking [21] and brining [11] which leads to increased lipid content. The statistically increased TL content of grilled and brined sardine was mainly attributed to the increased TPL content.
3.2. Fatty Acid Content of Raw, Brined and Grilled Fish Fillet Samples
Raw sardine fillet showed high contents of palmitic (16:0), steatic (18:0), eicosapentaenoic (EPA; 20:5ω-3) and docosahexanoic acid (DHA; 22:6ω-3) (Table 3).
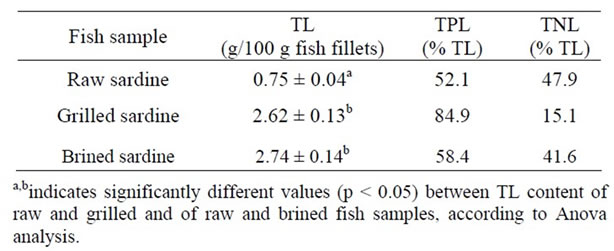
Table 2. TL expressed as g (100 g fish tissue)−1 [mean ± SD (n = 3)] and percentages of TPL and TNL content in raw, grilled and brined sardine (Sardina pilchardus) fillets.
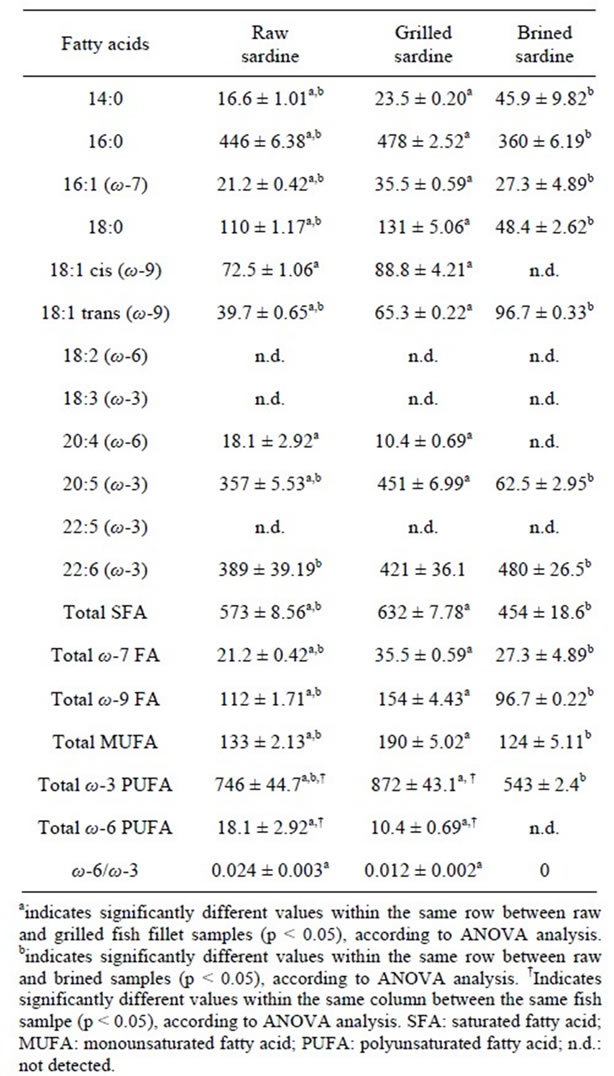
Table 3. Fatty acid profile of raw, grilled and brined sardine (Sardina pilchardus) fillets TL, expressed as mg (kg fish tissue)−1 [mean + SD (n = 3), p < 0.05].
These findings are in agreement with those obtained by other researchers [22,23]. In all sardine samples (raw, brined, grilled) polyunsaturated fatty acids (PUFAs) were the most abundant fatty acid class followed by saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) (Table 3). The ω-3 fatty acid content was significant higher than that of ω-6 fatty acids (Table 3). These results emphasize the high quality of sardine filletfat from a cardio-protective point of view [24].
Grilled fish sample exhibited significant increased PUFAs’ levels, whereas brined fish samples significant decreased (Table 3). The most abundant ω-6 fatty acid in raw and grilled specimens, was arachidonic acid (ArA; 20:4ω-6) where the most abundant ω-3 fatty acids were DHA and EPA (Table 3). Grilled sardine sample showed significant increased ω-3 fatty acids’ content, especially EPA, when compared to raw fish sample, probably as a consequence of the water loss that occurs during grilling (Table 3). A similar trend in sardine (i.e. increased levels of ω-3 fatty acids during grilling) has been reported [22]. In addition oven-baked sardines (20 min at 200˚C) rich in ω-3 PUFAs, especially EPA and DHA, has also been reported [21]. The same trend has also been observed in eel fillets, where grilled and microwave-cooked eel fillets were found to have the highest levels of EPA [25]. However, in catfish fillets, boiling, baking and grilling were found to marginally affect the fatty acid levels; some fatty acids (20:0; 22:0; 14:1ω-5 and 22:1ω-9) were not detected in raw fillets but they were found at low levels after these heating treatments [26]. Nevertheless grilling decreased the ω-6 content of the fish.
It was found that brining caused a significant decrease in the levels of some fatty acids, especially EPA and ArA, probably due to the effect of salt. Sodium chloride has been shown to catalyse lipid oxidation in muscle tissues, fish being one of them [27]. Chloride ion can be converted to a radical via a mechanism as observed with myeloperoxidase. It could then be added directly to a double bond or abstract a hydrogen [28]. Alternatively, the Na+ species may replace the iron from a cellular complex via an ion exchange reaction. The displaced iron may then participate in the initiation of lipid peroxidation.
During grilling, statistically significant increases were observed at the levels of total SFAs and total MUFAs of grilled sardine when compared to raw sardine (Table 3). On the contrary, brining led to a statistically significant decrease of the levels of SFAs and MUFAs, probably due to the effect of salt. Also grilling and brining affected statistically significant the ratio of ω-6/ω-3. The highest ratio was observed in raw sardine (0.024), followed by grilled sardine (0.012) and brined sardine (0) (Table 3). This could be explained as a result of the statistically significant decrease during grilling and brining of the levels of ArA.
3.3. Biological Activity of Sardine’s TPL and TLC Polar Lipid Fractions
Some food lipid constituents have been found to exhibit either agonistic (induce platelet aggregation) or inhibitory effect on PAF-induced biological activity. When they act as agonists of PAF they act as PAF (induce platelet aggregation) but they are thousands times less active than PAF thus they are practically acting as PAFinhibitors, whereas when they exhibit PAF-inhibitory effect they block PAF activity, acting as PAF-inhibitors, as well. PAF agonists (with aggregatory activity) have been found to have better in vivo antiatherogenic activity than PAF-inhibitors [5,29]. Therefore, the detection of lipids (TPL or TLC polar lipid fractions) that exhibit aggregatory and/or inhibitory actions is a strong indication that these lipids are biologically active compounds against PAF and consequently against atherogenesis.
With these in mind, a comparison of the biological activities of TPL and TLC polar lipid fractions of all fish samples (raw, grilled and brined) follows. The biological activities of TPL of all fish samples against washed rabbit platelets aggregation were studied for whether they induced platelet aggregation (PAF agonists) or inhibited the PAF-induced platelet aggregation (PAF inhibitors).
According to Table 4, all three TPL samples (1.42 μg of raw, 12.3 μg of grilled and 5.89 μg of brined TPL, respectively) have shown 95% - 100% inhibition of the biological activity against PAF. Thus, it could be suggested that compounds with strong inhibitory activity against PAF were present in TPL of raw, grilled and brined sardine but grilled and brined fish TPL were about 8 and 4 times, respectively, less biologically active, against PAF, than raw TPL.
The fact that the TPL of all three samples of sardines (raw, grilled and brined) have shown strong PAFinhibitory activities has prompted us to further fractionate the TPL of all three fish samples and study their biological properties. Therefore, approximately 60.0 mg TPL of raw, grilled and brined sardine fillets were further separated by preparative TLC (Figure 1) and the lipid
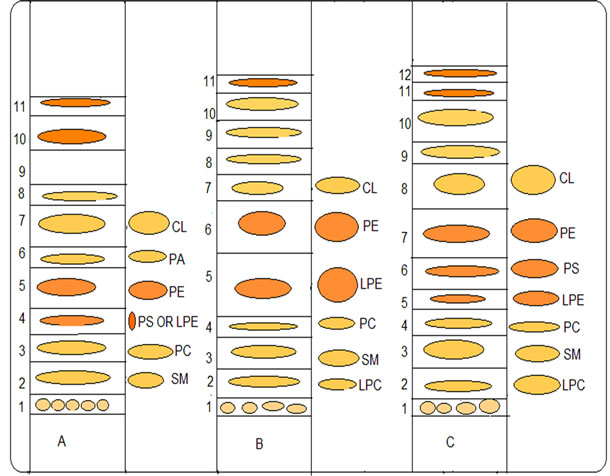
Figure 1. Typical profile of TPL separation of the fish samples on preparative TLC. A: raw sardine B: grilled sardine C: brined sardine, L-PC: lyso-phosphatidylcholine, SM: sphingomyelin, PC: phosphatidylcholine, PI: phosphatidylinositol, PS: phosphatidylserine, L-PE: lyso-phosphatidylethanolamine, PE: phosphatidylethanolamine, PA: phosphatidic acid, CL: cardiolipin. The developing system used for the separation of total polar lipids was chloroform:methanol:water 65:35:6 (v/v/v).
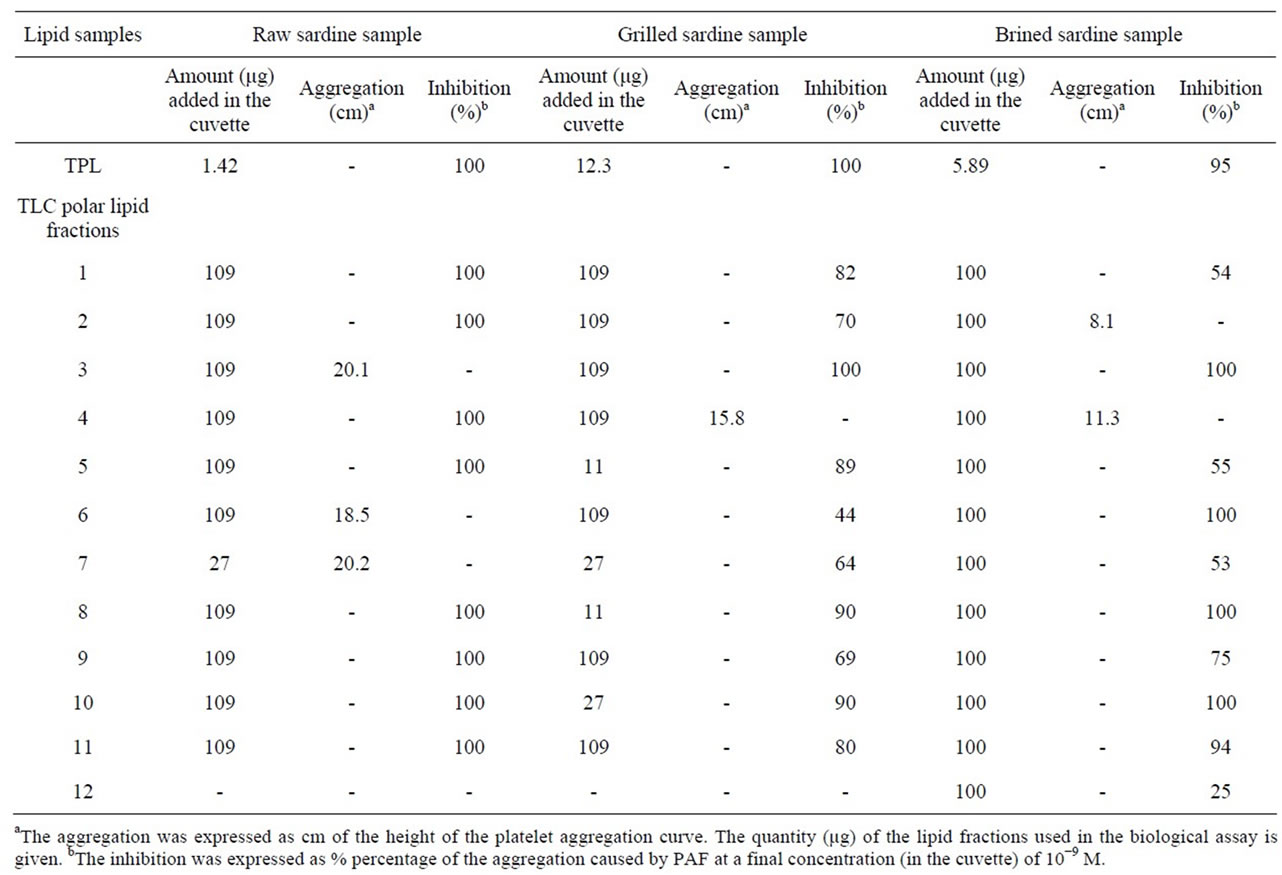
Table 4. In vitro biological activities, against platelet aggregation, of TPL and TLC polar lipid fractions of raw, grilled and brined sardine (Sardina pilchardus).
fractions obtained were tested for their ability to induce washed rabbit platelet aggregation or inhibit the PAF induced platelet aggregation. The quantity (in μL of raw TPL) was chosen in order to obtain 100% inhibition of PAF and the same quantity of grilled and brined TPL was assayed, in order to compare the biological activities of the individual TLC fractions of the three different fish samples.
The aggregation and inhibition values of each TLC polar lipid fraction from all samples are shown in Table 4. In order to keep Table 4 as simple as possible, it should be noticed that the numbers of the TLC fractions do not correspond to the same Retardation factor (Rf). Thus, the TLC lipid fractions need to be compared in both Figure 1 and Table 4.
The majority of TLC polar lipid fractions of raw, grilled and brined sardine fillets exhibited inhibitory activity (Table 4). As mentioned above, the TLC polar lipid fractions of raw sardine exhibited the maximum inhibitory activity and that was compared to the corresponding biological activities that equal amounts of TLC polar lipid fractions of grilled and brined exhibit. The observed inhibition of raw sardine was attributed to almost all TLC polar lipid fractions (1, 2, 4, 5, 8, 9, 10 and 11) apart from lipid fractions 3, 6 and 7 that exhibited aggregatory activities (Figure 1 and Table 4). TLC polar lipid fractions of grilled and brined sardine also exhibited inhibitory activities that could be attributed to almost all TLC polar lipid fractions. The only exceptions were observed for lipid fraction 4 of grilled samples and fractions 2 and 4 of brined samples that exhibited aggregatory activity. TLC polar lipid fraction 3 of raw sardine and lipid fraction 4 of grilled and brined sardine have similar Rf values to that of phosphatidylcholine (PC) (Figure 1). PC does not have aggregating properties but oxidized-PC, which has a similar Rf value to that of PC, may have PAF-like activity [30].
In detail, raw sardine TLC lipid fractions 3, 6, and 7 exhibited aggregatory properties while in grilled and brined sardine only the analogous TLC fractions 4 exhibited aggregatory properties. The analogous TLC fraction of 7 of raw sardine lipids, in grilled and brined sardine exhibited inhibitory properties (Figure 1 and Table 4).
Grilled sardine exhibited either similar or reduced inhibitory activity in comparison to raw sardine. TLC lipid fractions 1, 5, 8, 9, 10 and 11 showed reduced inhibitory activity in comparison to the analogues TLC polar lipid fractions of raw sardine (Figure 1 and Table 4).
Similarly, brined sardine exhibited either similar or reduced inhibitory activity in comparison to raw sardine. Lipid fractions 1, 5, 7, 9 and 12 of brined sardine were found to have reduced inhibitory activity whereas lipid fractions 3, 6, 10 and 11 showed similar inhibitory activity in comparison to analogues TLC polar lipid fractions of raw sardine (Figure 1 and Table 4).
When comparing grilled and brined sardine, their nutritional value (with emphasis against atherogenesis) seems to be similar since lipid fractions 1 and 5 of brined fish, exhibited reduced inhibitory activity in comparison to grilled sardine whereas lipid fractions 8, 10 and 11 of brined fish exhibited increased inhibitory activity in comparison to grilled sardine (Figure 1 and Table 4).
The existence of PAF antagonists in various foodstuffs is of major importance for their nutritional value, considering the importance of platelet activation and thrombosis in cardiovascular diseases. Moreover, protective intervention studies against atherogenesis have shown that only specific PAF inhibitors [31], fish polar lipids [5], olive oil polar lipids [32] and statins [33] are able to reduce atherogenesis in vivo.
3.4. Sensory Analysis
The scope of this analysis was to detect possible differences between samples that, a priori, are of good quality. The panelists scored the grilled and brined sardine samples using values between zero (0) and ten (10) (the lowest “0”, the highest “10”) for attributes describing taste, aftertaste and odour characteristics and the obtained scores are outlined in Τable 5.
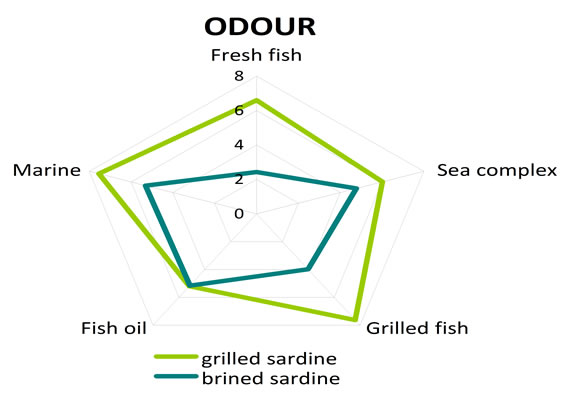
The assessors indicated statistically significant organoleptic differences among grilled and brined sardines. More specific brined sardine exhibited more salty taste (p = 0.001) (Table 5) in comparison to grilled sardine, as it was expected, while grilled sardine was evaluated as more sweet (p = 0.017) and more fresh (p = 0.001) (Table 5) when compared to brined sardine. The sensation of aftertaste of brined sardine was statistically more intensive for the attributes bitter (p = 0.001) and lasting (p = 0.002) (Table 5) in comparison to grilled sardine. On the other hand grilled sardine was found to be more sweet aftertaste (p = 0.023) (Table 5) than brined one. Finally the attributes of odour fresh fish and grilled fish had significant higher scores for grilled sardine when compared to the ones for brined sardine (Table 5). The sensory profiles of grilled and brined sardine in terms of taste, aftertaste and odour are given in Figure 2, where the scores of each fish (either grilled or brined) for the different attributes used are given in the form of spiderweb plots.
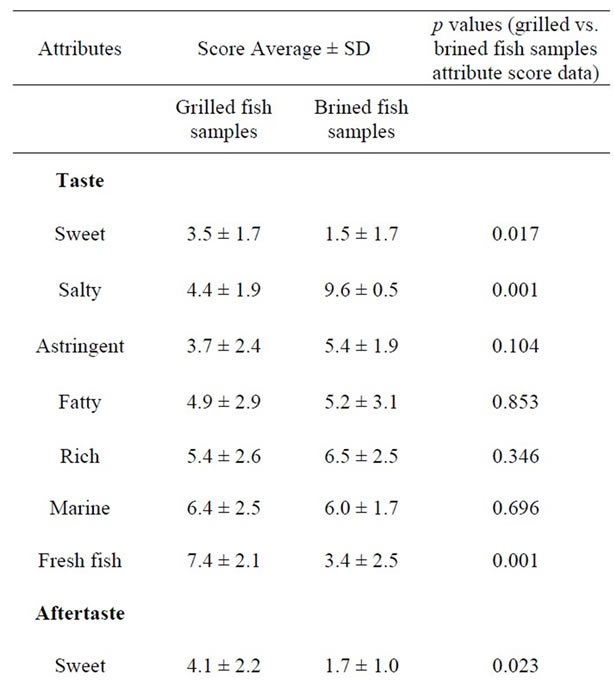

Table 5. Attribute score data of grilled and brined sardines after fish samples evaluation by ten trained assessors. The values are average scores of three replicates with statistical deviation of 99% confidence level; p values of each attribute produced from ANOVA analysis.
4. Conclusion
The obtained results underline the nutritional value of sardine against atherogenesis suggesting that its cardio-protective properties are not diminished during grilling and brining; a positive result given the fact that sardine is mainly consumed as grilled or brined. Therefore, the commercial choice between grilled and
 (a)
(a)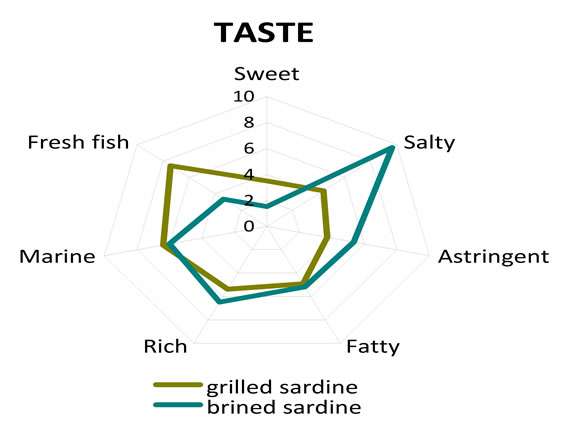 (b)
(b)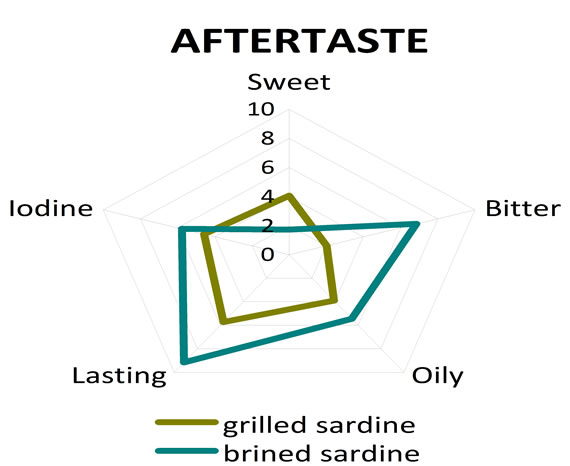 (c)
(c)
Figure 2. Comparison of sensory profiles of grilled and brined sardine. (a) Odour attributes scores; (b) Taste attributes scores; (c) Aftertaste attributes scores.
brined sardine should not only be based on terms of nutritional value as both processed fish have high nutritional value but it should also be based on which taste and odour attributes the consumers prefer.
REFERENCES
- F. Médale, F. Lefèvre and G. Corraze, “Qualité Nutritionnelle et Diététique des Poissons: Constituants de la Chair et Facteurs de Variations,” Cahiers de Nutrition et de Diététique, Vol. 38, No. 1, 2003, pp. 37-44.
- K. Gebauer, T. L. Psota, W. S. Harris and P. M. KrisEtherton, “ω-3 Fatty Acid Dietary Recommendations and Food Sources to Achieve Essentiality and Cardiovascular Benefits,” American Journal of Clinical Nutrition, Vol. 83, No. Suppl 6, 2006, pp. 1526-1535.
- D. Bhatnagar and P. N. Durrington, “Omega-3 Fatty Acids: Their Role in the Prevention and Treatment of Atherosclerosis Related Risk Factors and Complications,” International Journal of Clinical Practice, Vol. 57, No. 4, 2003, pp. 305-314.
- T. Nomikos, H. C. Karantonis, C. Skarvelis, C. A. Demopoulos and I. Zabetakis, “Antiatherogenic Properties of Lipid Fractions Obtained from Raw and Fried Fish Samples,” Food Chemistry, Vol. 96, No. 1, 2006, pp. 29-35. doi:10.1016/j.foodchem.2005.01.060
- C. Nasopoulou, H. C. Karantonis, D. N. Perrea, S. E. Theocharis, D. G. Iliopoulos, C. A. Demopoulos and I. Zabetakis, “In Vivo Anti-Atherogenic Properties of Cultured Gilthead Sea Bream (Sparus aurata) Polar Lipid Extracts in Hypercholesterolaemic Rabbits,” Food Chemistry, Vol. 120, No. 3, 2010, pp. 831-836. doi:10.1016/j.foodchem.2009.11.023
- C. A. Demopoulos, R. N. Pinckard and D. J. Hanahan, “Platelet Activating Factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphoryl-choline as the Active Component (a New Class of Lipid Chemicalmediators),” Journal of Biological Chemistry, Vol. 254, No. 19, 1979, pp. 9355-9358.
- C. A. Demopoulos, H. C. Karantonis and S. Antonopoulou, “Platelet Activating Factor—A Molecular Link between Atherosclerosis Theories,” European Journal of Lipid Science and Technology, Vol. 105, No. 11, 2003, pp. 705-716. doi:10.1002/ejlt.200300845
- G. Subbanagounder, N. Leitinger, P. T. Shih, K. F. Faull and J. A. Berliner, “Evidence That Phospholipid Oxidation Products and/or Platelet-Activating Factor Play an Important Role in Early Atherogenesis: in Vitro and in Vivo Inhibition by WEB 2086,” Circulation Research, Vol. 85, No. 4, 1999, pp. 311-318. doi:10.1161/01.RES.85.4.311
- S. Antonopoulou, T. Nomikos, H. C. Karantonis, E. Fragopoulou and C. Demopoulos, “PAF, a Potent Lipid Mediator,” In: A. D. Tselepis, Ed., Bioactive Phospholipids. Role in Inflammation and Atherosclerosis, Transworld Research Network, Kerala, 2008, pp. 85-134.
- A. Bognar, “Comparative Study of Frying to Other Cooking Techniques. Influence on the Nutritive Value,” Grasas Aceites, Vol. 49, No. 3-4, 1998, pp. 250-260. doi:10.3989/gya.1998.v49.i3-4.746
- S. Bellagha, A. Sahli, A. Farhat, N. Kechaou and A. Glenza, “Studies on Salting and Drying of Sardine (Sardinella aurita): Experimental Kinetics and Modeling,” Journal of Food Engineering, Vol. 78, No. 3, 2007, pp. 947-952. doi:10.1016/j.jfoodeng.2005.12.008
- E. G. Bligh and W. J. Dyer, “A Rapid Method of Total Lipid Extraction and Purification,” Canadian Journal of Biochemistry and Physiology, Vol. 37, No. 8, 1959, pp. 911-917. doi:10.1139/o59-099
- D. S. Galanos and V. M. Kapoulas, “Isolation of Polar Lipids from Triglyceride Mixtures,” Journal Lipid Research, Vol. 3, 1962, pp. 134-137.
- C. Nasopoulou, G. Stamatakis, C. A. Demopoulos and I. Zabetakis, “Effects of Olive Pomace and Olive Pomace Oil on Growth Performance, Fatty Acid Composition and Cardio Protective Properties of Gilthead Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax),” Food Chemistry, Vol. 129, No. 3, 2011, pp. 1108-1113. doi:10.1016/j.foodchem.2011.05.086
- C. Nasopoulou, T. Nomikos, C. A. Demopoulos and I. Zabetakis, “Comparison of Antiatherogenic Properties of Lipids Obtained from Wild and Cultured Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata),” Food Chemistry, Vol. 100, No. 2, 2007, pp. 560-567. doi:10.1016/j.foodchem.2005.09.074
- SO 10399, Sensory Analysis—Methodology—Duo-Trio Test, 2004.
- ISO 4120, Sensory Analysis—Methodology—Triangle Test, 2004.
- ISO 8587, Sensory Analysis—Methodology—Ranking, 2006.
- C. M. Meilgaard, G. V. Civille and B. T. Carr, “Sensory Evaluation Techniques,” 3rd Edition, CRC Press, London, 1999.
- M. J. Macfie, N. Bratchell, K. Greenhoff and L. V. Vallis, “Designs to Balance the Effect of Order of Presentation and First-Order Carry-Over Effects in Hall Tests,” Journal of Sensory Studies, Vol. 4, No. 2, 1989, pp. 129-148. doi:10.1111/j.1745-459X.1989.tb00463.x
- A. Zotos, A. Kotaras and E. Mikras, “Effect of Baking of Sardine (Sardina pilchardus) and Frying of Anchovy (Engraulis encrasicholus) in Olive Oil and Sunflower Oil on Their Quality,” Food Science and Technology International, Vol. 19, No. 1, 2013, pp. 11-23. doi:10.1177/1082013212442179
- M. T. Garcia-Arias, E. Alvarez Pontes, M. C. GarciaLinares, M. C. Garcia-Fernande and F. J. Sanchez-Muniz, “Cooking-Freezing-Reheating (CFR) of Sardine (Sardina pilchardus) Fillets. Effect of Different Cooking and Reheating Procedures on the Proximate and Fatty Acid Compositions,” Food Chemistry, Vol. 83, No. 3, 2003, pp. 349-356. doi:10.1016/S0308-8146(03)00095-5
- K. Bouderoua, J. Mourot, F. Benmehdi-Tabet-Aoull and G. Selselet-Attou, “The Effects of Season and Site of Catch on Morphometric Characteristics, Mineral Content, and Fatty Acids of Sardines (Sardina pilchardus) Caught on the Algerian Coast,” Journal of Aquatic Food Product Technology, Vol. 20, No. 4, 2011, pp. 412-420. doi:10.1080/10498850.2011.577272
- J. E. Kinsella, B. Lokesh and R. A. Stone, “Dietary ω-3 Polyunsaturated Fatty Acids and Amelioration of Cardiovascular Disease: Possible Mechanisms,” American Journal of Clinical Nutrition, Vol. 52, No. 1, 1990, pp. 1- 28.
- B. Ersoy, “Effects of Cooking Methods on the Proximate, Mineral and Fatty Acid Composition of European Eel (Anguilla anguilla),” International Journal of Food Science and Technology, Vol. 46, No. 3, 2011, pp. 522-527. doi:10.1111/j.1365-2621.2010.02546.x
- Y. Moradi, J. Bakar, A. A. Motalebi, S. H. Syed Muhamad and Y. Che Man, “A Review on Fish Lipid: Composition and Changes during Cooking Methods,” Journal of Aquatic Food Product Technology, Vol. 20, No. 4, 2012, pp. 379-390. doi:10.1080/10498850.2011.576449
- D. D. Nambudiry, “Lipid Oxidation in Fatty Fish: The Effect of Salt Content in Meat,” Journal of Food Science and Technology, Vol. 17, 1980, pp. 176-178.
- J. Kanner and J. E. Kinsella, “Lipid Deterioration Initiated by Phagocytic Cells in Muscle Foods: Caroene Destruction by a Myeloperoxidase-Hydrogenperoxidehalide System,” Journal of Agricultural and Food Chemistry, Vol. 31, No. 2, 1983, pp. 370-376. doi:10.1021/jf00116a047
- N. Tsantila, H. C. Karantonis, D. N. Perrea, S. E. Theocharis, D. G. Iliopoulos, S. Antonopoulou and C. A. Demopoulos, “Antithrombotic and Antiatherosclerotic Properties of Olive Oil and Pomace Polar Extracts in Rabbits,” Mediators of Inflammation, Vol. 2007, No. 1, 2007, pp. 1-11. doi:10.1155/2007/36204
- A. Tokumura, T. Sumida, M. Toujima, K. Kogure and K. Fukuzawa, “Platelet-Activating Factor (PAF)-Like Oxidized Phospholipids: Relevance to Atherosclerosis,” Biofactors, Vol. 13, No. 1-4, 2000, pp. 29-33. doi:10.1002/biof.5520130106
- R. Feliste, B. Perret, P. Braqueta and H. Chap, “Protective Effect of BN 52021, a Specific Antagonist of Platelet-Activating Factor (PAF Acether) against Diet-Induced Cholesteryl Ester Deposition in Rabbit Arta,” Atherosclerosis, Vol. 78, No. 2-3, 1989, pp. 151-158. doi:10.1016/0021-9150(89)90219-0
- H. C. Karantonis, S. Antonopoulou, D. N. Perrea, D. P. Sokolis, S. E. Theocharis, N. Kavantzas, D. G. Iliopoulos and C. A. Demopoulos, “In Vivo Antiatherogenic Properties of Olive Oil and Its Constituent Lipid Classes in Hyperlipidemic Rabbits,” Nutrition, Metabolism and Cardiovascular Diseases, Vol. 16, No. 3, 2006, pp. 174-185. doi:10.1016/j.numecd.2005.07.003
- A. S. Wierzbicki, R. Poston and A. Ferro, “The Lipid and Non-Lipid Effects of Statins,” Pharmacology and Therapeutics, Vol. 99, No. 1, 2003, pp. 95-112. doi:10.1016/S0163-7258(03)00055-X
Abbreviations
ArA, arachidonic acid;
BSA, bovine serum albumin;
CL, cardiolipin;
CVDs, cardiovascular diseases;
DHA, docosahexaenoic acid;
EPA, eicosapentaenoic acid;
L-PC, lyso-phosphatidylcholine;
L-PE, lyso-phosphatidyle-thanolamine;
MUFAs, monounsaturated fatty acids;
PAF, Platelet-Activating-Factor;
PA, phosphatidic acid;
PC, phosphatidylcholine;
PE, phosphatidylethanolamine;
PI, phosphatidylinositol;
PS, phosphatidylserine;
PUFAs, polyunsaturated fatty acids;
SFAs, saturated fatty acids;
SM, sphingomyelin;
TLC, thin layer chromatography;
TL, total lipids;
TNL, total neutral lipids;
NOTES
*Conflict of interests: All authors declare that no possible conflicts of interest exist in our submitted manuscript.
#Corresponding author.

