Pharmacology & Pharmacy
Vol.3 No.3(2012), Article ID:20633,6 pages DOI:10.4236/pp.2012.33042
Inhibitory Effect of Cigarette Smoke Extract on Experimental Lung Metastasis of Mouse Melanoma by Suppressing Tumor Invasion
![]()
1Department of Pharmacology, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Nishinomiya, Japan; 2Analytical Center, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Nishinomiya, Japan; 3Institute for Biosciences, Mukogawa Women’s University, Nishinomiya, Japan.
Email: lfp51193@mukogawa-u.ac.jp
Received March 14th, 2012; revised April 20th, 2012; accepted May 20th, 2012
Keywords: Cigarette Smoke Extract (CSE); Anti-Metastasis; B16-BL6 Mouse Melanoma Cells; Invasion; Migration
ABSTRACT
We investigated the effect of a nicotineand tar-free cigarette smoke extract (CSE) using an experimental metastasis mouse model which was intravenously injected with B16-BL6 mouse melanoma cells. Three-hour pretreatment of cells with various concentrations of CSE (0%, 0.1%, 0.3%, and 1%) dose-dependently reduced the number of lung metastatic nodules 14 days after tumor injection. To elucidate the mechanism of this anti-metastatic effect of CSE, we examined the invasion and migration activities of B16-BL6 cells pretreated with CSE for three hours in vitro. CSE significantly reduced the invasion of cells at 1% and the migration at 0.3% and 1%. Under the same pretreatment conditions, CSE had no effect on the proliferation of cells. These findings suggest that CSE contains some ingredients that suppress hematogenic lung metastasis via inhibition of the invasion and migration activities of mouse melanoma cells.
1. Introduction
Cigarette smoking has been accepted as the main cause of cancers of the head and neck, lung, and bladder and is a major contributing factor for cancers of the esophagus, pancreas, and kidney [1,2]. In previous in vivo studies, nicotine, its derivatives, and cigarette smoke condensate including almost all cigarette smoke components enhanced experimental metastasis [3,4].
Although cigarette smoke contains harmful ingredients as mentioned above, cigarette smoke, tobacco leaves and flowers contain many useful compounds [5]. Saito et al. reported the identification of cembratriene-4,6-diol as an antitumor-promoting agent from cigarette smoke condensate, a particle phase extract [6]. Also, tobacco cembratriene-4,6-diol was reported to inhibit tumor cell invasion [7]. However, there have been few studies determining whether nicotineand tar-free cigarette smoke extract (CSE), a gas phase extract, can enhance or suppress the metastasis of tumor cells.
In this study, we focused on active ingredients other than nicotine and tar in cigarette smoke, and investigated the effect of CSE on an experimental metastasis model where mice were intravenously injected with highly metastatic mouse melanoma cells. Furthermore, the invasion and migration activities of cells after a three-hour exposure to CSE in vitro were examined to elucidate the mechanism of the anti-metastatic action of CSE.
2. Materials and Methods
2.1. Materials
Frontier Lights brand, cigarettes containing 1 mg of tar and 0.1 mg of nicotine per cigarette, were purchased from Japan Tobacco, Inc. (Tokyo, Japan). Cambridge filters were used to remove 99.9% of all particles and 99.998% of nicotine from cigarette smoke, and were obtained from Heinr. Borgwaldt GmbH (Hamburg, Germany). Fetal bovine serum (FBS) was from BioWest Co. (Nuaillé, France). EDTA trypsin solution (EDTA: 2.2 mM, trypsin: 0.25%) was from Mediatech, Inc. (Manassas, VA, USA). Penicillin/streptomycin solution (penicillin: 50,000 U/ml, streptomycin: 50 mg/ml) was from Cosmo Bio Co., Ltd. (Tokyo, Japan). Dulbecco’s modified Eagle’s medium (DMEM) with L-glutamine was from Invitrogen Corp. (Carlsbad, CA, USA). Dulbecco’s phosphate-buffered saline without calcium and magnesium [DPBS (-)] was from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Growth factor-reduced Matrigel matrix and FALCON cell culture inserts were from Becton Dickinson Labware (Bedford, MA, USA).
2.2. Animals
Specific pathogen-free male C57BL/6Cr mice (7 weeks old) purchased from Japan SLC, Inc. (Hamamatsu, Japan) were used as metastatic melanoma syngeneic animals. Mice were maintained in an air-conditioned room (23˚C ± 2˚C and 60% ± 10% humidity) under an artificial 12-hour light/dark cycle (7:00 a.m. - 7:00 p.m.). Food and water were given ad libitum during the experimental period. All procedures followed the Guiding Principles for the Care and Use of Laboratory Animals approved by The Japanese Pharmacological Society.
2.3. Cell Cultures
The highly metastatic B16-BL6 mouse melanoma cell line was kindly provided by Dr. Futoshi Okada of Tottori University (Yonago, Japan). B16-BL6 cells were cultured in DMEM containing 10% FBS and 0.1% penicillin/streptomycin solution. In all experiments, sub-confluent B16-BL6 cells passaged fewer than 50 times were pretreated with CSE (0%, 0.1%, 0.3%, and 1%) for 3 hours at 37˚C, subsequently harvested with EDTA trypsin solution, and were resuspended in DPBS (-) or DMEM.
2.4. Preparation of CSE
CSE was prepared by a modification of the technique described in a previous report [8]. Briefly, CSE was prepared by bubbling the mainstream of smoke (Gas phase) into DPBS (-) (1 mL per three cigarettes), from which the particle phase, including tar and nicotine, had been almost completely removed by passage through a Cambridge filter using an aspiration pump (Nippon Rikagaku Kikai Co., Ltd., Tokyo, Japan). The pump flow rate was kept constant (1 L/min) and smoke was bubbled for 1 min after lighting cigarettes. The CSE-DPBS (-) solution was immediately filtrated through a 0.22-µm filter. This solution was regarded as 100% CSE, stored at –80˚C, and diluted to various concentrations with DPBS (-) when necessary. The final concentrations of these solutions are expressed as percent values.
2.5. Assay of Experimental Metastasis of Tumor Cells
B16-BL6 cells pretreated with CSE (0%, 0.1%, 0.3%, and 1%) were resuspended to appropriate densities in DPBS (-). Cells (1 × 105/200µL) were injected via a tail vein into syngeneic C57BL/6 mice anesthetized with diethyl ether. Mice were anesthetized with pentobarbital and sacrificed 14 days after tumor cell injection. The lung was excised and fixed in a formaldehyde neutral buffer solution. Nodules, visible as black forms in the lung, were enumerated with the aid of a magnifying glass.
2.6. Matrigel Invasion Assay
The invasion assay of B16-BL6 cells in vitro was carried out employing a modification of the technique described in a previous report [9]. Briefly, 6.4-mm-diameter Transwells were used with tracked-etched polyethylene terephthalate (PET) membrane filters (8-µm pore size) coated with 150 µg/mL matrigel in 100 µL DMEM for 4 hours at 37˚C. B16-BL6 cells pretreated with CSE (0%, 0.1%, 0.3%, and 1%) were resuspended to appropriate concentrations in DMEM. Five-hundred-microliter samples of 2 × 105 cells were placed in the upper chamber compartments. Lower chambers contained 750 µL of serum-free medium with 20 µg/mL fibronectin as a chemoattractant. After 24 hours of incubation in a tissue culture incubator, cells were fixed and stained with 0.1% crystal violet in 10% methanol, and non-invading cells on the upper side of the filter were completely removed by wiping with a cotton swab. Invading cells on the lower side of the filter were extracted with a lysis buffer containing 2% sodium acetate trihydrate, 1% acetic acid, and 50% methanol, and absorbance of the cell lysate was measured at 550 nm.
2.7. Migration Assay
The migration assay of B16-BL6 cells in vitro was performed according to a previous report [10]. Briefly, migrated cells from the upper to the lower chamber were counted microscopically after fixing with methanol and staining with 3% Giemsa in DPBS (-).
2.8. Growth Curves for Tumor Cells in Vitro
Sub-confluent B16-BL6 cells were pretreated with various concentrations of CSE (0%, 0.1%, 0.3%, and 1%) for three hours, harvested with EDTA trypsin solution, and resuspended to appropriate concentrations in DMEM containing 10% FBS with antibiotics. Using 1 × 105 cells/2 mL in each well of a 12-well culture plate, cells were incubated for 24, 48, and 72 hours in a CO2 incubator at 37˚C. Triplicate samples of viable cells were enumerated with a Coulter counter.
2.9. Statistical Analyses
Data are expressed as the mean ± S.E. Statistical analyses were performed with the Dunnett test using the Graphpad Prism 4 software package (Graphpad Software, Inc., San Diego, CA, USA). A difference was considered significant when p < 0.05.
3. Results
Mice injected with tumor cells after pre-incubation with 0%, 0.1%, 0.3%, and 1% CSE for 3 hours displayed visible lung nodules 14 days after tumor cell injection. Figure 1 shows a representative photograph of a typical lung with metastatic melanoma nodules. The mean number of lung nodules (n = 7, each group) after pre-incubation with 0%, 0.1%, 0.3%, and 1% CSE was 89.6 ± 13.6, 62.1 ± 18.9, 49.9 ± 11.8, and 41.0 ± 8.1, respectively. The number of nodules following pretreatment with 1% CSE showed a decrease of 54%, which was significantly lower than the control.
To clarify the mechanism of the anti-metastatic effect of CSE, invasion and migration assays were conducted using B16-BL6 cells since these are essential abilities to establish tumor metastasis. The invasion of B16-BL6 cells pretreated with CSE was dose-dependently suppressed, and the invasion at 1% CSE was decreased by 32%, which was significantly lower than the control (Figure 2). The migration of B16-BL6 cells pretreated with 0.3% and 1% CSE showed a dose-dependent decrease of 32 and 49%, respectively, which were significantly lower than the control (Figure 3).
To address the question of whether the inhibitory effects of CSE on the hematogenic metastatic potential, invasion, and migration of tumor cells were due to inhibition of the proliferation of tumor cells by CSE, we examined the effect of CSE on the growth curves of B16-BL6 cells. The growth curves of B16-BL6 cells pretreated with CSE (0.1%, 0.3%, and 1%) for 3 hours were
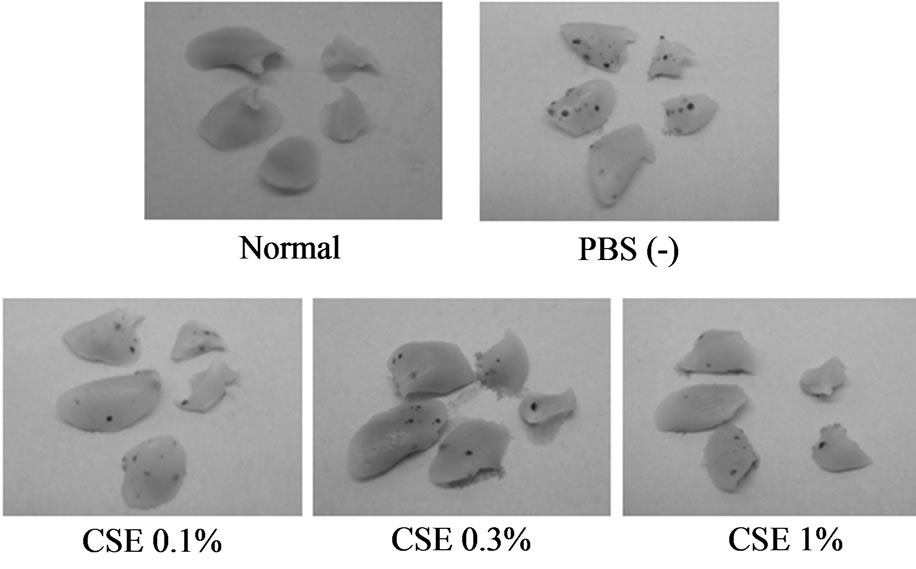
Figure 1. Appearance of the lungs of C57BL/6Cr mice injected intravenously with highly metastatic B16-BL6 melanoma cells (1 × 105), after pretreatment with 0%, 0.1%, 0.3%, and 1% CSE for 3 hours at 37˚C. Fourteen days later, mice were anesthetized with pentobarbital and the lungs were excised. Each photograph shows a representative specimen from each group.

Figure 2. Inhibitory effect of CSE on B16-BL6 melanoma cell invasion. Sub-confluent cells were pretreated with CSE (0%, 0.1%, 0.3%, and 1%) for 3 hours at 37˚C. Cells (2 × 105/500µL) obtained as a monodisperse suspension by trypsinization were seeded into the upper compartment of matrigel coated-Transwell chambers. Lower chambers contained serum-free medium with 20 µg/mL fibronectin as a chemoattractant. After incubation for 24 hours, invading cells on the lower surface were stained with crystal violet, and cell lysates were measured at 550 nm. Data are expressed as the mean ± S.E.M. of 6 samples. *p < 0.05 vs. control.
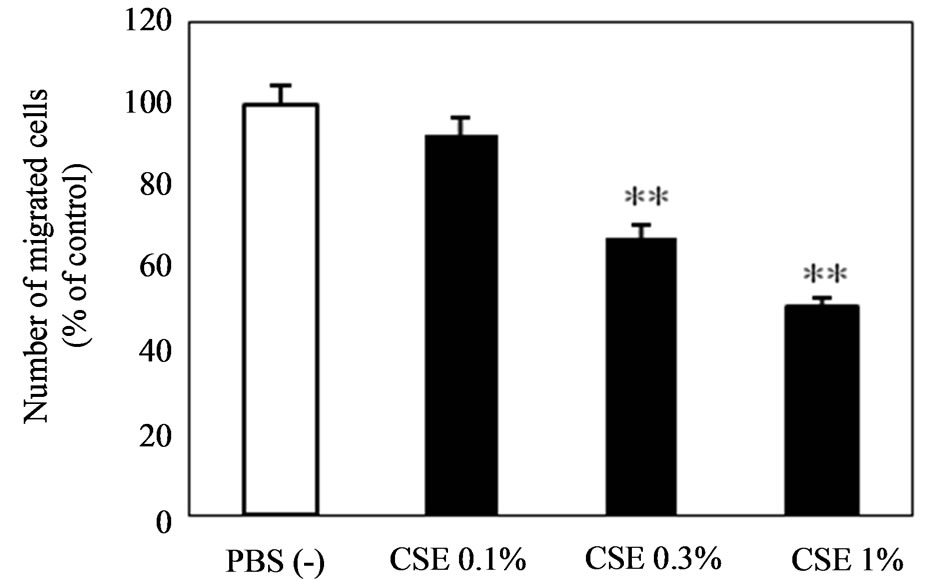
Figure 3. Inhibitory effect of CSE on B16-BL6 melanoma cell migration. Sub-confluent cells were pretreated with CSE (0%, 0.1%, 0.3%, and 1%) for 3 hours at 37˚C. Cells (2 × 105/500µL) obtained as a monodisperse suspension by trypsinization were seeded into the upper compartment of Transwell chambers. Lower chambers contained serumfree medium with 20 µg/mL fibronectin as a chemoattractant. After incubation for 6 hours, migrating cells on the lower surface were counted microscopically. Data are expressed as the mean ± S.E.M. of 6 samples. **p < 0.01 vs. control.
not different from the control (Figure 4).
4. Discussion
In the present study, we used an experimental metastasis model, which was produced by the injection of highly metastatic melanoma cells into syngeneic mice via the
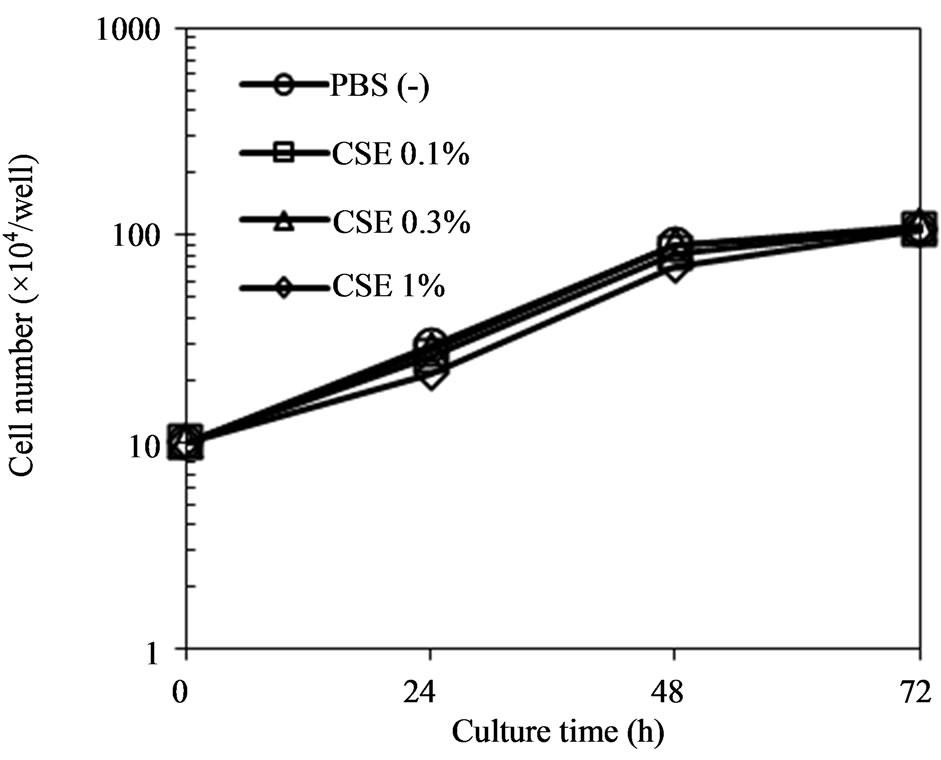
Figure 4. Effect of CSE on growth curves for B16-BL6 melanoma cells. Sub-confluent cells were pretreated with CSE (0%, 0.1%, 0.3%, and 1%) for 3 hours at 37˚C. At time 0, 1 × 105 cells in 2 mL of medium per well obtained as a monodisperse suspension by trypsinization were seeded into a 12- well culture plate. At the times indicated, triplicate cultures were trypsinized and viable cells in samples were enumerated using a Coulter counter.
tail vein to form metastatic nodules in the lung [11]. This model does not mimic all of the steps required for metastasis from a primary tumor. However, the model requires only a short experimental time period, can reproduce similar experimental results, and can measure the ability of malignant cells to extravasate and form tumors in the lungs. It is considered to be the most suitable first screening method for anti-metastatic drug candidates. The results of this study demonstrated that CSE contained such drug candidates. We are presently planning to conduct the next experiment to elucidate the effect of intraperitoneally administered CSE using the same or another metastasis model.
The metastasis of melanoma is completed through a number of steps. Tumor cells must acquire the capacity to detach from the primary focus and invade the basement membrane to intravasate to the vascular or lymphatic circulation. Circulating tumor cells must then extravasate to the stroma of targeted organs and proliferate to form the secondary focus. The inhibitory effect on tumor cell growth also contributes to anti-metastasis. It has been reported that cigarette smoke extract inhibited lung fibroblast proliferation [12]. However, pretreatment with CSE (0%, 0.1%, 0.3%, and 1%) did not alter the proliferation of melanoma cells. These findings indicate that some ingredients in CSE exert an anti-metastatic effect in vivo without inhibiting the proliferation of melanoma cells under our experimental conditions.
Some studies suggest that epidermal growth factors (EGF), secreted by macrophages along blood vessel walls, induce the intravasation of primary tumor cells into the circulation, whereas chemokines, secreted from bone, lymph nodes, and the brain, actively promote the extravasation of circulating tumor cells [13,14]. Thus, both intravasation and extravasation are mediated by chemotaxis, which is essential for cells to detect an extracellular gradient of chemical stimuli and migrate to the higher concentration site [13,15]. Actually, cancer cell metastases were found to be inhibited by blocking tumor cell invasion and migration [16]. In our experiments, pretreatment of tumor cells with CSE inhibited experimental metastasis of melanoma cells in vivo, and invasion and migration in vitro. Accordingly, it is considered that CSE-induced inhibition of metastasis would be due to inhibition of the invasion and migration of tumor cells. La Rocca et al. demonstrated that cigarette smoke exposure inhibited extracellular matrix metalloproteinase (MMP)-2 (gelatinase A) activity, an essential enzyme in tumor cell invasion, in human lung fibroblasts [17]. Overall, CSE may inhibit the invasiveness of tumor cells via the inhibition of not only migration, but also MMP activity.
Cigarette smoke is reported to contain some anticancer agents [5,7], although it is an extremely complex mixture of over 4800 chemicals including many carcinogenic compounds and toxins [18]. Cigarette smoke can be separated into the particle phase and gas phase using a Cambridge filter. The gas phase contains many components such as free radicals, oxidants, and pro-oxidants as well as the particle phase [19-21]. Although reactive oxygen spicies (ROS) in the gas phase, such as peroxynitrite and free radicals, are generally highly reactive, their life times are very short. Accordingly, ROS may not be active ingredients in the CSE to indicate anti-metastatic effect. On the other hand, the gas phase of cigarette smoke has been shown to inhibit the chemotaxis of polymorphonuclear leukocytes, an effect which was partially reversed by cysteine [22], and contains α,β-unsaturated aldehydes and ketones (e.g., acrolein and crotonaldehyde) [23], which are thiol-alkylating reagents [24]. Interestingly, these results support our present results. In fact, CSE contained α,β-unsaturated aldehydes and ketones such as acrolein, crotonaldehyde, and methyl vinyl ketone according to our analyses (unpublished data). These observations support the hypothesis that the CSEinduced inhibition of tumor cell metastasis, invasion, and migration probably occurs due to the presence of the above-mentioned aldehydes and ketones instead of ROS in CSE as effective components. It is clear that cigarette smoke is harmful to our health, and the best advice is to stop smoking. However, our results show that it may contain a few beneficial ingredients, and that it is important to remove nicotine and tar from cigarette smoke before aspirating it using suitable filters.
5. Conclusions
We demonstrated that pretreatment with CSE led to an anti-metastatic action on an experimental lung metastasis mouse model with melanoma cells. Furthermore, the anti-metastatic effect of CSE is suggested to be due to inhibition of the invasion and migration of melanoma cells. To address the question of whether these actions of CSE on the tumor cells were due to the cytotoxicity on cells, we examined the effect of CSE on the growth curves for cells. The growth curves for cells pretreated with CSE did not change compared with the control.
These results indicate that CSE contains some antimetastatic ingredients.
6. Acknowledgements
This study was supported, in part, by a Special Grant from the Smoking Research Foundation for Biomedical Research of Japan.
REFERENCES
- National Institutes of Health (US), “Tobacco and the Clinician: Interventions for Medical and Dental Practice,” Bethesda (MD), US Department of Health, NIH Publication No. 94-3693, 1994.
- US Office on Smoking and Health, “Smoking and Health: A Report of the Surgeon General,” Washington (DC), US Department of Health, Education, and Welfare, DHEW Publication No. 79-50066, 1979.
- R. Davis, W. Rizwani, S. Banerjee, M. Kovacs, E. Haura, D. Coppola and S. Chellappan, “Nicotine Promotes Tumor Growth and Metastasis in Mouse Models of Lung Cancer,” PLoS One, Vol. 4, 2009, p. e7524. doi:10.1371/journal.pone.0007524
- R. Gopalakrishna, Z. H. Chen and U. Gundimeda, “Tobacco Smoke Tumor Promoters, Catechol and Hydroquinone, Induce Oxidative Regulation of Protein Kinase C and Influence Invasion and Metastasis of Lung Carcinoma Cells,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 91, No. 25, 1994, pp. 12233-12237.
- K. A. E. I. Sayed and P. W. Sylvester, “Biocatalytic and Semisynthetic Studies of the Anticancer Tobacco Cembranoids,” Expert Opinion on Investigational Drugs, Vol. 16, No. 6, 2007, pp. 877-887.
- Y. Saito, H. Takizawa, S. Konishi, D. Yoshida and S. Mizusaki, “Identification of Cembratriene-4,6-diol as Antitumor-Promoting Agent from Cigarette Smoke,” Carcinogenesis, Vol. 6, No. 8, 1985, pp. 1189-1194. doi:10.1093/carcin/6.8.1189
- K. A. E. I. Sayed, S. Laphookhieo, H. N. Baraka, M. Yousaf, A. Hebert, D. Bagaley, F. A. Rainey, A. Muralidharan, S. Thomas and G. V. Shah, “Biocatalytic and Semisynthetic Optimization of the Anti-Invasive Tobacco (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol, Bioorganic and Medical Chemistry, Vol. 16, No. 6, 2008, pp. 2886-2893.
- M. Yokode, T. Kita, H. Arai, C. Kawai, S. Narumiya and M. Fujiwara, “Cholesteryl Ester Accumulation in Macrophages Incubated with Low Density Lipoprotein Pretreated with Cigarette Smoke Extract,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 85, No. 7, 1988, pp. 2344-2348.
- M. Ogasawara and H. Suzuki, “Inhibition by Evodiamine of Hepatocyte Growth Factor-Induced Invasion and Migration of Tumor Cells,” Biological and Pharmaceutical Bulletin, Vol. 27, No. 4, 2004, pp. 578-582. doi:10.1248/bpb.27.578
- C. Takahashi, Z. Sheng, T. P. Horan, H. Kitayama, M. Maki, K. Hitomi, Y. Kitaura, S. Takai, R. M. Sasahara, A. Horimoto, Y. Ikawa, B. J. Ratzkin, T. Arakawa and M. Noda, “Regulation of Matrix Metalloproteinase-9 and Inhibition of Tumor Invasion by the Membrane-Anchored Glycoprotein RECK,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 95, No. 22, 1998, pp. 13221-13226. doi:10.1073/pnas.95.22.13221
- I. J. Fidler, “Selection of Successive Tumour Lines for Metastasis,” Nature: New Biology, Vol. 118, 1973, pp. 148-149.
- Y. Nakamura, D. J. Romberger, L. Tate, R. F. Ertl, M. Kawamoto, Y. Adachi, T. Mio, J. H. Sisson, J. R. Spurzem and S. I. Rennard, “Cigarette Smoke Inhibits Lung Fibroblast Proliferation and Chemotaxis,” American Journal of Respiratory and Critical Care Medicine, Vol. 151, No. 5, 1995, pp. 1497-1503.
- A. Zlonik, “Chemokines Inneoplastic Progression,” Seminars in Cancer Biology, Vol. 14, No. 3, 2004, pp. 181- 185. doi:10.1016/j.semcancer.2003.10.004
- J. Condeelis and J. W. Pollard, “Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis,” Cell, Vol. 124, No. 5, 2006, pp. 263-266. doi:10.1016/j.cell.2006.01.007
- P. N. Devreotes and C. Janetopoulos, “Eukaryotic Chemotaxis: Distinctions between Directional Sensing and Polarization,” Journal of Biological Chemistry, Vol. 278, No. 23, 2003, pp. 20445-20448. doi:10.1074/jbc.R300010200
- A. Müller, B. Homey, H. Soto, N. Ge, D. Catron, M. E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. N. Wagner, J. L. Barrera, A. Mohar, E. Verástegui and A. Zlotnik, “Involvement of Chemokine Receptors in Breast Cancer Metastasis,” Nature, Vol. 410, 2001, pp. 50-56. doi:10.1038/35065016
- G. La Rocca, R. Anzalone, F. Magno, F. Farina, F. Cappello and G. Zummo, “Cigarette Smoke Exposure Inhibits Extracellular MMP-2 (Gelatinase A) Activity in Human Lung Fibroblasts,” Respiratory Research, Vol. 8, 2007, p. 23. doi:10.1186/1465-9921-8-23
- D. Hoffmann, I. Hoffmann and K. El-Bayoumy, “The Less Harmful Cigarette: A Controversial Issue. A Tribute to Ernst L. Wynder,” Chemical Research in Toxicology, Vol. 14, 2001, pp. 767-790. doi:10.1021/tx000260u
- M. Kunitomo, Y. Yamaguchi, S. Kagota, N. Yoshikawa, K. Nakamura and K. Shinozuka, “Biochemical Evidence of Atherosclerosis Progression Mediated by Increased Oxidative Stress in Apolipoprotein E-Deficient Spontaneously Hyperlipidemic Mice Exposed to Chronic Cigarette Smoke,” Journal of Pharmacological Sciences, Vol. 110, No. 3, 2009, pp. 354-361. doi:10.1254/jphs.09100FP
- W. A. Pryor and K. Stone, “Oxidants in Cigarette Smoke. Radicals, Hydrogen Peroxide, Peroxynitrate, and Peroxynitrite,” Annals of the New York Academy of Sciences, Vol. 686, 1993, pp. 12-28.
- B. Frei, T. M. Forte, B. N. Ames and C. E. Cross, “Gas Phase Oxidants of Cigarette Smoke Induce Lipid Peroxidation and Changes in Lipoprotein Properties in Human Blood Plasma. Protective Effects of Ascorbic Acid,” The Biochemical Journal, Vol. 277, 1991, pp. 133-138.
- R. B. Bridges, J. H. Kraal, L. J. Huang and B. M. Chancellor, “Effects of Tobacco Smoke on Chemotaxis and Glucose Metabolism of Polymorphonuclear Leukocytes,” Infection and Immunity, Vol. 15, No. 1, 1977, pp. 115- 123.
- D. G. Hatzinikolaou, V. Lagesson, A. J. Stavridou, A. E. Pouli, L. Lagesson-Andrasko and J. C. Stavrides, “Analysis of the Gas Phase of Cigarette Smoke by Gas Chromatography Coupled with UV-Diode Array Detection. Analytical Chemistry, Vol. 78, No. 13, 2006, pp. 4509-4516. doi:10.1021/ac052004y
- M. Dixon and E. C. Webb, “Enzymes,” Academic Press Inc., New York, 1964, pp. 937-943.

