American Journal of Plant Sciences
Vol. 4 No. 5A (2013) , Article ID: 32255 , 5 pages DOI:10.4236/ajps.2013.45A009
Differences of Wood Elements of Prosopis laevigata from Two Areas of Northeast Mexico
![]()
1Facultad de Ciencias Forestales, Universidad Untónoma de Nuevo León, Linares, México; 2Facultad de Ciencias Biológicas, Universidad Uutónoma de Nuevo León, San Nicolás de los Garza, México; 3Instituto de Silvicultura e Industria de la Madera, Universidad Juárez del Estado de Durango, Durango, México; 4Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Instituto Politécnico Nacional, Oaxaca de Juárez, México.
Email: *artemio.carrillopr@uanl.edu.mx
Copyright © 2013 Artemio Carrillo-Parra et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 13th, 2013; revised April 15th, 2013; accepted May 1st, 2013
Keywords: Wood Anatomy; Prosopis laevigata; Vessels; Fiber
ABSTRACT
Anatomical features of wood have a great variation among species as a result of genetic and environmental factors. The anatomical heartwood characteristics of Prosopis laevigata species from two areas with differences on temperature and rain precipitation on northeast Mexico were compared. Fibers length (µm), diameter of vessels (µm), and the area of the vessels (µm−2) were measured using light microscopy coupled with a digitized-image analysis system. The differences were statistically analyzed with analysis of variance. Statistical differences between fibers length (p < 0.0001) and diameter of the vessels (p < 0.001) from the two localities were found. The locality Linares, Nuevo León, Mexico, with higher precipitation and lower temperature, showed higher fiber length and higher diameter of the vessels than China, Nuevo León. Hard environmental conditions, where low precipitation values and high temperatures prevail, condition P. laevigata trees do reduce the risk of losing water.
1. Introduction
Physical, chemical, mechanical, and anatomical features of wood have a great variation among species, trees, and even between samples from the same tree as result of genetic and environmental factors such as temperature, light intensity, wind, frost, photoperiod, soil fertility, and water availability [1,2]. Studies on the relationship between environmental conditions to wood characteristics are mainly focused on wood density, pore distribution, vessel diameter, vessel lumen, and pits [3-5]. Light-demanding species grow faster than shade-tolerant species producing low-density wood. A fast development of stem produced thinner cell wall and wider lumen that slow growing species. Ring-porous species tolerate lower degrees of drought stress than diffuse-porous species [6].
The vessels characteristics as the number of vessels, diameter, length, density, distribution and thickness are affected by some climatic factors such as temperature, water availability as well as latitude, altitude, and differences in habitats [7-9]. Vessels also show forming groups in dry environment conditions. On the other hand, in humid environments they are only solitary and rarely grouped [10].
Few reports showing the relation between cell length and climate have been developed. Reference [11] did not find relationship between climatic conditions and tracheid length. According to [12] temperature plays the major role in regulating cambial activity and phenologic events in most plants, while the availability of water in dry conditions is the limiting factor.
The study of Prosopis wood anatomy and its relationship with environmental conditions is a good opportunity to increase the knowledge on effect of weather condition to wood because it comprises about 44 species of trees and shrubs grown naturally in arid and semi-arid zones of the world [13-16]. Wood anatomical characteristics of Prosopis species have been described by [16-23] and some differences in pore distribution, fiber length, vessel diameter, and lumber area have been found because of multiple environmental conditions where the species grow. The main objective of the present study is to determine the anatomical characteristics of wood from P. laevigata that grow in two different environmental conditions in Northeast Mexico.
2. Materials and Methods
2.1. Description of the Study Area
The study was developed in Northeast Mexico, the climate is classified as semi-dry to moderate warm, the average annual temperature from the whole area ranges from 14.7˚C to 22.0˚C, the average annual precipitation is from 400 to 812 mm and potential evapotranspiration is estimated on 1150 mm, the rain season is in the summer, but it is interrupted by a dry period [24,25]. The native vegetation is classified as Tamaulipan thornscrub [26]. The origin of rocky type soils are Upper Cretaceous, rich in calcite and dolomite, the dominant soils are deep, dark grey, lime-clay vertisol results from alluvial and colluvial processes, high clay and calcium carbonate content, a pH from 7.0 to 8.0, and low organic matter content.
Figure 1 shows the two study areas selected. The selection was done according to areas with higher differential in rain precipitation and temperature in the Northeast distribution of P. laevigata species. Table 1 shows the climatic conditions of Rancho Saltilleros municipality of China and La Reforma municipality of Linares, both in Nuevo León, Mexico.
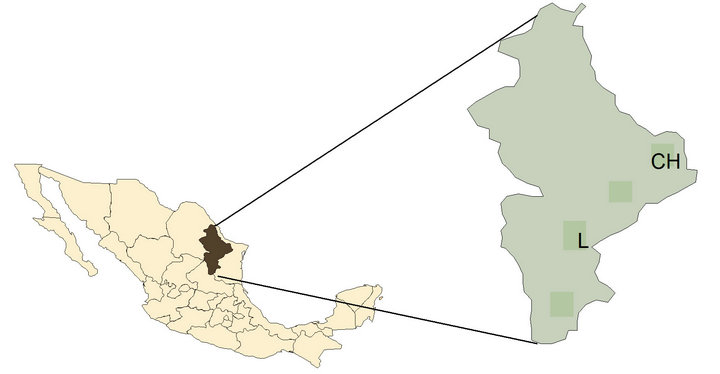
Figure 1. Localization of sampling areas of P. laevigata wood in northeast Mexico. CH, local area Rancho Saltilleros municipality of China. L, local area Ejido la Reforma municipality of Linares.
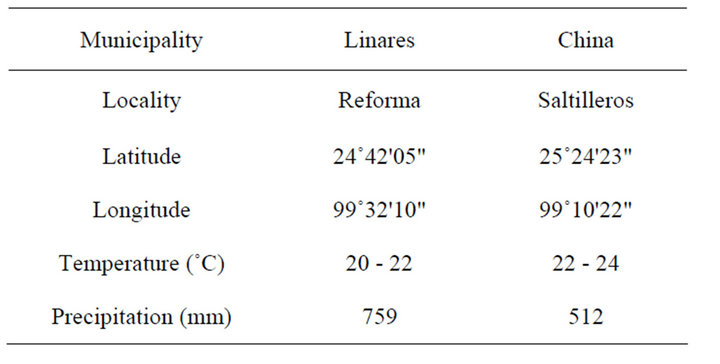
Table 1. Climatic condition of the two localities selected in Northeast Mexico.
2.2. Sampling Trees for Anatomical Description
Three trees, free of knots, cracks, gum, visually damaged by insects with a diameter at breast height (DBH) from 0.3 to 0.4 m, were selected from natural forest stand in each locality. One disk of 0.1 m thick at the height of DBH was sawn from each tree. In order to use mature wood from each tree, the outer section of heartwood in each disk was used to prepare wooden samples of 2 × 2 × 2 cm for anatomical study.
The samples were softened during one hour in boiling water. Twenty µm thickness sections were obtained from cross, radial, and tangential directions. The length of the fiber was determined using wooden parts from the same heartwood section with toothpicks characteristics then, they were macerated with the solution of nitric acid and chromic acid diluted. They were placed for 10 minutes at 60˚C. The measurements for anatomic structures were developed with a Nikon Eclipse E600 microscope equipped with the Dxm 1200 digital camera and computer Lucia (Lucia image version 4.82). The data generated by the program were exported to a Microsoft Excel spreadsheet.
The characterization of wood was carried out based on the list of the International Association of Wood Anatomists (IAWA), which determines the anatomical characteristics for the hardwood species. Average and standard deviation of the length of the fiber, diameter of the vessel (µm), vessel area (µm−2) as well as the number of vessels per square mm was calculated. Differences between wood anatomical characteristics were determined using analysis of variance regarding to locality (2) and sample tree (3).
3. Results and Discussions
3.1. Length of the Fiber
The average length of P. laevigata fibers from both localities was 382 µm (1312 - 49 µm maximum, minimum). The average fiber length from Linares locality was 462 µm (1312 - 49 µm maximum, minimum); the length was higher than China’s samples that showed an average of 232 µm (1144 - 52 µm maximum, minimum). According to Table 2, there were highly significant statistical differences (p < 0.0001) on fiber length between localities, trees, as well as the interaction between localities and trees.
The fiber length of both localities was distributed in seven length classes from 200 to 1400 µm (Figure 2). China locality with low precipitation values and high temperature, presented high frequency values for the smallest length classes (200 and 400 µm). In contrast, Linares locality with high precipitation values and low temperature presented the highest frequencies values on the higher length classes (600 up to 1400 µm).
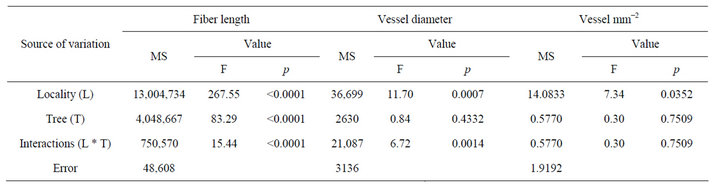
Table 2. Analysis of variance of fiber length, vessel diameter and number of vessel per square millimeter of P. laevigata wood form two localities of Northeast Mexico.
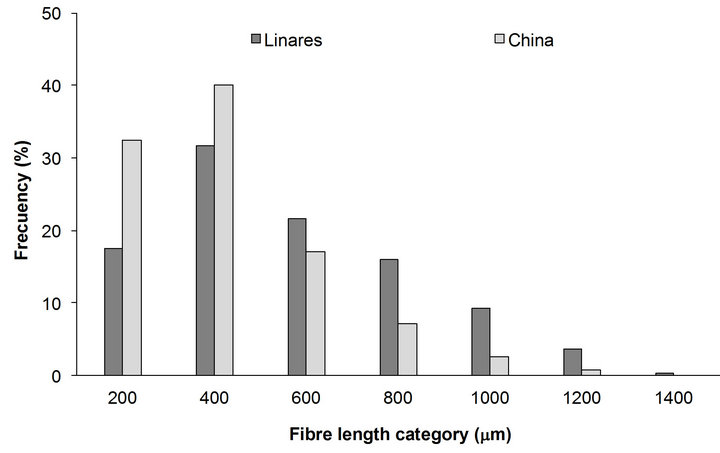
Figure 2. Fiber length distribution of P. laevigata wood from two localities of Northeast Mexico.
Define abbreviations and acronyms the first time they are used in the text, even after they have been defined in the abstract. Abbreviations such as IEEE, SI, MKS, CGS, sc, dc, and rms do not have to be defined. Do not use abbreviations in the title or heads unless they are unavoidable.
3.2. Vessels
Prosopis laevigata is semi-porous or diffuse-porous species; the vessels distribution does not present any characteristic pattern. Most of the vessels are mainly in groups of two, three and four vessels. The average vessels diameter of P. laevigata from both localities was 92 µm (420 - 8 µm maximum, minimum). Linares samples presented 103 µm (420 - 8 µm maximum, minimum); the value was higher than China vessels with an average of 81 µm (241 - 7 µm maximum, minimum). According to Table 2, there were significant statistical differences (p < 0.001) on fiber length between localities, there were no statistical differences between trees (p > 0.1). Interactions between localities * trees showed statistical differences (p < 0.01).
Vessel, diameter of P. laevigata from both localities was distributed in 14 length classes from 20 to 300 µm (Figure 3). Vessels are adaptive elements developed to enhance water transport especially in species that grow on water stress. China locality with low precipitation values and high temperature presented 81% of the vessels in the smallestest length classes (20, 40 and 60 µm). Linares locality with high precipitation values and low temperature presented 77% of the vessels on higher length classes (40 up to 140 µm).
Vessel area for Linares was 11,435 µm−2 and 7168 µm−2 for China vessels, [27] found a positive correlation between the number and total area of vessels with the precipitation on Prosopis flexuosa. A positive influence of precipitation was also found for vessel density on Quercus ilex [28].
3.3. Number of Vessels
The The average number of vessels of P. laevigata from both localities was 9.3 vessels mm−2 (12.4 - 7.2 vessels mm−2, maximum, minimum). The average number of vessels from Linares locality was 9.3 vessels mm−2 (12.5 - 7.5 vessels mm−2, maximum, minimum); the number of vessels was higher than China’s samples that showed an average of 8.0 vessels mm−2 (10.2 - 6.8 vessels mm−2 maximum, minimum).
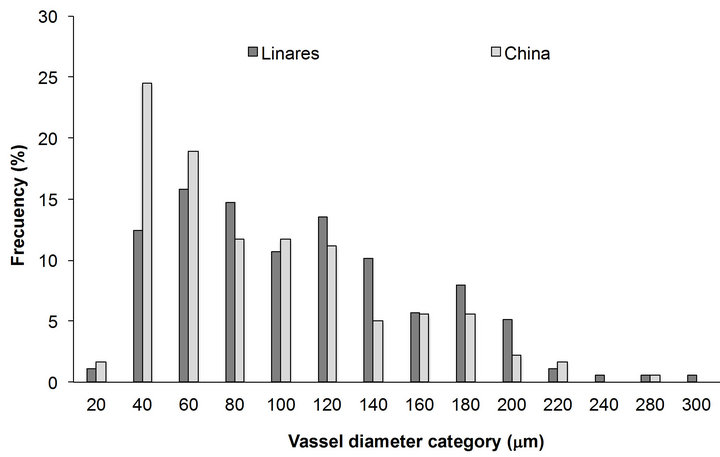
Figure 3. Vessel diameter classes of P. laevigata wood form two localities of Northeast Mexico.
4. Conclusion
After the text edit has been completed, the paper is ready for the template. Duplicate the template file by using the save as command, and use the naming convention prescribed by your journal for the name of your paper. In this newly created file, highlight all of the contents and import your prepared text file. You are now ready to style your paper.
REFERENCES
- L. Poorter, L. Bongers and F. Bongers, “Architecture of 54 Moist-Forest Tree Species: Traits, Trade-Offs, and Functional Groups,” Ecology, Vol. 87, No. 5, 2006, pp. 1289-1301. doi:10.1890/0012-9658(2006)87[1289:AOMTST]2.0.CO;2
- T. J. Wodzicki, “Natural Factors Affecting Wood Structure,” Wood Science and Technology, Vol. 35, No. 1-2, 2001, pp. 5-26. doi:10.1007/s002260100085
- L. Poorter, I. McDonald, A. Alarcón, E. Fichtler, J. Licona, M. Peña-Claros, et al., “The Importance of Wood Traits and Hydraulic Conductance for the Performance and Life History Strategies of 42 Rainforest Tree Species,” New phytologist, Vol. 185, No. 2, 2010, pp. 481- 492. doi:10.1111/j.1469-8137.2009.03092.x
- A. E. Zanne, M. Westoby, D. S. Falster, D. D. Ackerly, S. R. Loarie, S. E. J. Arnold, et al., “Angiopsperm Wood Structure: Global Patterns in Vessel Anatomy and Their Relation to Wood Density and Potential Conductivity,” American Journal of Botany, Vol. 97, No. 2, 2010, pp. 207-215. doi:10.3732/ajb.0900178
- N. J. B. Kraft, M. R. Metz, R. S. Condit and J. Chave, “The Relationship between Wood Density and Mortality in a Global Tropical Forest Data Set,” Vol. 188, No. 4, New Phytologist, 2010, pp. 1124-1136. doi:10.1111/j.1469-8137.2010.03444.x
- J. Chirkova, I. Andersone, I. Irbe, B. Spince and B. Andersons, “Lignins as Agents for Bio-Protection of Wood,” Holzforschung, Vol. 65, 2011, pp. 497-502. doi:10.1515/hf.2011.092
- P. Bass, “Systematic, Phylogenetic and Ecological Wood Anatomy-History and Perspectives,” In: P. Bass, Ed., New Perspectives in Wood Anatomy, Martinus Nijhoff, Boston, 1982, pp. 23-58.
- N. A. V. D. Graaff and P. Baas, “Wood Anatomical Variation in Relation to Latitude and Altitude,” Blumea, Vol. 22, No. 1, 1974, pp. 101-121. doi:10.1007/978-94-017-2418-0_2
- S. Carlquist, “Comparative Wood Anatomy,” SpringerVerlag, Berlin, 1988, p. 457.
- J. Barajas-Morales, “Wood Structural Differences between Trees of Two Tropical Forests in Mexico,” International Aviation Womens Association, Vol. 6, No. 4, 1985, pp. 355-364.
- R. Wimmer and M. Grabner, “A Comparison of TreeRing Features in Picea abies as Correlated with Climate,” International Association of Wood Anatomists Journal, Vol. 21, No. 4, 2000, pp. 403-416. doi:10.1163/22941932-90000256
- A. Fahn and E. Werker, “The Vascular Cambium,” Taunton, 1990.
- P. E. Villagra, R. Villalba and J. A. Boninsegna, “Dendrocronología de los Algarrobales de la Zona Árida Argentian,” SECEDOC, 2002, pp. 53-57.
- A. Burkart, “A Monograph of the Genus Prosopis (Leguminosae subfam. Mimosoideae),” Journal of Arnold Arboretum, Vol. 57, 1976, pp. 219-249, 450-525.
- USDA, ARS, National Genetic Resources Program, Germplasm Resources Information Network—(GRIN) National Germplasm Resources Laboratory, Beltsville, Maryland. http://www.ars-grin.gov/cgi-bin/npgs/html/taxgenform.pl?language=en
- R. Villalba, “Xilem Structure and Cambial Activity in Prosopis flexuosa D.C.,” IAWA Bulletin, Vol. 6, No. 2, 1985, pp. 119-130.
- M. Iqbal and A. K. M. Ghouse, “An Analytical Study on Cell Size Variation in Some Arid Zone Trees of India: Acacia nilotica and Prosopis spicigera,” IAWA Bulletin, Vol. 4, No. 1, 1983, pp. 46-52.
- M. A. Castro, “Maderas Argentinas de Prosopis,” Secretaría General de la Presidencia de la Nación, Atlas Anató- mico, Buenos Aires, 1994.
- P. Villagra, “Wood Structure of Prosopis alpataco and P. argentina Growing under Different Edaphic Conditions,” IAWA Journal, Vol. 18, No. 1, 1997, pp. 37-51.
- H. G. Richter, “Commercial Timbers: Descriptions, Illustrations, Identification and Information Retrieval in English, French, German, and Spanish,” 2000. http://www.biologie.uni-hamburg.de/b-online/wood/english/
- B. López, “Wood Anatomy, Description of Annual Rings, and Responses to ENSO Events of Prosopis pallida H.B.K., a Wide-Spread Woody Plant of Arid and SemiArid Lands of Latin America,” Journal of Arid Environments, Vol. 61, No. 4, 2005, pp. 541-554. doi:10.1016/j.jaridenv.2004.10.008
- G. Scholz, “Holzeigenschaften und Verwendungspotentiale der Baumarten Prosopis Kuntzei Harms. und Schinopsis cornuta Loes. aus dem Chaco Paraguays,” Holztechnologie, Vol. 46, 2005, pp. 18-25.
- A. Carrillo, I. Mayer, G. Koch and F. Hapla, “Wood Anatomical Characteristics and Chemical Composition of Prosopis laevigata Grown in the Northeast of Mexico,” IAWA Journal, Vol. 29, No. 1, 2008, pp. 25-34. doi:10.1163/22941932-90000167
- J. Navar and R. B. Bryan, “Fitting the Analytical Model of Rainfall Interception of Gash to Individual Shrubs of Semiarid Vegetation in Northeastern Mexico,” Agricultural and Forest Meteorology, Vol. 68, No. 93, 1994, pp. 133-143. doi:10.1016/0168-1923(94)90032-9
- J. Návar, F. Charles and E. Jurado, “Spatial Variations of Interception Loss Components by Tamaulipan thornscrub in Northeastern Mexico,” Forest Ecology and Management, Vol. 124, No. 2-3, 1999, pp. 231-239. doi:10.1016/S0378-1127(99)00077-8
- J. Rzedowski, “Vegetación de México,” Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, 2006.
- M. Giantomasi, F. Juñent, P. Villagra and A. Srur, “Annual Variation and Influence of Climate on the Ring Width and Wood Hydrosystem of DC Trees Using Image Analysis,” Trees—Structure and Function, Vol. 23, No. 1, 2009, pp. 117-126.
- L. Corcuera, J. Camarero and E. Gil-Pelegrín, “Effects of a Severe Drought on Quercus Ilex Radial Growth and Xylem Anatomy,” Trees, Vol. 18, No. 1, 2004, pp. 83-92. doi:10.1007/s00468-003-0284-9
NOTES
*Corresponding author.

