American Journal of Plant Sciences
Vol.5 No.11(2014), Article ID:45999,12 pages DOI:10.4236/ajps.2014.511172
Assessing the Potential Impacts of Elevated Temperature and CO2 on Growth and Health of Nine Non-Vascular Epiphytes: A Manipulation Experiment
Liang Song1*, Wenyao Liu1*, Yongjiang Zhang2, Zhenghong Tan1, Su Li1, Jinhua Qi3, Yuanlin Yao1,4
1Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Kunming, China
2Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, USA
3National Forest Ecosystem Research Station at Ailao Mountains, Jingdong, China
4Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
Email: *liuwy@xtbg.ac.cn, *songliang@xtbg.ac.cn
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 20 March 2014; revised 19 April 2014; accepted 30 April 2014
ABSTRACT
The consequences of sharp rise in atmospheric carbon dioxide concentration ([CO2]) and global warming on vascular plants have raised great concerns, but researches focusing on non-vascular epiphytes remain sparse. We transplanted nine common cryptogamic epiphyte species (3 bryophytes, 6 lichens) from field sites to growth chambers (control, elevated [CO2], elevated temperature, elevated [CO2] and temperature) and monitored their growth and health at regular intervals in a subtropical montane forest in Ailao Mountains in southwestern China. Our results implied a dim future for nonvascular epiphytes, especially lichens, in a warming world. The initial rise in temperature and decrease in water availability from field sites to the control chamber had remarkable negative impacts on growth and health of nonvascular epiphytes, many of which turned brown or died back. Although elevated [CO2] in chambers had no significant effects on growth of any of the experimental species, further warming caused significant negative impacts on growth of Lobaria retigera (Bory) Trev. In addition, elevated [CO2] and temperature have a significant interaction on growth of four experimental lichens. Considering the ecological importance of epiphytic bryophytes and lichens for the subtropical montane forest ecosystems and high sensitivity to environmental changes, people may underestimate global change impacts to nonvascular epiphytes, or even the whole forest ecosystems.
Keywords:Bryophyte, Climate Change, Lichen, Global Warming, Transplantation

1. Introduction
Over the last 650 thousand years, atmospheric carbon dioxide concentration ([CO2]) varied between 180 and 290 ppm around a mean of 240 ppm [1] . Starting with the industrial revolution, [CO2] has increased significantly due to human activities such as fossil fuel combustion and forest destruction, and reached ca. 380 ppm recently [2] . This increase in concentrations of greenhouse gases, especially CO2, has been suggested to cause global warming [3] . The predicted increase in atmospheric [CO2] reaching 600 to 850 ppm by the end of this century will increase atmospheric temperature by 1.8˚C to 4.0˚C [3] .
The consequences of sharp rise in [CO2] and global warming on plants and forests have raised great concerns because vegetation represents the major potential carbon sinks to alleviate [CO2] emission [4] . Because CO2 is the substrate for photosynthesis and temperature will directly influence plant physiological processes, [CO2] and temperature will no doubt have direct and substantial impacts on plants [5] . Over the last decades, a large body of literature on actual and predicted impacts of elevated [CO2] and temperature on the physiology and productivity of plants has accumulated [4] -[6] .
In the tropics and some subtropics, epiphytes are a diverse plant group and are thought to be particularly vulnerable to changes in climate and atmospheric deposition due to their tight coupling to atmospheric inputs [7] . Although epiphytes represent almost 10% of the global flora (up to 50% in some tropical montane cloud forests) [7] [8] , and the interest in elevated [CO2] and climate change effects on canopy communities is strongly increasing [9] -[11] , knowledge of the potential effects of global climate change on epiphytes remains scarce. Among the few studies that assessed the potential impacts of elevated [CO2] and global warming on epiphytes, most evidence has been derived from vascular epiphytes [12] [13] . Non-vascular epiphytes are poikilohydric and lack roots and an outer waxy cuticle; they absorbed all the required water and nutrients directly from air and precipitation through the entire plant surface [14] . Thus, non-vascular epiphytes are probably more sensitive to changes in atmospheric environment than vascular epiphytes [15] [16] . However, empirical evidences concerned about impacts of elevated [CO2] and global warming on non-vascular epiphytes remain scarce.
Montane moist evergreen broad-leaved forest (MMEBF) is a subgroup of evergreen broad-leaved forest, and occurs mainly in tropical and subtropical mountains at high altitude in Yunnan Province, south-western China [17] . Ailao Mountain MMEBF is included in the Indo-Burma biodiversity hotspot and is one of the most diverse regions of China [18] . Due to the high rainfall, high relative humidity (RH), the presence of large trees, and absence of widespread human disturbance, the MMEBF located in the Ailao Mountains is especially rich in epiphytes [19] [20] . In this study, we transplanted 9 common cryptogamic epiphyte species (3 bryophytes, 6 lichens) from field sites to growth chambers and monitored their growth and health at regular intervals in a subtropical MMEBF in the Ailao Mountains. The main objective of this study is to assess the potential impacts of predicted elevated [CO2] and temperature on the growth, and health of 9 common non-vascular epiphytes in the MMEBF.
2. Materials and Methods
2.1. Study Site
We conducted this study in four growth chambers in the Xujiaba region of south-western China (24˚32'N, 101˚01'E). The chambers locate in Ailao Mountains National Nature Reserve, which is surrounded by primary montane moist evergreen broad-leaved forests (MMEBFs) (23˚35' - 24˚44'N, 100˚54' - 101˚01'E). The MMEBF is primarily co-dominated by Lithocarpus hancei (Benth.) Rehder, Castanopsis rufescens (Hook.f.et Th.) Huang et Y.T. Chang, and L. xylocarpus (Kurz) Markgr. [21] . This forest has been classified to be an old-growth forest according to the presence of large, old trees, and absent of widespread human disturbance, which harbors plenty of epiphytic flora [21] . Especially, nonvascular epiphytes in the subtropical MMEBFs are abundant, including 176 species of epiphytic bryophytes [22] and 217 species of epiphytic lichens [23] .
The study site is affected by both the south sub-current of the west current from India and Pakistan and the southwestern monsoon, so it experiences a striking alternation of dry and wet conditions [24] . The following averages were recorded from the weather station in Xujiaba region between 2000 and 2010: average annual temperature, 11.1˚C (mean = 5.6˚C in January; 15.3˚C in July); mean annual precipitation, 1874 mm, with 87% of the rain in the rainy season (May to October); mean annual RH, 84% [11] . The annual temperature is predicted to increase by 2.2˚C by the 2050s, compared with the current condition in the MMEBF [11] .
2.2. Experimental Design and Measurement
Six epiphytic macrolichens (Sticta nylanderiana A. Zahlbr., Lobaria retigera (Bory) Trev., Lobaria isidiophora Yoshim., Nephromopsis pallescens (Schaer.) Y.S. Park, Usnea florida (L.) Wigg., Sulcaria sulcata (Levl.) Bystr.ex Brodo er Hawksw.)and three epiphytic bryophytes (Sinskea phaea (Mitt.) Buck, Calyptothecium hookeri (Mitt.) Broth., Homaliodendron flabellatum (Sm.) Fleisch.) were selected as the target species for translocation, as they are widespread in Asia (some in the worldwide) [25] and easy to sample in the study region. As in moist montane forests, bryophytes usually dominated the lower canopy, while lichens dominated the upper or outer canopy [26] , we sampled bryophyte materials from tree trunks at 1 - 2 m height and lichen materials from middle and upper canopies at 20 - 25 m height using single rope technique [27] . We used pendant transplants of thalli fragments of lichens and shoots of bryophytes to assess the biomass increment in different environmental conditions following McCune et al. [28] . In early April 2010, lichen thalli and bryophyte shoots were collected from the MMEBF. The lichen and bryophyte fragments were air-dried for 24 hours at room temperature and weighed. Pieces (0.1 - 0.2 g) were attached to two to three cm nylon monofilament loop using a silicone sealant (Figure 1(b), Figure 1(c)). After a further 24 hours of air-drying, all thalli were reweighed, including the weight of the device. For each species, 10 similar-sized samples were transplanted to the sample sites (upper canopy (ca. 24 m) for epiphytic lichens and lower canopy (ca. 1.5 m) for bryophytes) as field control, while 40 samples were transplanted randomly to 4 closed-top chambers (E-sheng Tech. Co., Beijing, China) (10 samples per chamber) in April 2010 (Figure 1(a)). Considering the logistics and costs of controlling [CO2] and temperature are great, a split-plot design was used in this study, with treatment as the between-plot effect and plant species as the within-plot effect followed by Stiling et al. [29] and Lei et al. [30] when controlling [CO2] or temperature.
The close-top chambers are located in Ailaoshan Station for Subtropical Forest Ecosystem Studies (24˚32'N, 101˚01'E, elevation 2450 m), Jingdong County, Yunnan Province, SW China. There were four types of treatments in chambers: control; elevated [CO2] (580 - 780 ppm); elevated temperature (+2˚C - 3˚C compared with the control); and elevated both [CO2] and temperature (580 - 780 ppm, +2˚C - 3˚C). [CO2] and temperature in each chamber were recorded at a 15 s interval and adjusted to the above ranges (data not shown). A computer-controlled CO2 supply system (LT/ACR-ePLC, E-Sheng Tech. Co., Beijing, China) was used to control [CO2]. The detailed structure and material of the chambers can be found in Lei et al. [30] . The chambers were covered with black shade nets to avoid direct sunshine (Figure 1(a)). Ten transplants of each studied species were included in each chamber. Each chamber was divided into two sections and each section contained five transplants of each species to decrease the potential influence of possible environmental heterogeneity within each chamber. Transplants were randomly arranged in each section. To provide similar nutrient levels with the field sites, we watered the transplants with the same amount of rainfall every rain events using an aerosol sprayer in all the chambers.
After that, samples were removed, transported to the lab and air-dried for 24 hours before re-measuring, and then been put back every three months until January, 2011. Because the weights of air-dried lichens and bryophytes may be affected by humidity and temperature, water content of thalli and shoots was adjusted using the reference sample method [28] .
We ranked the health of each lichen thallus or bryophyte shoot: 0 = fully brown, dead looking; 1 = with many brown patches (>50% brown); 2 = some brown or dieback (10% - 50% brown); 3 = healthy (<10% brown). Relative measures of growth of all the species were calculated following Song et al. [11] .
To document air temperature, water and light availability, we measured the air temperature, air RH, and photosynthetic active radiation (PAR) by placing an automatic weather station (Hobo U30, Onset Computer Corporation) at ca. 1.5 m height beneath the canopy (field control site for bryophytes) and one at ca. 24 m height upon the canopy (field control site for lichens). Microclimate measurements were recorded every 30 minutes. The mean vapor pressure deficits (VPD) were calculated according to the Goff-Gratch formulae [31] .

Figure 1. Study methods and materials. Detailed legend: Photos of study methods and materials. (a) Closed-top chambers; (b) Pendent transplants of epiphytic lichens; (c) Pendent transplants of epiphytic bryophytes.
2.3. Statistical Analysis
Effects of plant species, treatment, and their interactions on growth were tested using a split-plot ANOVA according to Lei et al. [30] . Then, differences in growth among different treatments and observation periods were analyzed using repeated measures General Linear Model (GLM) for each species [32] . Differences in growth among different treatments within each observation period were analyzed with two or three-way ANOVA. All the above analysis was divided into the following two steps: 1) treatment of translocation from field site to the control chamber, and 2) treatment of elevated [CO2] and temperature. The categorical health data were analyzed with non-parametric methods (Kruskal-Wallis H). All analyses were conducted using the SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Microclimate Comparisons among Field Sites and the Control Chamber
Mean air temperature in the control chamber was 21.6˚C, which was remarkably higher than temperature at the field site at 24 m in the upper canopy (16.1˚C) and 1.5 m beneath the canopy (15.6˚C). The mean air RH in the control chamber was 90.2%, while 96.2% and 95.7% at the field site at 24 m and 1.5 m height, respectively. The VPD were 3.17, 0.85, and 0.79 kPa at the above three locations, respectively. The mean PAR showed a similar trend with air temperature, with mean values of 143.4 μmol·m−2·s−1, 119.0 μmol·m−2·s−1, 3.8 μmol·m−2·s−1, respectively. Additional data of microclimate are given in electronic supplemental material_1 (ESM_1).
3.2. Effects of Plant Species, Treatment, and Their Interactions
The split-plot ANOVA results showed that, for the first step, both plant species (F8,125 = 56.395, P < 0.001) and treatment of translocation from field sites to the control chamber (F1,125 = 24.304, P < 0.001) had a significant negative impact on the growth of the experimental materials. For the second step, although treatment effects of elevated [CO2] (F1,245 = 0.472, P = 0.493) and temperature (F1,245 = 0.003, P = 0.953) were not significant, their interaction (F1,245 = 4.124, P = 0.043) was significant. In addition, plant species (F8,245 = 159.480, P < 0.001) also had a significant impact on the growth of the experimental materials in chambers.
3.3. Response of Growth and Health of Epiphytic Lichens after Transplants
Translocation from field sites to the control chamber had a significant negative impact on the growth in L. retigera, U. florida, and N. pallescens, while marginally significant for S. nylanderiana (P = 0.051), many of which turned brown or died back (Table 1, Figure 2, ESM_2). No significant effect of elevated [CO2] had been detected for any of the experimental species, while significant negative effect of elevated temperature had been observed in L. retigera (Table 1). Further, significant interaction between elevated [CO2] and temperature had been detected in S. nylanderiana, L. isidiophora, U. florida, and S. sulcata (Table 1).
During the 9 months experimental period, samples of S. nylanderiana, L. retigera, U. florida, L. isidiophora, N. pallescens grew best at the field site, while transplants in all the four chambers showed limitation on growth after transplantation in different extent except S. sulcata (Figures 2(a)-(f), ESM_3(a)-(f)). Especially, samples of L. retigera were losing their biomass after been transplanted into the chambers (Figure 2(b)). We detected no significant differences at different treatments (field site, the control chamber, elevated [CO2], elevated temperature, elevated [CO2] and temperature) among the initial biomass of the samples of S. nylanderiana (F4,35 = 2.124, P = 0.099), L. retigera (F4,35 = 1.342, P = 0.274), L. isidiophora (F4,32 = 0.389, P = 0.815), N. pallescens (F4,34 = 0.270, P = 0.895) (Figures 2(a)-(c), Figure 2(e)). Three months after transplantation, there were significant differences in biomass of S. nylanderiana (F4,35 = 4.595, P = 0.004) and L. retigera (F4,35 = 15.381, P < 0.001) among treatments (Figure 2(a), Figure 2(c)). Another three months after this, we detected significant differences in biomass of L. isidiophora (F4,32 = 3.985, P = 0.010) and N. pallescens (F4,34 = 4.587, P = 0.005) among different treatments (Figure 2(b), Figure 2(e)). We detected significant differences among different treatments
Table 1. Results of repeated measure GLM for growth of different epiphytes among different treatments.
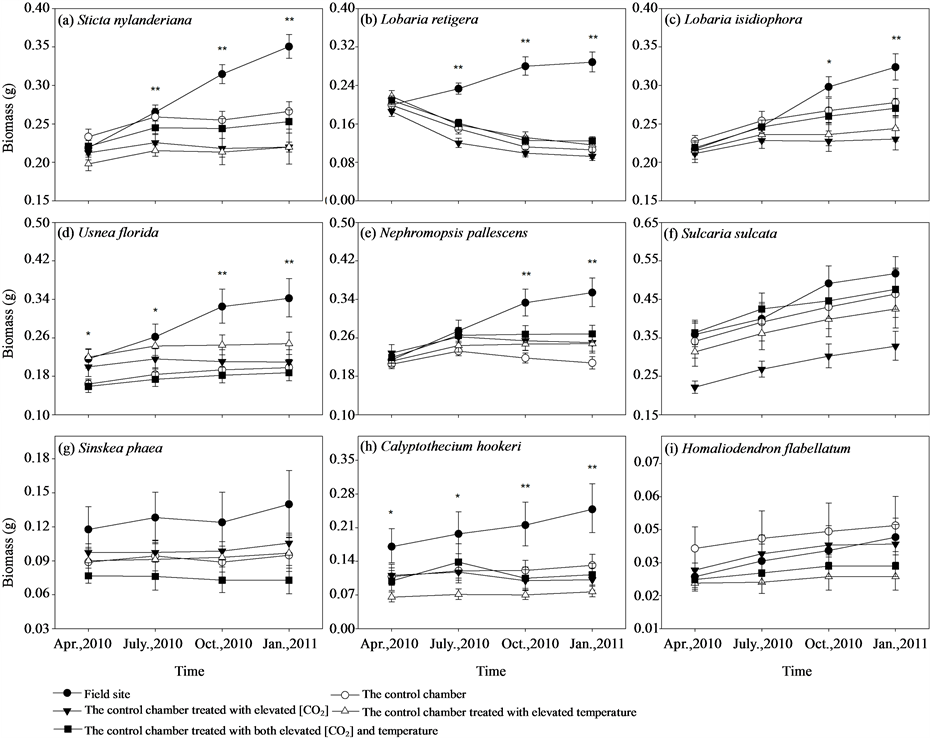
Figure 2. Treatment effects of different chambers on growth (represented by biomass (g)) of different epiphytic species. Bars indicate ± SE. * and ** represent differences among treatments within the observation period are significant at the 0.05 level and the 0.01 level, using ANOVAs, respectively.
in the initial biomass of the samples of U. florida (F4,35 = 3.156, P = 0.026). Differences of biomass of U. florida increased and reached highly significant difference among different treatments 6 months after transplantation (F4,35 = 5.843, P = 0.001) (Figure 2(d)).
The initial samples were all in very good health for all lichens; however, health of samples in all chambers markedly deteriorated, with parts of transplants turning brown or were dying back after transplantation. Health of samples in the field site was not affected (ESM_2(a)-(f)). Three months after transplantation, there was a highly significant difference among treatments in health rating of L. retigera (Kruskal-Wallis H χ2 = 29.061, P < 0.001). Many transplants of L. retigera in the chambers died back (ESM_2(b)). Another three months after this, we detected a significant difference in health rank of S. sulcata (Kruskal-Wallis H χ2 = 11.069, P = 0.026), S. nylanderiana (Kruskal-Wallis H χ2 = 14.952, P = 0.005), L. isidiophora (Kruskal-Wallis H χ2 = 14.133, P = 0.007), U. florida (Kruskal-Wallis H χ2 = 19.473, P = 0.001), and N. pallescens (Kruskal-Wallis H χ2 = 17.296, P = 0.002) among different treatments (ESM_2(a), ESM_2(c)-(f)).
3.4. Response of Growth and Health of Epiphytic Bryophytes after Transplants
Treatment effects of translocation from field sites to the control chamber was marginally negatively significant on growth of C. hookeri (P = 0.074, Table 1), many of which turned brown or died back (ESM_2(h)). In addition, marginally significant effect of further warming in chambers have been detected on growth of H. flabellatum (P = 0.070, Table 1).
Similarly, samples grew best at the field site, while transplants hanging in the chambers showed relatively slow or negative growth during the 9 months’ experimental period (Figures 2(g)-(i), ESM_3(g)-(i)). Health of the bryophyte samples in the chambers markedly deteriorated, with parts of transplants turning brown or dying back after transplantation, while health of samples in the field site had no obvious change (ESM_2(g)-(i)).
4. Discussions
4.1. Treatment Effects of Translocation from Field Sites to the Control Growth Chamber
Translocation from field sites to the control chamber significantly negatively affected the growth of L. retigera, U. florida, and N. pallescens, while marginally significantly for S. nylanderiana (P = 0.051) and C. hookeri (P = 0.074), many of which turned brown or died back within 9 months (Table 1, Figure 2, ESM_2). There is no apparent detrimental effect of transplantation because transplants in the field sites showed relatively high growth rates and good health (Figure 2, ESM_2). Difference of microclimate conditions including water availability and air temperature between field sites and the control chamber should be the possible causes.
Water availability has usually been considered to be the overriding environmental determinant of poikilohydric epiphytes [33] . For example, the pattern of epiphytes being more abundant in a tropical lowland cloud forest compared with a nearby lowland rain forest in French Guiana was attributed to the prolonged water availability in the cloud forest [34] [35] . Even in forest ecosystems without continuous moisture input, species composition of epiphyte communities changed drastically when the environment became drier following disturbance [36] . In addition, the transplantation experiments in the moist forest indicated the detrimental effect of decreased water availability on epiphytic bryophytes [11] . Thus, the decline of epiphytes from field sites to the control growth chamber in this study may be attributed to water limiting because RH in the control chamber was lower, while VPD was much higher than field sites (ESM_1).
In addition, elevated temperature (3.5˚C - 4˚C) from field sites to the chamber control may be another important cause of significant adverse impacts on growth and health of experimental non-vascular epiphytes (Table 1, Figure 2, ESM_2). Similarly, translocation experiment in Bolivia indicated that elevated temperature may shift the structure of nonvascular epiphytic communities [9] . Another translocation study along the altitudes showed that transplantation to the warmer, drier sites resulted in remarkably reduced rates of growth and detrimental effects on the health of non-vascular epiphytes in southwestern China [11] . Furness and Grime [37] reported that all the 40 experimental bryophyte species were killed when kept continuously at temperatures above 30˚C, although maintained in a continuously moist condition. Elevated temperature will negatively affect carbon balances of lichens or even kill them [38] . It was shown that Peltigera scabrosa had been stressed when thallus temperature as low as 25˚C [39] . Normally, most lichens died when the thallus temperature exceeds 35˚C to 43˚C [40] .
It was reported that, in tropic, the ratio of daily carbon gain to respiration for non-vascular epiphytes is much lower than vascular plants, because photosynthesis is often strongly reduced due to desiccation during daytime, while these epiphytes are usually moist and actively respiring during nighttime with relative high air temperature [15] . This situation will no doubt become worse under warming condition based on the following two reasons. Firstly, higher temperatures may result in stronger respiration [33] . Secondly, higher temperatures will cause high VPD, and thus dehydration of poikilohydric bryophytes and lichens, thereby restricting the time available for carbon gain in the day time. This situation is exacerbated by the fact that lichens and bryophytes have small quantities of chlorophyll per unit area or mass compared to vascular plants [41] . It is suggested that, for the lichen Parmotrema endosulphureum, a predicted temperature increase of 3˚C without acclimatization would make it necessary to photosynthesize at maximum rates for >90% of the day to achieve a positive carbon balance [42] . This is clearly impossible because lichens show net photosynthesis for only 30% - 80% of the light period, and at mostly suboptimal rates [43] . As a result, many lichens and bryophytes are incapable of high photosynthetic rates required to overcome respiratory energy losses under warm circumstance [44] . These studies imply that lichens and bryophytes in subtropical or tropical forests are already living close to the edge of their physiological abilities under current conditions [15] and thus they cannot tolerate further warming. Considering temperature in the MMEBF is predicted to increase by 2.2˚C by the 2050s [11] , many epiphytic bryophyte and lichen species may be negatively affected or even face extinction.
4.2. Further Treatment Effects of Elevated [CO2] and Temperature in Chambers
Nonvascular epiphytes such as bryophytes and lichens can benefit from elevated [CO2] [45] . It is suggested that the negative effects of warming on the carbon balance of bryophytes and lichens may be at least partly counteracted by increases in atmospheric CO2 levels, although the inability to regulate water loss in poikilohydric plants limits the possible responses to CO2 as compared to those of homeohydric plants [15] . For instance, in the lichen Lobaria pulmonaria (L.) Hoffm., nitrogenase activity was approximately doubled maintained in doubled [CO2]; this, somehow, can be beneficial for them or even the whole forest ecosystem [46] . The moss Tortula ruralis and the lichen Cladonia convulata maintained their positive response to elevated [CO2], which showed increased net CO2 uptake in the material grown at high CO2 by more than 30% and 50%, respectively [45] [47] . However, some other studies gave the opposite evidence. After been exposure to elevated [CO2] for 30 d, photosynthetic capacity of green algal lichen (Parmelia sulcata Taylor) was found to be reduced, associated with a parallel decline in the amount of Rubisco in the pyrenoid of algal chloroplasts [48] . The moss Polytrichum formosum clearly down-regulated its chlorophyll and RuBisco contents after several months at 700 ppm CO2 [47] . Our result indicated a third response possibility for bryophytes and lichens at elevated [CO2] as there was no significant effect of elevated [CO2] for any of the experimental species, although significant interaction between elevated [CO2] and temperature had been detected in S. nylanderiana, L. isidiophora, U. florida, and S. sulcata. It was unfortunately that a large amount of experimental materials had already been killed by the dramatic microclimatic changes transplanted from field sites to the control chamber, which equals to a treatment of +3.5˚C in temperature and +2.38 kPa in VPD on average. This situation, to a certain degree, limited the possibility of discussing the further detailed effects of elevated temperature and [CO2] in chambers.
4.3. Implications
Our results indicate the sensitivity of nonvascular epiphytes to microclimate changes. Warming is directly deleterious to nonvascular epiphytes because it induces higher respiration, which means higher carbon loss. Furthermore, higher temperature usually causes higher VPD, which may lead to dehydration of poikilohydric nonvascular epiphytes, and thus dormancy of their photosynthesis and, of course, lower carbon gain. If the above situation lasts, it will no doubt break the balance of the carbon budget of nonvascular plants. The study implies that it is necessary to consider the indirect effects of increase in VPD when we discuss the global warming effects on poikilohydric plants because their physiological activities are closely linked with water availability. Furthermore, translocation from field sites to the control chamber in this study is kind of an approximative proxy of logging, both of which dramatically change a wide range of key microclimatic factors including increase in temperature and irradiance, while decrease in relative humidity [49] . Considering a sudden exposure to higher temperature and lower water availability can rapidly damage some epiphytic bryophytes and lichens, we are warmed to take much more careful evaluation before logging during the practice of forest management and conservation.
In addition to the obvious threats posed by deforestation and air pollution, epiphytic lichens and bryophytes around the world face the menace of other global events such as climate change [7] [11] . This study, as well as a former study in the study region [11] , provides experimental evidence on the sensitivity of epiphytic lichens and bryophytes in response to warming and dryness. Our results imply a dim future for nonvascular epiphytes in a warming world, although interactions between elevated [CO2] and temperature in some species such as L. isidiophora raise uncertainty to a certain degree. Given the fact that nonvascular epiphytes represent a large amount of biodiversity and biomass, play an important role in hydrological and nutrient cycles of the subtropical montane forests [19] [20] [22] , and show high sensitivity to environmental changes in the MMEBF [11] [16] , we may underestimate global change impacts to epiphytic flora, or even the whole forest ecosystems.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31300382, U1133605), the QCAS Biotechnology Fund (No. GJHZ1130), and West Light Foundation of the Chinese Academy of Sciences. Ailaoshan Station for Subtropical Forest Ecosystem Studies and the Ecological Invasion Group of Xishuangbanna Tropical Botanical Garden are thanked for granting permission and facilitating this research. We wish to thank Prof. Gerhard Zotz for his constructive suggests on the manuscript.
References
- Petit, J.R., Jouzel, J., Raynaud, D., Barkov, N.I., Barnola, J.M., et al. (1999) Climate and Atmospheric History of the Past 420,000 Years from the Vostok Ice Core, Antarctica. Nature, 399, 429-436. http://dx.doi.org/10.1038/20859
- CDIAC (2005) http://cdiac.esd.ornl.gov/ftp/trends/co2/maunaloa.co2
- IPCC (2007) Climate Change 2007: Synthesis Report. IPCC, Geneva.
- Rawson, H.M. (1992) Plant Responses to Temperature under Conditions of Elevated CO2. Australian Journal of Botany, 40, 473-490. http://dx.doi.org/10.1071/BT9920473
- Hovenden, M.J., Miglietta, F., Zaldei, A., Vander Schoor, J.K., Wills, K.E., et al. (2006) The Tasface Climate-Change Impacts Experiment: Design and Performance of Combined Elevated CO2 and Temperature Enhancement in a Native Tasmanian Grassland. Australian Journal of Botany, 54, 1-10. http://dx.doi.org/10.1071/BT04194
- Körner, C. (2000) Biosphere Responses to CO2 Enrichment. Ecological Applications, 10, 1590-1619. http://dx.doi.org/10.2307/2641226
- Gradstein, S.R. (2008) Epiphytes of Tropical Montane Forests—Impact of Deforestation and Climate Change. In: Gradstein, S.R., Gansert, D. and Homeier, J., Eds., The Tropical Montane Forest—Patterns and Processes in a Biodiversity Hotspot Biodiversity and Ecology Series, University of Göttingen Press, Göttingen, 51-65.
- Nadkarni, N.M., Mewin, M.C. and Niedert, J. (2001) In: Levin, S.A., Ed., Encyclopedia of Biodiversity, Academic Press, California, 27-40. http://dx.doi.org/10.1016/b0-12-226865-2/00126-7
- Jácome, J., Gradstein, S.R. and Kessler, M. (2011) Responses of Epiphytic Bryophyte Communities to Simulated Climate Change in the Tropics. In: Tuba, Z., Slack, N.G. and Stark, L.R., Eds., Bryophyte Ecology and Climate Change, Cambridge University Press, Cambridge, 191-208. http://dx.doi.org/10.1017/CBO9780511779701.011
- Hsu, R.C.C., Tamis, W.L.M., Raes, N., De Snoo, G.R., Wolf, J.H.D., et al. (2012) Simulating Climate Change Impacts on Forests and Associated Vascular Epiphytes in a Subtropical Island of East Asia. Diversity and Distributions, 18, 334-347. http://dx.doi.org/10.1111/j.1472-4642.2011.00819.x
- Song, L., Liu, W.-Y. and Nadkarni, N.M. (2012) Response of Non-Vascular Epiphytes to Simulated Climate Change in a Montane Moist Evergreen Broad-Leaved Forest in Southwest China. Biological Conservation, 152, 127-135. http://dx.doi.org/10.1016/j.biocon.2012.04.002
- Gouk, S.S., He, J. and Hew, C.S. (1999) Changes in Photosynthetic Capability and Carbohydrate Production in an Epiphytic Cam Orchid Plantlet Exposed to Super-Elevated CO2. Environmental and Experimental Botany, 41, 219-230. http://dx.doi.org/10.1016/S0098-8472(99)00006-4
- Monteiro, J.A.F., Zotz, G. and Körner, C. (2009) Tropical Epiphytes in a CO2-Rich Atmosphere. Acta Oecologica, 35, 60-68. http://dx.doi.org/10.1016/j.actao.2008.08.001
- Farmer, A.M., Bates, J.W. and Bell, J.N.B. (1992) Ecophysiological Effects of Acid Rain on Bryophytes and Lichens. In: Bates, J.W. and Farmer, A.M., Eds., Bryophytes and Lichens in a Changing Environment, Clarendon Press, Oxford, 284-313.
- Zotz, G. and Bader, M.Y. (2009) Epiphytic Plants in a Changing World-Global: Change Effects on Vascular and Non-Vascular Epiphytes. Progress in Botany, 70, 147-170. http://dx.doi.org/10.1007/978-3-540-68421-3_7
- Song, L., Liu, W.Y., Ma, W.Z. and Qi, J.H. (2012) Response of Epiphytic Bryophytes to Simulated N Deposition in a Subtropical Montane Cloud Forest in Southwestern China. Oecologia, 170, 847-856. http://dx.doi.org/10.1007/s00442-012-2341-9
- Wu, Z.Y. (1987) Vegetation of Yunnan (in Chinese). Science Publishing Agent, Beijing.
- Myers, N., Mittermeier, R.A., Mittermeier, C.G., Da Fonseca, G.A.B. and Kent, J. (2000) Biodiversity Hotspots for Conservation Priorities. Nature, 403, 853-858. http://dx.doi.org/10.1038/35002501
- Xu, H.Q. and Liu, W.Y. (2005) Species Diversity and Distribution of Epiphytes in the Montane Moist Evergreen Broad-Leaved Forest in Ailao Mountain, Yunnan. Biodiversity Science, 13, 137-147. http://dx.doi.org/10.1360/biodiv.040123
- Li, S., Liu, W.Y., Wang, L.S., Ma, W.Z. and Song, L. (2011) Biomass, Diversity and Composition of Epiphytic Macrolichens in Primary and Secondary Forests in the Subtropical Ailao Mountains, SW China. Forest Ecology and Management, 261, 1760-1770. http://dx.doi.org/10.1016/j.foreco.2011.01.037
- You, C.X. (1983) Classification of Vegetation in Xujiaba Region in Ailao Mts. In: Wu, Z.Y., Ed., Research of Forest Ecosystem on Ailao Mountains, Yunnan, Yunnan Science and Technology Press, Kunming, 74-117.
- Ma, W.Z., Liu, W.Y. and Li, X.J. (2009) Species Composition and Life Forms of Epiphytic Bryophytes in Old-Growth and Secondary Forests in Mt. Ailao, SW China. Cryptogamie Bryologie, 30, 477-500.
- Li, S., Liu, W.Y. and Li, D.W. (2013) Epiphytic Lichens in Subtropical Forest Ecosystems in Southwest China: Species Diversity and Implications for Conservation. Biological Conservation, 159, 88-95. http://dx.doi.org/10.1016/j.biocon.2012.12.027
- Qiu, X.Z. and Xie, S.C. (1998) Studies on the Forest Ecosystem in Ailao Mountains Yunnan, China. Yunnan Science and Technology Press, Kunming, 1-11.
- Wu, J.L. (1987) Iconography of Chinese Lichen. Zhanwang Press, Beijing.
- Sillett, S.C. and Rambo, T.R. (2000) Vertical Distribution of Dominant Epiphytes in Douglas-Fir Forests of the Central Oregon Cascades. Northwest Science, 74, 44-49.
- Perry, D.R. (1978) A Method of Access into the Crowns of Emergent and Canopy Trees. Biotropica, 10, 155-157. http://dx.doi.org/10.2307/2388019
- McCune, B., Derr, C.C., Muir, P.S., Shirazi, A., Sillett, S.C. and Daly, W.J. (1996) Lichen Pendants for Transplant and Growth Experiments. Lichenologist, 28, 161-169. http://dx.doi.org/10.1006/lich.1996.0014
- Stiling, P., Moon, D.C., Hunter, M.D., Colson, J., Rossi, A.M., Hymus, G.J. and Drake, B.G. (2003) Elevated CO2 Lowers Relative and Absolute Herbivore Density across All Species of a Scrub-Oak Forest. Oecologia, 134, 82-87. http://dx.doi.org/10.1007/s00442-002-1075-5
- Lei, Y.B., Feng, Y.L., Zheng, Y.L., Wang, R.F., Gong, H.D. and Zhang, Y.P. (2011) Innate and Evolutionarily Increased Advantages of Invasive Eupatorium adenophorum over Native E. japonicum under Ambient and Doubled Atmospheric CO2 Concentrations. Biological Invasions, 13, 2703-2714. http://dx.doi.org/10.1007/s10530-011-9940-y
- Smithsonian Institution (1984) Smithsonian Meteorological Tables. Smithsonian Institution Press, Washington.
- Garson, G.D. (2008) GLM Repeated Measures. http://faculty.chass.ncsu.edu/garson/pa765/glmrepeated.htm
- Sillett, S.C. and Antoine, M.E. (2004) Lichens and Bryophytes in Forest Canopies. In: Lowman, M.D. and Nadkarni, N.M., Eds., Academic Press, Forest Canopies. http://dx.doi.org/10.1016/B978-012457553-0/50013-7
- Gehrig-Downie, C., Obregón, A., Bendix, J. and Gradstein, S.R. (2011) Epiphyte Biomass and Canopy Microclimate in the Tropical Lowland Cloud Forest of French Guiana. Biotropica, 43, 591-596. http://dx.doi.org/10.1111/j.1744-7429.2010.00745.x
- Normann, F., Weigelt, P., Gehrig-Downie, C., Gradstein, S.R., Sipman, H.J.M., Obregon, A. and Bendix, J. (2010) Diversity and Vertical Distribution of Epiphytic Macrolichens in Lowland Rain Forest and Lowland Cloud Forest of French Guiana. Ecological Indicators, 10, 1111-1118. http://dx.doi.org/10.1016/j.ecolind.2010.03.008
- Gradstein, S.R. (1992) The Vanishing Tropical Rain Forest as an Environment for Bryophytes and Lichens. In: Bates, J.W. and Farmer, A.M., Eds., Bryophytes and Lichens in a Changing Environment, Clarendon Press, Oxford, 232-256.
- Furness, S. and Grime, J. (1982) Growth Rate and Temperature Responses in Bryophytes: II. A Comparative Study of Species of Contrasted Ecology. The Journal of Ecology, 70, 525-536. http://dx.doi.org/10.2307/2259920
- Maphangwa, K.W., Musil, C.F., Raitt, L. and Zedda, L. (2012) Experimental Climate Warming Decreases Photosynthetic Efficiency of Lichens in an Arid South African Ecosystem. Oecologia, 169, 257-268. http://dx.doi.org/10.1007/s00442-011-2184-9
- MacFarlane, J. and Kershaw, K. (1980) Physiological-Environmental Interactions in Lichens. IX Thermal Stress and Lichen Ecology. New Phytologist, 84, 669-685. http://dx.doi.org/10.1111/j.1469-8137.1980.tb04780.x
- Beckett, R., Kranner, I. and Minibayeva, F.V. (2008) Stress Physiology and the Symbiosis. In: Nash III, T.H., Ed., Lichen Biology, 2nd Edition, Cambridge University Press, Cambridge, 134-151. http://dx.doi.org/10.1017/CBO9780511790478.009
- Green, T.G.A. and Lange, O.L. (1994) Photosynthesis in Poikilohydric Plants: A Comparison of Lichens and Bryophytes. In: Schulze, E.-D. and Caldwell, M.M., Eds., Ecophysiology of Photosynthesis, Springer, Berlin, 319-341. http://dx.doi.org/10.1007/978-3-642-79354-7_16
- Zotz, G., Schultz, S. and Rottenberger, S. (2003) Are Tropical Lowlands a Marginal Habitat for Macrolichens? Evidence from a Field Study with Parmotrema endosulphureum in Panama. Flora, 198, 71-77. http://dx.doi.org/10.1078/0367-2530-00077
- Lange, O.L., Büdel, B., Meyer, A., Zellner, H. and Zotz, G. (2004) Lichen Carbon Gain under Tropical Conditions: Water Relations and CO2 Exchange of Lobariaceae Species of a Lower Montane Rainforest in Panama. Lichenologist, 36, 329-342. http://dx.doi.org/10.1017/S0024282904014392
- Frahm, J.P. (1990) The Effect of Light and Temperature on the Growth of the Bryophytes of Tropical Rain Forests. Nova Hedwigia, 51, 151-164.
- Tuba, Z., Csintalan, Z., Szente, K., Nagy, Z. and Grace, J. (1998) Carbon Gains by Desiccation-Tolerant Plants at Elevated CO2. Functional Ecology, 12, 39-44. http://dx.doi.org/10.1046/j.1365-2435.1998.00173.x
- Norby, R.J. and Sigal, L.L. (1989) Nitrogen Fixation in the Lichen Lobaria pulmonaria in Elevated Atmospheric Carbon Dioxide. Oecologia, 79, 566-568. http://dx.doi.org/10.1007/BF00378677
- Tuba, Z., Proctor, M.C.F. and Takács, Z. (1999) Desiccation-Tolerant Plants under Elevated Air CO2: A Review. Zeitschrift Fur Naturforschung C, 54, 788-796.
- Balaguer, L., Valladares, F., Ascaso, C., Barnes, J.D., De Los Rios, A., Manrique, E. and Smith, E.C. (1996) Potential Effects of Rising Tropospheric Concentrations of CO2 and O3 on Green-Algal Lichens. New Phytologist, 132, 641-652. http://dx.doi.org/10.1111/j.1469-8137.1996.tb01882.x
- Stoutjesdijk, P. and Barkman, J. (1987) Microclimate, Vegetation and Climate. Opulus Press AB, Knivstad.
Abbreviations
ESM, electronic supplemental material MMEBF, primary montane moist evergreen broad-leaved forest
Electronic Supplemental Materials
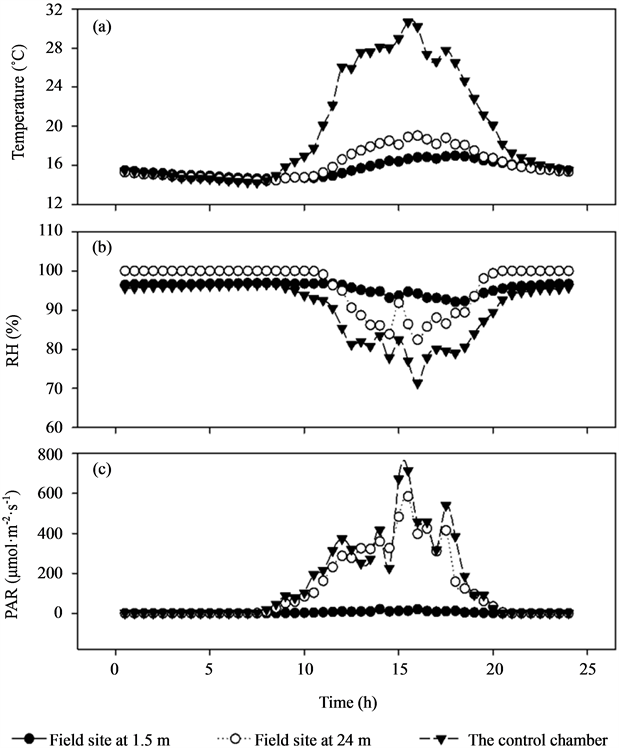
ESM_1. Comparison of mean air temperature, relative humidity (RH), vapour pressure deficit (VPD), and photosynthetic active radiation (PAR) among field site at 1.5 m, field site at 24 m, and the control chamber.
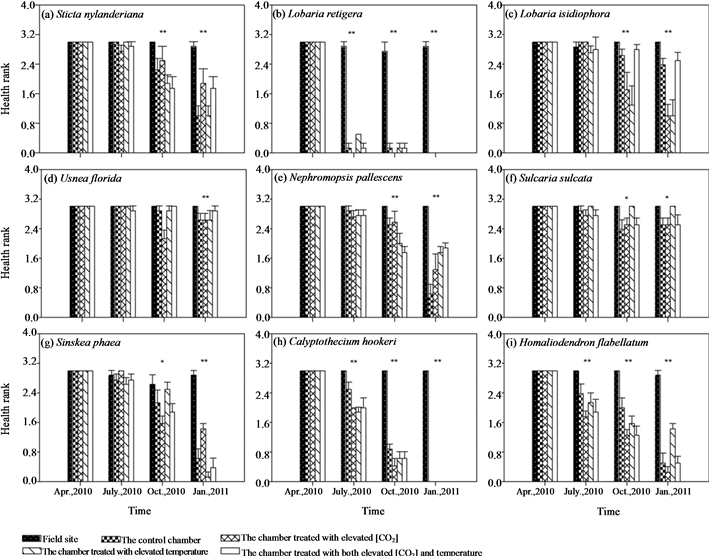
ESM_2. Treatment effects of different chambers on health rank of different epiphytic species. Bars indicate ± SE. * and ** indicates that differences among treatments within the observation period are significant at the 0.05 and 0.01 level using Kruskal-Wallis H’s nonparametric test.
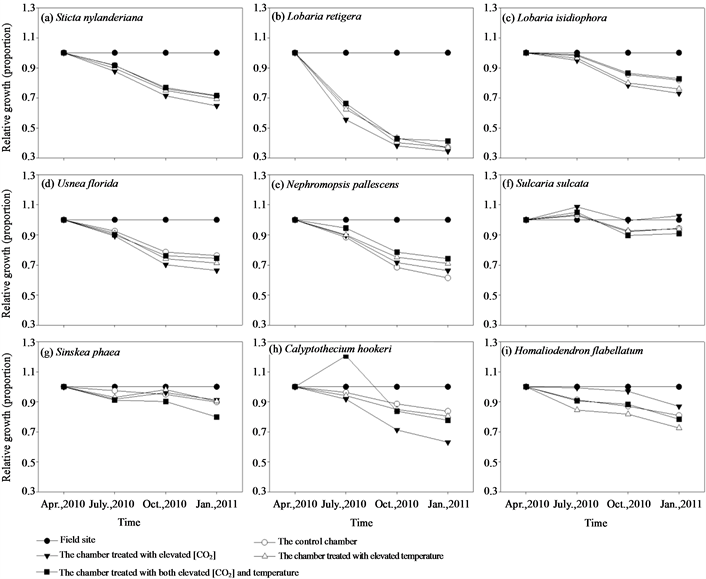
ESM_3. Treatment effects of different chambers on relative growth (use samples in the field as control) of different epiphytes.
NOTES

*Corresponding authors.


