American Journal of Plant Sciences
Vol.4 No.6A(2013), Article ID:33323,12 pages DOI:10.4236/ajps.2013.46A009
Commiphora wightii (Arnott) Bhandari—A Natural Source of Guggulsterone: Facing a High Risk of Extinction in Its Natural Habitat
![]()
Plant Cell and Molecular Biology, Indian Institute of Advanced Research, Gandhinagar, India.
Email: *rajanisn@iiar.res.in
Copyright © 2013 Neeraj Jain, Rajani S. Nadgauda. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 9th, 2013; revised May 9th, 2013; accepted June 5th, 2013
Keywords: IUCN; Conservation; Commiphora wightii; Somatic Embryogenesis; Threatened
ABSTRACT
The plants are the primary producers and an indispensible source of food and nutrition, medicine and fuel/biofuel. Unsustainable overharvesting and indiscriminate felling of plants due to ever increasing needs of population pressure are causes of great concern. The IUCN includes, the species facing a high risk of extinction in the wild as threatened, and “endangered” is one of the sub-categories under “threatened” category. Commiphora wightii (Arnott) Bhandari is an arid region plant, highly valued for its medicinally important guggul gum-resin as a source of guggulsterone. It is listed in IUCN’s Red Data List of threatened plants and now it is becoming endangered. Its population is fast depleting in its natural habitat, primarily due to over-exploitation, unsustainable and destructive methods of gum-extraction coupled with natural dry-arid habitat, slow growth and poor regeneration of the plant. Several other reasons have also been indicated for its declining population. Therefore, it demands severe measures for its conservation before we completely lose this important medicinal plant. A lot of research and study is underway but has vast scope for improvement, requiring efforts to supplement with such information that would aid transgenic development and breeding programmes for production and cultivation of improved varieties. The article presents the importance of this plant and its conservation in a nut-shell.
1. Introduction: Endangered Plant Species
The plants are the primary producers utilized, both, by herbivores (directly) and carnivores (indirectly). The plants are an indispensible source of food and nutrition, medicine and fuel/biofuel. Unsustainable overharvesting due to their utilization for above resources and indiscriminate felling of plants for urbanization due to ever increasing needs of population pressure are causes of great concern. In absence of proper measures for replenishing the vegetative cover, the number of trees in general is reduced and some specific ones acquire the threatened status. The IUCN includes, the species facing a high risk of extinction in the wild as threatened, and “endangered” is one of the sub-categories under “threatened” category. The Endangered Species Act of 1973 defines an “endangered” species as “an animal or plant species in danger of extinction throughout all or a significant portion of its range.” and “threatened” species as “an animal or plant species likely to become endangered within the foreseeable future throughout all or a significant portion of its range.” A “rare” species is “one that has only a few populations in the state and that faces threats to its continued existence”. Endangered species of plant thus face a high risk of extinction in near future and indicates the conservation status. The “Endangered” category is the most alarming conservation status of any species because plants which are a source of food, fuel and medicine face a threat of extinction in near future. The rarity of even the wild plants is alarming as these plants are a source of gene-pool. There are numerous reasons to the plants being categorized as “endangered”. Some of these are: over-exploitation, lack of systematic cultivation, invasion of foreign species in the natural habitat, abiotic factors etc.
Commiphora wightii (Arnott) Bhandari is a plant of immense medicinal importance and is listed in IUCN’s Red Data List of threatened plants [1]. This plant is becoming endangered and demands severe measures for its conservation. This article deals 1) briefly with various aspects of this plant species per se, like its distribution range, economic importance, chemical constituents of its oleo-gum resin; and 2) detailed discussion about its conservation status, the possible reasons for its declining population and the existing conservation strategies based on literature. On the basis of this survey, the authors conclude and suggest the future potential strategies to save the existing population and also to replenish it. The article is not meant to be a review article but it is an effort to present to the readers the importance of this plant and its conservation in a nut-shell.
2. About the Plant and Its Distribution
2.1. The Plant: Commiphora wightii (Arnott) Bhandari
Myrrh genus Commiphora (Family: Burseraceae) has about 165 [2] to 185 species [3]. It is widely distributed in tropical regions of Africa, Madagascar, Asia [4], Australia and Pacific Islands [5]. Six species of this genus are found in Indian sub-continent [5] and three of these (viz., C. wightii, C. agallocha and C. berryi) occur in India [5, 6]. Atal et al. [7] reported four species from India. Of these species, Commiphora wightii is highly valued for its medicinally important guggul gum-resin. It is regarded as threatened species as its population is depleting fast in its natural habitat [3].
Commiphora wightii is commonly known as guggul in Hindi and Indian myrrh or Indian bdellium in English. It was earlier known as C. mukul (Hook. ex Stocks) Engl. or Blasmodendron mukul Hook. ex Stocks (1849), then was renamed C. roxburghii by Santapau (1849). Later Bhandari validated its name to the present one—C. wightii (Arn.) Bhandari [5]. It is a perennial, highly branched, thorny and woody shrub (Figure 1). It thrives well in arid, semi-arid and rocky regions with scanty rainfall. It is a slow growing plant with a long dormant phase and deciduous nature. The plant is dimorphic in nature, i.e., one having bisexual and male flowers and other having female flowers with staminodes [8]. Yadava et al. [9] reported the occurrence of only female plants from Rajasthan [10]. The plant has been documented to be apomictic [11] and polyembryonic in nature [11,12]. Apomixis is not the main method of reproduction [11] and the seeds are produced by cross pollination resulting in heterozygous population. Two types of seeds-black and white are observed in mature fruits. While the black ones

Figure 1. Commiphora wightii (Arnott) Bhandari growing in medicinal garden at IIAR.
are viable, the whitish-black ones are non-viable due to absence of embryo [12].
2.2. Distribution Range
C. wightii is distributed in pockets in India (Rajasthan, Gujarat, Maharashtra and Karnataka [7], Madhya Pradesh [5]) and adjoining countries of Sind, Baluchistan and Afghanistan. In India, Rajasthan and Gujarat have been identified as the main commercial centers [13]. Sabins and Rao [14] reported that the species is confined to a smaller area of Northern Gujarat and districts of Kutch and Jamnagar. Shetty and Singh [15] reported that, in Rajasthan, the distribution range of this species is in Indian Desert and Aravallis.
3. Guggul Gum-Resin (Guggulu)
3.1. Extraction and Changing Economics of Guggulu
Guggulu, the gum-resin exudate from the tree, C. wightii, is extracted by the method of tapping. For tapping of gum-resin, an incision is made in the bark of the plant and the gum exudate is collected as it oozes out of the bark. A C. wightii plant, generally takes 10 years to reach maturity under the dry climatic conditions and the thick branches are incised during the winter to extract the oleo gum resin [16]. Guggul gum yield depends on a number of factors and varies from plant to plant, including the age of the trees in the same areas. However, between 200 - 500 gm dry guggul gum is usually obtained from a healthy plant in a single season, applying tapping practices through incision [17]. Generally, the oleo gum-resin of the plant is collected by the tribal people using the traditional method i.e., they give several deep incisions on the stem to get maximum yield and also apply a paste which is fatal to the plant. This crude method, besides over exploitation, is the main reason for the declining population of this species in its natural habitat.
It was earlier estimated that the domestic demand of the country is about 300 tons, while the production is only 75 tons. The rest of the material is being imported mostly from Pakistan’s Sindh area, adjoining Rajasthan, to meet the demand of the country [17]. According to Yadava [17], the raw material is already insufficient in the country to meet the demand of the indigenous medicines. He reports that presently, the collection of guggul gum from Rajasthan and Gujarat has much declined and it is being imported from countries like Pakistan and the only big market of the crude drug is Amritsar where presently, it is being sold for Rs.400 to Rs.430 per kg. According to Lal and Kasera [5], the price of guggul gum increased from Rs.100 - 400 per kg indicating many fold increase in its use as well as decrease in natural plant stand. The Forest Department collected 30 tons of gumresin in 1963 which was drastically reduced to 2.42 tons in 1999 [18].
3.2. Economic Importance
The plant has vast economic value and a wide array of medicinal uses in both ancient and modern therapeutics. The plant yields medicinally important natural gum resin. Guggul was first introduced to the scientific world by an Indian Medical Researcher, G. V. Satyavati, in 1966 [19]. In 1986, guggul was approved for marketing in India as a hypolipidaemic drug [19]. In the middle 1990s, guggul was introduced into the Western world [20]. Guggul is available in the United States and other Western countries as an over-the-counter dietary supplement [19].
It has wide ethnobotanical usage by Garsarias, Saharia and Kalbelia tribes [21]. Guggul-gum is known to be hypolipidemic, hypocholestrolemic and anti-obesity [22- 25] astringent and antiseptic [26], anti-arthritic [27,28], antimicrobial [29], anti-inflammatory [30,31], and anticancerous [32]. It is also reported for the treatment of thrombosis [22] and chronic bronchitis [33], nodulocystic acne [34], spongy gums, chronic tonsillitis and teeth carries [35]. The gum is also used in perfumery, calicoprinting, fumigation, dyeing silk and cotton and as incense [3].
Some reports contradict the hypocholestrolemic efficacy of C. wightii [36]. Guggul has been also reported to cause side effects like gastrointestinal discomfort, possible thyroid problems and generalized skin rash [37].
Several ayurvedic formulations containing guggul are available in Indian market and some of which have been tested experimentally for their activity are Maha Yogaraja Guggulu and Chandraprabha Vati [38], and Triphala guggulu [39].
3.3. Chemical Composition of Guggulu or Oleo Gum Resin
Guggul gum is a mixture of 61% resin, 29.3% gum [40], 6.1% water, 0.6% volatile oil and 3.2% foreign matter [41]. Guggulu, the gum-resin exudate from the tree Commiphora mukul, is a complex mixture of steroids, diterpenoids, aliphatic esters, carbohydrates and a variety of inorganic ions, besides minor amounts of sesamin and other unidentified constituents [42]. Bose and Gupta [43] separated the gum resin into a soluble resin and insoluble carbohydrate gum, by alcohol extraction. According to Fatope et al. [44], guggul contains more than 150 compounds and new compounds continue to be reported. Guggulsterones E and Z (Figure 2) are believed to be hypolipidaemic and the most important components of the guggul gum resin. These two compounds and several others have been reported from time to time by various workers [31,42,44-48]. Jain and Gupta [49] estimated the presence of essential oils (0.37%) in guggul gum and also analyzed some of its components.
Guggul gum-resin essentially consists of an ethyl acetate-soluble fraction (45%) and insoluble carbohydrate gum (55%). The desired biological activity lies entirely in the soluble cut and insoluble fraction is toxic to rats and devoid of any hypolipidaemic activity [10]. The crude gum guggul was found to contain 2% guggulsterone and ethyl acetate extract contains 4% - 4.5% guggulsterones. E and Z guggulsterones have been reported to be 10% [50]. According to Ramawat et al. [6], the two isomers Eand Z-guggulsterones are interconvertible in callus and cell cultures.
4. Conservation Status of C. wightii
C. wightii has been included in IUCN Red data list [1] v2012.2 under Data deficient v 2.3. UNDP has listed this species as “critically endangered” [51]. The Govt. of India has banned the export of this species [52]. It is considered to be a threatened plant [14] and over-exploited species in the country [53]. Despite its utility and rare occurrence, this native medicinal shrub has received little attention by agricultural scientists [8].
Observations revealed that this species is under threat in its entire range of distribution in its main centers of distribution in India-Rajasthan and Gujarat [3]. According to a report by Parmar [54], within a period of 10 years or so, as a whole, the population has been shrunk to less than 50% with scattered and dissected sub-popu-
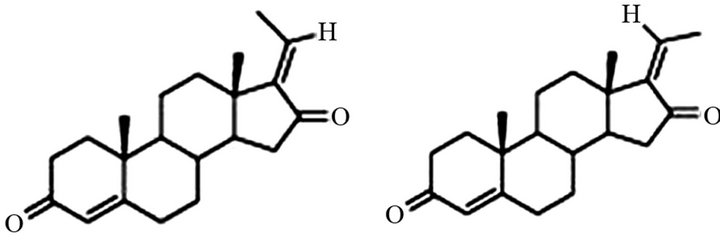
Figure 2. Structure of Eand Z-isomers of guggulsterone.
lation of few individuals in each. The study conducted by Kumar and Shankar [4] reported 10 plants per hectare in the Falna Aravalli range of Pali district and Mevanagar Hills of Barmer district.
A recent study by Reddy et al. [3], based on survey conducted throughout Rajasthan during 2007-2009, showed that the species was present only in 2% of the sample plots and confirms its rarity. On the basis of their conservation threat assessment data collected during their study they suggested to include this plant under endangered category. They found the extent of occurrence of this species to be <5000 km2 and total area of occupancy <500 km2, on the basis of which they confirmed its status as “endangered”. They proposed that the species be prioritized for “endangered category” conservation. Their study revealed that C. wightii was facing severe threat and extinction risk due to various factors like over-exploitation, narrow extent of occurrence, small area of occupancy, severe fragmentation of populations, very low regeneration and invasion of alien species to its habitat.
Of the 185 species of Commiphora, C. wightii is known to be a natural source of guggulsterone, the principle active compound of the plant. Therefore, it becomes very important to save this species from the danger of becoming extinct.
5. Possible Reasons for Its Decline
Several reasons are cited for the plant being listed as endangered species and some of these are considered to be of greater consequences affecting its population survival. The various factors are: slow growth, poor seed-setting and seed germination rate, lack of cultivation, unsustainable over-exploitation, excessive and unscientific tapping method, invasion of alien species [3,13,55]. These have been discussed below, one by one.
5.1. Unscientific Method of Tapping and Overexploitation
The traditional and unscientific method of tapping and overexploitation of the species by humans, for its economically important natural gum resin, are the primary reasons for its diminishing numbers in natural habitat. On maturity, the gum is extracted by tapping method by making an incision in the bark which leads to gradual death of the plant within a year or two. The tribals use traditional method of making several deep incisions in the stem to maximize the yield [16]. To increase the yield of the gum through multiple tappings, various pastes are applied around the incision e.g. a mixture of horse or wild ass urine, oleo-gum resin and copper sulphate [7,16]. Progressive drying and darkening of the incised branch is a common feature at Anand and Mangliawas, which extends from incised branch to the others and the plant dies within 8 - 10 months [8]. The plant is believed to undergo slow death in couple of years probably due to injurious effect of copper sulphate [56]. An alternative method developed by Bhatt et al. [55] described use of “mitchie golledge” knife coupled with ethephon. The method, no doubt, enhances gum production several folds compared to control, but in the long run, it too, exhausts and kills the plant [16]. Heavy tapping injures the cambium and curtails the life span of the tree on account of poor wound-healing process [10]. Therefore, the existing methods are destructive and unscientific. Destructive harvesting of oleo-gum resin for pharmaceutical and industrial uses is one of the major causes of the decline in population [13].
5.2. Low Seed Setting
The plant has a limitation of low seed-setting. Sriram and Dalal [57] observed that, percent flowers that set seeds in female and bisexual plants were only 1.25 and 5.50, respectively. Kumar et al. [58] reported that the seed set is ~16% in Aravalli ranges and even lower in drier parts of Western Rajasthan. Gupta et al. [59] described pollen-stigma interaction as the basis of low seed set. The plant is dimorphic in nature and number of male plants is nature is very limited [60]. Gupta et al. [11] reported the presence of only two male plants along with the large population of female plants, thus limiting the availability of pollens for fertilization. Therefore, dimorphism and limited number of male plants could also be some of the reasons for low seed-setting in C. wightii.
5.3. Regeneration through Seeds
Regeneration through seeds is very poor [17]. In nature, very few seedlings develop from seeds which was verified during field studies in different parts of Rajasthan and observation in the Farm by Yadava [17]. The experimental study at Guggul Herbal Farm, Mangliawas, during 1981, 1988, 1989 and 1990 indicated 1.4% germination (out of 1480 seeds sown). Natural regeneration through seeds has been rarely observed below the parent plants in the farm land and in the forests [9]. Dalal and Patel [8] reported a low germination percentage of 5%. C. wightii is a plant of dry and arid region. Arid zone soil is sandy with poor nutrient contents and soil moisture which affects seedling growth, therefore, may lead to low survival percentage [61]. A hard endocarp on seed [9], combined with dry/arid or naturally salty habitat and prevailing drought conditions are believed to be responsible for poor seed germination rate under natural conditions [60]. Prakash et al. [12] observed two types of seeds-black and white. The white ones were found to be non-viable whereas the black ones were viable and polyembryonic. Out of the total germinated seeds, 58.62% produced single seedling and 3.45% produced four seedlings.
5.4. Invasion of Alien Species
The invasion of alien species [3,62] has also been cited as one of the reasons for the decline of the population. Sharma and Raghubanshi [62] are of the opinion that invasive species may create demographic instability among the species and reduce the diversity which may lead to changes in the structure of the forest. According to Reddy et al. [3], the species with few individuals would be highly vulnerable due to impact of invasive species. They found that the species like Prosopis juliflora and Lantana camara have been found to be invading C. wightii habitats in Rajasthan region. The invasion of these species is suggested to synergistically affect regeneration along with grazing pressure and over-exploitation.
5.5. Other Reasons
Reddy et al. [3] stated, narrow extent of occurrence, small area of occupancy and severe fragmentation, as other reasons for conservation threat and extinction risk. The same is evident through the study of Haque et al. [63] about the evolution of C. wightii on the basis of study of ITS region sequences of nuclear ribosomal DNA of 32 individuals. From the study, it was concluded that the plants of C. wightii evolved under reproductive isolation, probably due to population fragmentation and habitat loss. The reproductive isolation, coupled with the reported apomictic behaviour may have detrimental effect on genetic variation. Since the population differentiation is high, the population continuum has been disrupted, possibly due to over exploitation, unsustainable utilization and other andropogenic activities. Availability of broad base is must for initiating breeding programmes in any crop [60].
Another reason for its population becoming scarce is that, it has never been brought under cultivation [64] till very lately and there has been hardly any commercial cultivation [13]. Due to lack of cultivation, the natural regeneration is almost negligible as compared to its depletion in nature [60].
Last but not the least, the changing environment and existing natural populations of this species has been affected by climatic conditions, soil erosion, low rainfall, termite infestation, over-grazing by domestic animals and mining activities. These prevailing abiotic factors coupled with the mass destruction, poor regeneration and non-survival due to poor tapping methods has caused a major setback to the plant stands in natural habitats [10].
6. Strategies for Its Conservation
The diminishing population and also the gum production level are a cause of great concern. In this scenario, there is a demand for immediate solution in form of effective conservation strategies. As a means to conservation there is a need of in situ conservation through survey, documentation and creating awareness, development of scientific or non-destructive methods, replenishments through macroand micro-propagation methods, production of guggulsterone in cell suspension cultures, molecular studies related to diversity and guggulsterone production. Several programs and studies are going on in various institutes and agencies for conservation of C. wightii, which have been discussed in this section.
6.1. Research Institutes and Other Agencies Active in the Conservation Programmes
Presently a few institutes in India viz., CIMAP (Central Institute of Medicinal and Aromatic Plants, Lucknow), CDRI (Central Drug Research Institute, Lucknow) and CAZRI (Central Arid Zone Research Institute, Jodhpur), are carrying out research on this species mostly related to the conservation of genetic resources and development of superior oleo-gum resin [16]. In the year 1972, the Central Council for Research in Ayurveda & Siddha (CCRAS, Govt. of India), initiated a project at Mangliawas (Ajmer) [17]. In 2008, India’s National Medicinal Plants Board (NMPB) launched a big project in Kutch District (Gujarat) to cultivate 500 to 700 hectares (1200 to 2000 acres) of guggal for three years as an initiative for conservation and cultivation of this highly traded medicinal plant in this border district. Our group at IIAR (Indian Institute of Advanced Research, Gandhinagar, Gujarat) is actively involved in studying various aspects related to this species especially somatic embryogenesis [65].
CEC member, an associate of IUCN, Vineet Soni, is carrying out education awareness programmes in his “Save Guggul Movement” to raise awareness among local rural and tribal people in Rajasthan about the IUCN red-listed plant Commiphora wighti [66]. This conservation work initially received support from the IUCN Sir Peter Scott Fund. Some efforts have recently been undertaken for effective conservation by promoting community participation and through planting schemes in Aravallis [67].
6.2. Vegetative Propagation
One of the limitations of the desert plants is their slow growth and development as compared to areas with high rain fall. For maintenance of hereditary characters and habitat adaptability, vegetative propagation is the most common method [61]. The traditional methods of propagation by seed germination, stem cuttings and air-layering are very common for C. wightii but, each one has its own limitation.
The plant is known for low seed germination percentage in nature therefore, various studies have been carried out to improve and enhance seed germination. Mechanical scarification with sand paper followed by running water for 24 h was found to be essential for germination in nursery conditions [68]. Several other methods tried by Prakash et al. [12] and Kasera and Chawan [69] failed to improve germination. Fortnightly irrigation helps in enhancing growth [69,70]. Seed sowing methods especially, depth and soil mixture was shown to play an important role in the seedling emergence in C. wightii [26]. It has been observed that the plants raised through seeds in the farm were having deep root system but growth was very slow as compared to the plants raised through cuttings, which have shallow root system and are comparatively fast growing [17], and healthier [68]. Lal and Kasera [71] reported a potassium nitrate pre-treatment method for the seed germination improvement.
Stem cutting method for plant production has been studied by several workers [17,68,69,72-74] as a means to conservation. Their study reports various growth regulators, season and method of collection, and planting of cuttings, for improved establishment and growth.
Air-layering experiments for vegetative propagation of C. wightii have been attempted by different workers [10,75]. These studies reported the effect of season, guggul solution and ceradik treatments at different concentrations, on root development and number, and survival percentage under field conditions. Kasera and Prakash [10] have shown that the survival was higher in plants raised through air layering technique than the other plants raised through seeds and stem cuttings after 24 months under field conditions.
6.3. Phytosociological Studies and Apomixis
Phytosociological studies provide baseline information on structure and floristic composition, particularly important for rare species and have been strongly recommended as a prerequisite for conservation of these species [76].
The plant has been documented to be apomictic. The production of seeds through apomixis, bypassing the process of fertilization, would produce plants which have genetically identical makeup. This process ensures the multiplication of plants in nature, however, such a population would be vulnerable to wipe out during disease epidemics and there would be loss of genetic variability [60]. The loss of variability would hamper breeding programmes due to loss of gene pool.
6.4. Diversity Studies and Cultivation of Suitable Land Races
The gum production can be enhanced by cultivation of suitable land races [64]. For the identification of such land races, it is necessary to document the diversity of the existing population. There are a few studies which report the diversity in C. wightii existing at morphological, phytochemical and molecular or genetic level. Sinha et al. [64] studied five provenances from extremely arid parts of India. The study brought out that a considerable amount of genetic variability exists in this species with respect to growth performance, which offers scope for selection and breeding, beneficial for conservation of genetic variability and future improvement schemes. Lal and Kasera [5] studied various morphological parameters of guggul plants growing under different natural habitats of Western India. Soni and Swarnakar [16] identified high guggulsterone yielding ecotypes from Rajasthan region which paved way for selecting plants and suitable geographical locality for large scale cultivation. Such plants can be used both for macroand in vitro micro -propagation. Kalpesh and Mohan [77] analyzed the crude enzyme extracts of 22 accessions of C. wightii and identified two accessions that should be conserved and maintained in the field gene bank. Molecular marker studies using RAPD [78,79] and ISSR [79] primers have been used to find genetic variation among different populations and genotypes of C. wightii. Genetic variation is important because it provides the “raw material” for natural selection. For conservation of this endangered species it is necessary to understand the nature of population structure [63].
6.5. In Vitro Propagation
In vitro propagation or micropropagation is the method of producing complete plants in sterile laboratory conditions and then their transfer into field. It is considered an efficient method for economically important but threatened species like C. wightii in view of limited stock for macropropagation for mass multiplication. The in vitro propagation method can be used for clonal propagation of selected germplasm, genetic improvement, production of active compound in cell culture.
In vitro propagation in C. wightii has been attempted through organogenesis and somatic embryogenesis methods by various researchers. Organogenesis has been induced through axillary shoot proliferation from nodal segments, seedling explants, shoot tips, internodes and leaves, by various workers [2,80-83]. Limited number of plantlet production has been achieved in all these studies and also their rate of establishment in the field has been low [60]. Recently, Parmar and Kant [84] reported efficient propagation and field survival percentage of in vitro raised plants from nodal segments of mature plants. Sterilization of explants, phenolic exudation, recalcitrant nature of plant, limited availability of preferred explants like tender shoots (due to long dormant phase) and zygotic embryos (due to low seed-setting) are some of the causes identified for low efficiency during in vitro regeneration by organogenesis [2,58].
Somatic embryogenesis is another method for in vitro mass multiplication. It is preferred over other methods, as root and shoot are produced in single step [60] and can be scaled up in bioreactors [85]. Immature zygotic embryos have been found suitable for this method [58]. Kumar et al. [86] reported secondary somatic embryogenesis. Asynchronous embryo development, low conversion rate of embryos into plantlets and survival of plantlets remain major limitations and need to be resolved in C. wightii [60].
Our group at IIAR has successfully developed an explant dependant, high frequency repetitive somatic embryogenesis protocol [65]. The protocol was developed using immature apomictic embryos from fruits collected throughout the year. Various parameters were studied to develop an efficient somatic embryogenesis method for in vitro regeneration and multiplication. In this study the season, age of fruit and size of embryo explants was optimized for best results. During the study it was found that the embryogenic mass was induced from 5 - 8 mm long immature embryo (explant) from 61 - 75 days old fruits, on MS media supplemented with 2, 4D when incubated in dark. This response was observed for 75% of the cultures. Long term proliferation of embryogenic mass could also be carried out on this media. This embryogenic mass differentiated into somatic embryos (Figure 3) when transferred in MS media devoid of 2, 4 D and incubated in light. The woody and recalcitrant nature of the species was a major challenge in the way of
Figure 3. Somatic embryogenesis in C. wightii.
developing an efficient in vitro method for multiplication. Once the protocol for somatic embryo development was standardized, the next step of conversion was another major challenge. The somatic embryos were subjected to pre-germination treatment using mannitol, polyethylene glycol (PEG-8000) and desiccation (air-drying), for conversion. Desiccation treatment gave the best results and the conversion of somatic embryos into well developed seedlings could be achieved. The study emphasizes explant dependency of the response towards somatic embryogenesis [65]. The work is being carried out to enhance the conversion frequency which would then in strict terms be an efficient conservation strategy for replenishment of the diminishing population of this species.
In C. wightii, genetic transformation studies have yet not been initiated due to lack of standardized micropropagation protocols and field trials [60].
6.6. Guggulsterone Production through Cell Culture
In case of an over-exploited species like C. wightii, there is always a limitation of plant in nature for microand macro-propagation and also for its product to meet the demand supply gap. An alternative approach to overcoming this limitation is, production of active compound in cell culture. Several efforts have been made for the production and its enhancement in cell culture. From among 185 species of Commiphora, C. wightii is the guggulsterone producing species, therefore, it becomes essential to develop a high throughput method for production of guggulsterone in vitro [2,87]. According to Ramawat et al. [6] and Suri and Ramawat [88], the cytodifferentiation in callus and cell cultures is primary prerequisite for production of secondary metabolites which are produced in complex tissue systems like laticifers or resin canals. Kumar et al. [89] reported the formation of resin canals but the slow growth of cultures was a limitation. Mathur et al. [90] reported the guggulsterone production in cell cultures without cyto-differentiation. Several elicitors and growth retardants have been used by various workers to enhance production of guggulsterone in cell culture [91-93]. Nitrogen, phosphate, calcium and sugars have been shown to affect the guggulsterone production [90,94]. Kumar et al. [86] reported immobilized cells and callus for guggulsterone production. The studies reveal that further work need to be carried out to enhance the production and at the same time maintain the growth of the cultures which has been observed to be slow or retarded [60,95] during the production. The yield can be increased by manipulation of the biosynthetic pathway through addition of elicitors and precursors [96] which brings out the necessity of study of its pathway.
During the study by our group [65], two types of morphologically distinguishable callus cultures could be identified which were embryogenic and non-embryogenic, respectively. This identification aids in efficient somatic embryogenesis. Additionally it could also be very useful in further studies involving production of guggulsterone in cell culture. Mathur et al. [90] and Tanwar et al. [91] reported the production of guggulsterone in nonembryogenic callus cultures. Mathur et al. [90] observed correlation between guggulsterone content in callus cultures and in vivo in plants. It was found to be maximum during January to July, the period of exudation in nature. The study by our group [65] also indicated that the induction of somatic embryos was best from explants excised from fruits collected during February to June. Such information could be combined together to maximize plantlet and guggulsterone production in nature in C. wightii.
The in vitro propagation and production methods show potential but require further improvements by detailed study of, diversity for selection of germpalsm and biosynthetic pathway.
7. Conclusion
C. wightii is medicinally a very important plant especially, as a source of guggulsterone. Of the 185 species of Commiphora, C. wightii is a species known to produce guggulsterone, the principle active compound of the plant. Therefore, it becomes very important to save this species from the danger of becoming extinct. The plant faces a high risk of endangerment due to various natural and man-made reasons which have been evaluated by various workers. These include slow growth, poor seedsetting and seed germination rate, lack of cultivation, unsustainable over-exploitation, excessive and unscientific tapping method, and invasion of alien species. To replenish the declining population several research institutes and Government agencies are putting in their efforts to conserve this species. Through their study at field and bench-level, researchers have come out with various methods for replenishment of C. wightii population in nature and alternative methods of guggulsterone production through cell culture. Of the existing methods, in vitro plant and guggulsterone production seem to hold maximum potential. In vitro somatic embryogenesis method produces a large number of somatic embryos, each a potential plant which is true to type. The production of guggulsterone in cell culture system is an alternative to extraction from plant gum. These efforts need to be supplemented by studies to improve methods of seed germination, in vitro plant and guggulsterone production by study of biosynthetic pathway, markers for sex determination and means to balance male and female population in nature. Such studies would help to cultivate superior genotypes, take up transgenic studies and conduct breeding experiments for propagation of improved germplasm. Last but not least, the methods for in situ conservation to conserve existing trees in natural habitats are extremely necessary so as to save the natural gene pool from becoming extinct.
8. Acknowledgements
The authors are grateful to IIAR, Puri Foundation; DBT (Department of Biotechnology), New Delhi; and CSIR (Council of Scientific and Industrial and Research), New Delhi, for financial support at various stages for related work.
REFERENCES
- S. Natesh and H. Y. Mohan Ram, “An Update of Green Medicine,” Journal of Indian Botanical Society, Vol. 78, 1999, pp. 13-23.
- D. M. Barve and A. R. Mehta, “Clonal Propagation of Mature Elite Trees of Commiphora wightii,” Plant Cell Tissue and Organ Culture, Vol. 35, No. 3, 1993, pp. 237- 244. doi:10.1007/BF00037276
- C. S. Reddy, S. L. Meena, P. H. Krishna, P. D. Charan and K. C. Sharma, “Conservation Threat Assessment of Commiphora wightii (Arn.) Bhandari—An Economically Important Species,” Taiwania, Vol. 57, No. 3, 2012, pp. 288-293.
- S. Kumar and V. Shanker, “Medicinal Plants of Indian Desert: Commiphora wightii (Arn.) Bhandari,” Journal of Arid Environments, Vol. 5, 1982, pp. 1-11.
- H. Lal and P. K. Kasera, “Status and Distribution Range of Guggal: A Critically Endangered Medicinal Plant from the Indian Thar Desert,” Science & Culture, Vol. 76, No. 11-12, 2010, pp. 531-533.
- K. G. Ramawat, M. Mathur, S. Dass and S. Suthar, “Guggulsterone: A Potent Natural Hypolipidemic Agent from Commiphora wightii—Problems, Perseverance, and Prospects,” In: K. G. Ramawat and J. M. Merillon, Eds., Bioactive Molecules and Medicinal Plants, Springer, Heidelberg, 2008, pp. 101-121. doi:10.1007/978-3-540-74603-4_5
- C. K. Atal, O. P. Gupta and S. H. Abag, “Commiphora mukul: Source of Guggul in Indian Systems of Medicine,” Economic Botany, Vol. 29, No. 3, 1975, pp. 208-218. doi:10.1007/BF02873167
- K. C. Dalal and M. A. Patel, “Guggal,” In: K. L. Chadha and R. Gupta, Eds., Advances in Horticulture, Vol. 11- Medicinal and Aromatic Plants, Malhotra Publishing House, New Delhi, 1995, pp. 491-501.
- B. B. L. Yadava, K. V. Billore, J. G. Joseph and D. D. Chaturvedy, “ Cultivation of GUGGULU,” Central Council in Ayurveda and Siddha (Ayush), New Delhi, 1999, pp. 1-87.
- P. K. Kasera and J. Prakash, “Ecology and Cultivation Practices of Guggul (Commiphora wightii): An Endangered Medicinal Plant of the Thar Desert in India,” In: D. K. Majumadar, J. N. Govil, V. K. Singh and R. K. Sharma, Eds., Recent Progress in Medicinal Plants, Vol. 9— Plant Bioactives in Traditional Medicine, Stadium Press LLC, Houston, 2005, pp. 403-423.
- P. Gupta, K. R. Shivanna and H. Y. Mohan Ram, “Apomixis and Polyembryony in the Guggul Plant, Commiphora wightii,” Annals of Botany, Vol. 78, No. 1, 1996, pp. 67-72. doi:10.1006/anbo.1996.0097
- J. Prakash, P. K. Kasera and D. D. Chawan, “A Report on Polyembryony in Commiphora wightii from Thar Desert, India,” Current Science, Vol. 78, No. 10, 2000, pp. 1185- 1187.
- R. S. Mertia, N. K. Sinha, B. K. Kandpal and D. Singh, “Evaluation of Indian Myrrh (Commiphora wightii) Landraces for Hyper Arid Thar Desert,” Indian Journal of Agricultural Sciences, Vol. 80, No. 10, 2010, pp. 869-871.
- S. D. Sabins and K. S. S. Rao , “Rare and Endangered Endemics of South Eastern Kutch,” Assessment of Threatened Plants of India, Proceedings of Seminar, Dehra Dun, 14-17 September 1983, pp. 71-77.
- B. V. Shetty and V. Singh, “Flora of Rajasthan,” Vol. 1, Botanical Survey of India, Kolkata, 1993, p. 445.
- V. Soni and P. L. Swarnakar, “Conservation Strategies for Commiphora wightii. An Important Medicinal Plant Species,” Medicinal Plant Conservation, Vol. 12, 2006, pp. 40-42.
- B. B. L. Yadava, “Commiphora wightii (Gum-Guggul) Present Status in India: An Overview,” Herbal Tech Industry, Vol. 8, No. 1, 2011, pp. 24-28.
- M. Dixit and S. V. S. Rao, “Observation on Distribution and Habitat Characteristics of Guggul (Commiphora wighti) in the Arid Region of Kachch, Guajarat, India,” Tropical Ecology, Vol. 41, No. 1, 2000, pp. 81-83.
- R. Deng, “Therapeutic Effects of Guggul and Its Constituent Guggulsterone: Cardiovascular Benefits,” Cardiovascular Drug Reviews, Vol. 25, No. 4, 2007, pp. 375- 390.
- R. B. Singh, M. A. Niaz and S. Ghosh, “Hypolipidemic and Antioxidant Effects of Commiphora mukul as an Adjunct to Dietary Therapy in Patients with Hypercholesterolemia,” Cardiovascular Drugs and Therapy, Vol. 8, No. 4, 1994, pp. 659-664. doi:10.1007/BF00877420
- V. Singh and R. P. Pandey, “Biodiversity of Desert National Park, Rajasthan,” Botanical Survey of India, Kolkata, 2006, p. 344.
- S. N. Tripathi, V. V. S. Sastri and G. V. Satyavati, “Experimental and Clinical Studies on the Effects of Guggul (C. mukul) in Hyperlipidemia and Thrombosis,” Journal of Research in Indian Medicine, Vol. 2, No. 2, 1968, p. 10.
- G. V. Satyavati, C. Dwarkanath and S. N. Ttripathi, “Experimental Studies on the Hypocholesterolemic Effect of Commiphora mukul Engl. (Guggul),” Indian Journal of Medical Research, Vol. 57, No. 10, 1969, pp. 1950-1962.
- A. D. Bhatt, D. G. Dalal, S. J. Shah, B. A. Joshi, M. N. Gajjar, R. A. Vaidya, A. B. Vaidya and D. S. Antarkar, “Conceptual and Methodologic Challenges of Assessing the Short-Term Efficacy of Guggulu in Obesity: Data Emergent from a Naturalistic Clinical Trial,” Journal of Postgraduate Medicine, Vol. 41, No. 1, 1995, pp. 5-7.
- N. L. Urizar and D. D. Moore, “GUGULIPID: A Natural Cholesterol-Lowering Agent,” Annual Review of Nutrition, Vol. 23, 2003, pp. 303-313. doi:10.1146/annurev.nutr.23.011702.073102
- P. K. Kasera, J. Prakash and D. D. Chawan, “Effects of Different Seed Sowing Methods on Seedling Emergence in Commiphora wightii, an Endangered Medicinal Plant,” Annals of Forestry, Vol. 10, No. 1, 2002, pp. 176-178.
- G. N. Chaturvedi and R. H. Singh, “Experimental Studies on Anti-arthritic Effect of Certain Indigenous Drugs,” Indian Journal of Medical Research, Vol. 53, No. 1, 1965, pp. 71-80.
- J. N. Sharma and J. N. Sharma, “Comparison of the Anti Inflammatory Activity of Commiphora mukul (an Indigenous Drug) with those of Phenylbutazone and Ibuprofen in Experimental Arthritis Induced by Mycobacterial Adjuvant,” Arzneimittel-Forschung, Vol. 27, No. 7, 1977, pp. 1455-1457.
- K. B. Ishnava, Y. N. Mahida and J. S. S. Mohan, “In Vitro Assessments of Antibacterial Potential of Commiphora wightii (Arn.) Bhandari Gum Extract,” Journal of Pharmacognosy and Phytotherapy, Vol. 2, No. 7, 2010, pp. 91-96.
- M. Gupta, S. N. Tripathi and D. Prasad, “Effect of Extract of Gum Guggulu on Estrogen Induced Hyerlipidaemia in Chicks,” Journal of Research in Indian Medicine, Vol. 9, No. 2, 1974, pp. 4-11.
- I. Kimura, M. Yoshikawa, S. Kobayashi, Y. Sugihara, M. Suzuki, H. Oominami, T. Murakami, H. Matsuda and V. V. Doiphode, “New Triterpenes, Myrrhanol A and Myrrhanone A, from Guggul-Gum Resins, and their Potent Anti-Inflammatory Effect on Adjuvant-Induced Air-Pouch Granuloma of Mice,” Bioorganic and Medicinal Chemistry Letters, Vol. 11, No. 8, 2001, pp. 985-989. doi:10.1016/S0960-894X(01)00111-1
- D. Xiao and S. V. Singh, “Z-Guggulsterone, a Constituent of Ayurvedic Medicnal Plant Commiphora mukul Inhibits Angiogenesis in Vitro and in Vivo,” Molecular Cancer Therapeutics, Vol. 7, No. 1, 2008, pp. 171-180. doi:10.1158/1535-7163.MCT-07-0491
- R. K. Sinha and S. Sinha, “Ethnobiolgy (Role of Indigenous and Ethnic Societies in Biodiversity Conservation, Human Health Protection and Sustainable Development),” Surabhi Publications, Jaipur, 2001, p. 335.
- D. M. Thappa and J. Dogra, “Nodulocystic Acne: Oral Gugulipid versus Tetracycline,” Journal of Dermatology, Vol. 21, No. 10, 1994, pp. 729-31.
- R. Raghunathan and R. Mitra, “Pharmacognosy of Indigenous Drugs,” Vol. 1, The Central Council for Research in Ayurvedic Sciences, New Delhi, 1999, pp. 354- 375.
- P. O. Szapary, M. L. Wolfe, L. T. Bloedon, A J. Cucchiara, A H. DerMarderosian, M. D. Cirigliano and D. J. Rader, “Guggulipid for the Treatment of Hypercholesterolemia: A Randomized Controlled Trial,” The Journal of the American Medical Association, Vol. 290, No. 6, 2003, pp. 765-772. doi:10.1001/jama.290.6.765
- A. Nohr, B. Rasmussenb and J. Straandc, “Resin from the Mukul Myrrh Tree, Guggul, Can It Be Used for Treating Hypercholestrolaemia? A Randomized Controlled Study,” Complementary Therapies in Medicine, Vol. 17, No. 1, 2009, pp. 16-22. doi:10.1016/j.ctim.2008.07.001
- M. S. Bagul, H. Srinivasa, N. S. Kanaki and M. Rajani, “Anti-Inflammatory Activity of Two Ayurvedic Formulations Containing Guggul,” Indian Journal of Pharmacology, Vol. 37, No. 6, 2005, pp. 399-400. doi:10.4103/0253-7613.19080
- V. N. Sumantran, A. A. Kulkarni, A. Harsulkar, A. Wele, S. J. Koppikar, R. Chandwaskar, V. Gaire, M. Dalvi and U. V. Wagh, “Hyaluronidase and Collagenase Inhibitory Activities of the Herbal Formulation Triphala Guggulu,” Journal of Biosciences, Vol. 32, No. 4, 2007, pp. 755-761. doi:10.1007/s12038-007-0075-3
- A. T. Dutt, S. Ghosh and R. N. Chopra, “Chemical Investigation of Gum Resin from Blasmodendron mukul,” Indian Journal of Medical Research, Vol. 30, No. 2, 1942, pp. 331-334.
- Anonymous, “The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products,” Vol. II CSIR, New Delhi, 1950, p. 427.
- V. D. Patil, U. R. Nayak and Sukh Dev, “Chemistry of Ayurvedic Crude Drugs—I: Guggulu (Resin from Commiphora mukul)—1: Steroidal Constituents,” Tetrahedron, Vol. 28, No. 8, 1972, pp. 2341-2352. doi:10.1016/S0040-4020(01)93577-X
- S. Bose and K. C. Gupta, “Structure of C. mukul -Part II. Structure of the Degraded Gum,” Indian Journal of Chemistry, Vol. 2, 1964, pp. 156-158.
- M. O. Fatope, S. K. S. Al-Burtomani, J, Ochei, A. O. Abdulnur, M. Z. Al-Kindy and Y. Takeda, “Muscanone: A 3-O-(1'',8",14"-Trimet Hylhexadecanyl)Naringenin from Commiphora wightii,” Phytochemistry, Vol. 62, No. 8, 2003, pp. 1251-1255. doi:10.1016/S0031-9422(02)00686-6
- V. D. Patil, U. R. Nayak and S. Dev, “Chemistry of Ayurvedic Crude Drugs—III: Guggulu (Resin from Commiphora mukul)-3 Long-Chain Aliphatic Tetrols, a New Class of Naturally Occurring Lipids,” Tetrahedron, Vol. 29, No. 11, 1973, pp. 1595-1598. doi:10.1016/S0040-4020(01)83402-5
- K. K. Purushothaman and S. Chandrasekaran, “Guggulsterols from Commiphora wightii (Burseraceae),” Indian Journal of Chemistry, Vol. 14B, No. 10, 1976, pp. 802- 804.
- A. G. Bajaj and S. Dev, “Chemistry of Ayurvedic Crude Drugs,” Tetrahedron, Vol. 38, No. 14, 1982, pp. 2049- 2054.
- N. Verma, S. K. Singh and R. C. Gupta, “Simultaneous Determination of the Stereoisomers of Guggulsterome in Serum by High Performance Liquid Chromatography,” Journal of Chromatography, Vol. 708, No. 1-2, 1998, pp. 243-248.
- A. Jain and V. B. Gupta, “Chemistry and Pharmacological Profile of Guggul-A Review,” Indian Journal of Traditional Knowledge, Vol. 5, No. 4, 2006, pp. 478-483.
- B. Mesorb, M. R. Nesbitt and C. R. Pandey, “High Performance Liquid Chromatographic Method for Fingerprinting and Quantitative Determination of Eand ZGuggulsterones in Commiphora wightii,” Journal of Chromatography, Vol. 720, No. 1-2, 1998, pp. 189-196.
- UNDP, “Rajasthan Red Listed Medicinal Plants,” 2008, pp. 22-23. http://www.frlht.org
- http://www.iucnredlist.org/details/31231/0
- K. V. Billore, “Some Threatened Medicinal Plants of Rajasthan and Their Conservation,” The Indian Forester, Vol. 115, No. 8, 1989, pp. 595-599.
- P. J. Parmar, “Loss of Commiphora wightii (Arn.) Bhandari in Indian Desert,” Bulletin of Botanical Survey of India, Vol. 45, 2003, pp. 77-90.
- J. R. Bhatt, M N. B. Nair and H. Y. Mohan Ram, “Enhancement of Oleo Gum Resin Production in Commiphora wightii by Improved Tapping Technique,” Current Science, Vol. 58, No. 7, 1989, pp. 349-357.
- S. Kshetrapal and R. Sharma, “Studies on the Effect of Various Plant Extracts on the Sprouting Behaviour of the Cuttings of Commiphora wightii (Arn.) Bhandari and C. agallocha Engl.,” Journal of Indian Botanical Society, Vol. 72, 1993, pp. 73-75.
- S. Sriram and K. C. Dalal, “Studies on Floral Biology in Commiphora wightii,” Ninth Workshop of All India Coordinated Research Project on Medicinal and Aromatic Plants (Tribal Data Book), 1991, pp. 393-397.
- S. Kumar, S. S. Suri, K. C. Sonie and K. G. Ramawat, “Establishment of Embryonic Cultures and Somatic Embryogenesis in Callus Culture of Guggul-Commiphora wightii (Arn.) Bhandari,” Indian Journal of Experimental Biology, Vol. 4, No. 2003, pp. 69-77.
- P. Gupta, K. R. Shivanna and H. Y. Mohan Ram, “Pollen-Pistil Interaction in a Non-Pseudogamous Apomict, Commiphora,” Annals of Botany, Vol. 81, No. 5, 1998, pp. 589-594. doi:10.1006/anbo.1998.0598
- [61] A. Kulhari, A. Sheorayan, S. Kalia, A. Chaudhury and R. K. Kalia, “Problems, Progress and Future Prospects of Improvement of Commiphora wightii (Arn.) Bhandari, an Endangered Herbal Magic, through Modern Biotechnological Tools: A Review,” Genetic Resources and Crop Evolution, Vol. 59, No. 6, 2012, pp. 1223-1254. doi:10.1007/s10722-012-9854-2
- [62] P. K. Kasera, J. Prakash and J. K. Shukla, “Conservation of Commiphora wightii and Leptadaenia reticulata—Two Important Endangered Medicinal Plants of Indian Desert” In: B. R. Pandit, Ed., Emerging Areas in Plant Sciences, Department of Life Sciences, Bhavnagar University, Bhavnagar, 2001, pp. 36-40.
- [63] Sharma, G. P. and A. S. Raghubanshi, “Effect of Lantana camara L. Cover on Local Depletion of Tree Population in the Vindhyan Tropical Dry Deciduous Forest of India,” Applied Ecology and Environmental Research, Vol. 5, No. 1, 2007, pp. 109-121.
- [64] I. Haque, R. Bandopadhyay and K. Mukhopadhyay, “Intraspecific Variation in Commiphora wightii Populations Based on Internal Transcribed Spacer (ITS-5.8 S-ITS2) Sequence of r-DNA,” Diversity, Vol. 1, No. 2, 2009, pp. 89-101.
- [65] N. K. Sinha, R. S. Mertia, B. K. Kandpal, R. N. Kumawat, P. Santra and D. Singh, “Morphological Characterization of Guggal (Commiphora wightii) Provenances from Extremely Arid Parts of India,” Forests, Trees and Livelihoods, Vol. 1, No. 1, 2012, pp. 63-69. doi:10.1080/14728028.2012.669579
- [66] S. Kumar, N. Jain and R. Nadgauda, “An Explant Dependent, High Frequency, Repetitive Somatic Embryogenesis and Plant Regeneration in Commiphora wightii,” Journal of Tropical Medicinal Plants, Vol. 10, No. 2, 2009, pp. 195-207.
- [67] “Education and Awareness in the Save Guggul Movement,” IUCN News. 31 July 2010. http://iucn.org/news_homepage/news_by_date/?5797/cecsave-guggul-movement
- [68] V. Soni, “Conservation of Commiphora wightii (Arn.) Bhandari: An Endangered Medicinal Shrub, through Propagation and Planting, and Education Awareness Programs in the Aravaalli Hills of Rajasthan, India,” Conservation Evidence, Vol. 7, 2010, pp. 27-31.
- [69] K. C. Dalal, M. A. Patel, N. V. Upadhyay, D. H. Patel, R. B. Patel, B. V. Hirpara, C. Patel, P. B. Patel and R. Gupta, “Germplasm Collection and Therapeutic, Botanical, Agricultural, Chemical, and Clinical Aspects of the Endangered Indigenous Plant, Guggul Commiphora wightii (Arn.) Bhandari-a Status cum Review Report, In: Presentation of Tribal Data: Eighth Workshop of All India Coordinated Research Project on Medicinal and Aromatic Plants,” Faizabad, 1989, pp. 67-79.
- [70] P. K. Kasera and D. D. Chawan, “Cultivation and Evaluation of Medicinal Plants under Desert Conditions of North-West India,” Final Technical Progress Report, ISMH Project, J. N. Vyas University, Jodhpur, 2001, pp. 65.
- [71] I. I. Chaudhari, “Scope of Plant Introduction in Agriculture and Forestry of West Pakistan,” The Pakistan Journal of Forestry, Vol. 9, 1959, pp. 208-211.
- [72] H. Lal and P. K. Kasera, “Seed Germination Improvement in Commiphora wightii (Guggal) Through Potassium Nitrate Pre-treatment-a Critically Endangered Plant from the Indian Desert,” International Journal of Plant Science, Vol. 3, No. 8, 2012, pp. 174-180.
- [73] R. R. Shah, D. B. Patel, D. H. Patel and K. C. Dalal, “Hormonal Effect on Germination of Guggal Cuttings,” Indian Drugs, Vol. 20, No. 11, 1983, pp. 435-437.
- [74] P. Singh, M. L. Sharma and S. Mukherjee, “Effect of Indole Butyric Acid on Sprouting in Plant Cuttings in Commiphora wightii (Arnott) Bhandari,” Indian Drugs, Vol. 26, 1989, pp. 515-516.
- [75] R. S. Mertia and M. Nagarajan, “Successful Rooting in Cuttings of Commiphora wightii (Arnott) Bhandari,” Annals of Arid Zone, Vol. 39, No. 1, 2000, pp. 87-88.
- [76] J. Prakash, P. K. Kasera and D. D. Chawan, “Multiplication of Commiphora wightii through an Air-Layering Technique in Indian Desert,” Science and Culture, Vol. 67, No. 1-2, 2001, pp. 65-67.
- [77] C. V. S. Gunnatilleke and I. A. U. N. Gunnatilleke, “Phytosociology of Sinharaja—A Contribution to Rain Forest Conservation in Sri Lanka,” Biological Conservation, Vol. 31, No. 1, 1985, pp. 21-40. doi:10.1016/0006-3207(85)90032-1
- [78] B. I. Kalpesh and J. S. S. Mohan, “Intraspecific Isozyme Variation in Commiphora wightii (Arn.) Bhandari: A Traditional Hypocholestrolaemic Medicinal Shrub from Gujarat, India,” Journal of Herb, Spices and Medicinal Plants, Vol. 13, No. 2, 2008, pp. 25-40. doi:10.1300/J044v13n02_03
- [79] I. Haque, R. Bandopadhyay and K. Mukhopadhyay, “Population Structure of the Endangered and Endemic Medicinal Plant Commiphora wightii,” Molecular Biology Reporter, Vol. 37, No. 2, 2009, pp. 847-854.
- [80] S. Samantaray, K. A. Geetha, K. P. Hidayath and S. Maiti, “Identification of RAPD Markers Linked to Sex Determination in Guggal [Commiphora wightii (Arnott.)] Bhandari,” Plant Biotechnology Reports, Vol. 4, No. 1, 2010, pp. 95-99. doi:10.1007/s11816-009-0113-8
- [81] V. Soni, “Efficacy of in Vitro Tissue Culture versus Stem Cuttings for Propagation of Commiphora wightii in Rajasthan, India,” Conservation Evidence, Vol. 7, 2010, pp. 91-93.
- [82] A. Yusuf, T. S. Rathore and N. S. Shekhawat, “Micropropagation of Commiphora wightii (Arn.) Bhandari-a Threatened Medicinal Plant of Semi-Arid Region,” Indian Journal of Plant Genetics Research, Vol. 12, No. 3, 1999, pp. 371-375.
- [83] T. Kant, S. Prajapati and A. K. Parmar, “Efficient Micropropagation from Cotyledonary Node Cultures of Commiphora wightii (Arn.) Bhandari, an Endangered Medicinally Important Desert Plant,” Journal of Plant Development, Vol. 17, No. 1, 2010, pp. 37-48.
- [84] N. Singh, A. Garg, K. Yadav and S. Kumari, “Influence of Growth Regulators on the Explants of Commiphora mukul (Hook ex Stocks) Engl. Under in Vitro Conditions,” Researcher, Vol. 2, No. 7, 2010, pp.41-48.
- [85] A. K. Parmar and T. Kant, “Efficient Micropropagation and Evaluation of Genetic Fidelity of in Vitro Raised Plants of commiphora wightii (Arn.) Bhandari—A Medicinally Important red Listed Species of Arid Regions,” Journal of Plant Development, Vol. 19, No. 1, 2012, pp. 29-40.
- [86] A. Jain, G. R. Rout and S. N. Raina, “Somatic Embryogenesis and Plant Regeneration from Callus Culture of Phlox paniculata Linn.,” Scientia Hoticulturae, Vol. 94, No. 1-2, 2002, pp. 137-143. doi:10.1016/S0304-4238(01)00369-7
- [87] S. Kumar, M. Mathur, A. K. Jain and K. G. Ramawat, “Somatic Embryo Proliferation in Commiphora wightii and Evidence for Guggulsterone Production in Culture,” Indian Journal of Biotechnology, Vol. 5, No. 2, 2006, pp. 217-222.
- [88] M. Mathur, A. K. Jain, S. S. Dass and K. G. Ramawat, “Guggulsterone Production in Cell Suspension Culture in Commiphora wightii Grown in Shake Flask and Bioreactors,” Biotechnology Letters, Vol. 29, No. 6, 2007, pp. 979-982. doi:10.1007/s10529-007-9342-5
- [89] S. S. Suri and K. G. Ramawat, “Effect of Calotropis Latex on Laticifers Differentiation of Calotropis procera,” Biologia Plantarum, Vol. 38, No. 2, 1996, pp. 185-190. doi:10.1007/BF02873844
- [90] S. Kumar, S. S. Suri, K. C. Sonie and K. G. Ramawat, “Development of Resin Canals during Somatic Embryogenesis in Callus Cultures of Commiphora wightii (Arn.) Bhandari,” Indian Journal of Biotechology, Vol. 3, No. 2, 2004, pp. 267-270.
- [91] M. Mathur, A. K. Jain, S. S. Dass and K. G. Ramawat, “Optimization of Guggulsterone Production in Callus Culture of Commiphora wightii (Arn.) Bhandari,” Indian Journal of Biotechnology, Vol. 6, No. 4, 2007, pp. 525- 531.
- [92] Y. S. Tanwar, M. Mathur and K. G. Ramawat, “Morphactin Influence Guggulsterone Production in Callus Culture of Commiphora Wightii,” Plant Growth Regulators, Vol. 51, No. 1, 2007, pp. 93-98. doi:10.1007/s10725-006-9151-1
- [93] S. Suthar, S. Thul, A. K. Kukreja and K. G. Ramawat, “RAPD Markers Reveal Polymorphism in Commiphora wightii, an Endangered Medicinal Tree,” Journal of Tissue Culture Research, Vol. 8, No. 2, 2008, pp. 1477- 1480.
- [94] S. S. Dass and K. G. Ramawat, “Elicitation of Guggulsterone Production in Cell Cultures of Commiphora wightii by Plant Gums,” Plant Cell Tissue and Organ Culture, Vol. 96, No. 3, 2009, pp. 349-353. doi:10.1007/s11240-008-9493-7
- [95] S. S. Dass and K. G. Ramawat, “Calcium Deprivation Greatly Enhances Guggulsterone Accumulation in Cell Cultures of Commiphora wightii,” Current Science, Vol. 96, No. 2009, pp. 1022-1024.
- [96] S. S. Dass Y. S. Tanwar and K. G. Ramawat, “Commiphora wightii Callus Cultures—A New Source of Anthocyanin,” Journal of Herb and Medicinal Toxicology, Vol. 2, No. 1, 2008, pp.17-20.
- [97] S. Shisodia, B. Kuzhuvelil, D. S. Harikumar, K. G. Ramawat and B. B. Agarwal, “The Guggul for chronic Diseases: Ancient Medicine, Modern Targets,” Anticancer Research, Vol. 2, No. 6A, 2008, pp. 3647-3664.
NOTES
*Corresponding author.

