Pharmacology & Pharmacy
Vol.3 No.2(2012), Article ID:18723,6 pages DOI:10.4236/pp.2012.32026
Pharmacokinetic Disposition of Streptomycin Sulfate in Japanese Eel (Anguilla japonica) after Oral and Intramuscular Administrations
![]()
1Zhejiang Institute of Freshwater Fisheries, Huzhou, China; 2Zhejiang Sanmen Food Inspection Center, Sanmen, China.
Email: *sjinyu@126.com
Received January 20th, 2012; revised February 26th, 2012; accepted March 8th, 2012
Keywords: Pharmacokinetic; Streptomycin Sulfate; Japanese Eel; Intramuscular; Oral Gavage
ABSTRACT
Pharmacokinetics and residue elimination of streptomycin sulfate (STR) are important in the determination of optimal dosage regimens and in establishing safe withdrawal periods in farmed fishes. The pharmacokinetics of STR was studied after a single dose (50 mg/kg) of intramuscular (i.m.) or oral gavage (p.o.) administration to Japanese eel (Anguilla japonica) in freshwater at 25˚C. Eight fish per sampling point were examined after treatment. Plasma and muscle were collected and analyzed by high-performance liquid chromatography (HPLC) method with 0.05 μg/ml detection limit. The data of pharmacokinetics conformed to the two-compartment open model for intramuscular and one-compartment open model for oral administrations. After intramuscular administration, the elimination half-life (t1/2β) was calculated to be 11.346 h, the maximum plasma concentration (Cmax) to be 29.524 μg/ml, the time to peak plasma streptomycin concentration (Tmax) to be 0.218 h, and the area under the plasma concentration-time curve (AUC) to be 90.206 μg/ml·h. Following p.o. administration, the corresponding estimates were 13.239 h, 0.346 μg/ml, 11.960 h, and 12.356 μg/ml·h. After intramuscular administration, a therapeutic concentration of the drug was maintained for 12 hours in the plasma, however, a therapeutic level could not be achieved after oral administration, and the results suggest that the drug can be used clinically by intramuscular administration against streptomycin susceptible systemic infections in Japanese eel.
1. Introduction
Streptomycin sulfate (STR) is an aminoglycoside antibiotic widely used in medical and veterinary practices to treat infections from gram-negative bacteria and is now used in export eel farming as a chemotherapeutic agent in China. However, incorrect use of this drug can lead to the presence of residues of this drug and may lead to direct toxic effects on consumers, allergic reactions of those sensitive to the antibiotics or may indirectly cause problems through the induction of resistant strains of bacteria [1].
Japanese eel (Anguilla japonica) is one kind of the most economically important freshwater fishes in China and have become a consumer and farmers’ favorite for its nutritional value, taste, strong adaptability, and efficient growth. However, bacterial disease resulted in huge losses in recent years; drug residue problems have now become serious owing to the increasing and unreasonable use of the drug. Therefore, pharmacokinetics (PK) and residue elimination studies are important in determination of optimal dosage regimens and in establishing a safe withdrawal time. The PK of STR has been studied extensively in many animal species, including camels [2], buffaloes [3], dog [4], horse [5], goat [6], and human being [7]. To the best of our knowledge, no data on STR PK and residue elimination in Japanese eel are available. The objective of the present study was to obtain information on the PK and residue elimination of STR for Japanese eel, and to determine reasonable dosage regimens of the drug in Japanese eel.
2. Materials and Methods
2.1. Chemicals
Streptomycin sulfate (712 U/mg, No. k0051201) were purchased from China Institute of Veterinary Drug Control, acetonitrile, methanol, perchloric acid, ammonium ferric sulfate, sodium 1-heptanesulfonate were of HPLC or analytical grade.
2.2. Fish
Clinically healthy Japanese eel, weighing 95.4 ± 5.2 g, were obtained from aquatic fry farm of Zhejiang Institute of Freshwater Fisheries in China and maintained in ponds (1 m3), supplied with circulating water and oxygen continuously by an inflation pump. The water temperature was maintained by circulating water system (Shanghai Haisheng Company) at 25˚C ± 1˚C. The fish were allowed to acclimate for three days, fed a drug-free commercial diet. The fish had no previous exposure to any antibiotic, and no drugs were given to the animals during the acclimation or study periods. To rule out the influence of food content on the absorption of STR, the fish were starved for 24 h before drug administration.
2.3. Standard Calibration Curve
Working calibration curves were prepared at concentrations of 0.25, 1.25, 2.5, 6.25, 12.5, 25, 125 and 250 μg/ml was drawn by plotting the known STR concentrations against the average peak area. The STR concentrations in unknown samples were calculated from the standard curves.
2.4. Dosing and Sampling
Japanese eel were divided randomly into two groups for i.m. and oral administrations, respectively. The STR solution for i.m. (50 mg/ml) and oral (50 mg/ml) was prepared by dissolving the pure STR powder in distilled water. Individual fish was injected with STR solution at the dorsal fin (i.m.) or drenched with STR solution with gavage needle (p.o.) at the dose of 50 mg/kg (b.w.). The blood samples were taken from tail sinus of eight fish at 0.25, 0.5, 1, 3, 6, 9, 12, 18, 24, 48, 72, 120, 168 h, 5, 7, 9, 12, 15 d after i.m. and p.o. treatment, respectively. All samples were immediately frozen and stored at −20˚C until analysis.
2.5. STR Analysis Protocol
To 15 ml graduated plastic-stoppered centrifuge tubes was added 1 ml plasma (1 g of ground tissue) sample and 5 ml of perchloric acid. Each sample was whirlmixed for 5 min and then centrifuged for 15 min at 5000 rpm. The remaining tissue pellet in the centrifuge tube was extracted again for another two times, the supernatant were combined and transferred to another plastic-stoppered centrifuge tube and adjusted to pH 2.4 with perchloric acid, 5 ml of 0.2 M Sodium 1-heptanesulfonate was added to each tube, then were transferred to Bond Elute C-18 solid-phase extraction columns pre-washed with methanol (10 ml) and distilled water (30 ml), and allowed to run dry for at least 2 min. STR was eluted from C-18 column with 10 ml methanol. The methanol eluate was collected and evaporated under reduced pressure in a vacuum rotary evaporator for the complete removal of solvents. The dried eluate were dissolved in 200 μl distilled water, 80 μl of 0.05 M NaOH were added and mixed for 1 min, then standing at 65˚C for 1 h, 40 μl of 0.2% ammonium ferric sulfate were added when the solution temperature were 25˚C, and diluted to 500 μl with distilled water. An aliquot (20 μl) of the diluted sample was analyzed with HPLC (Agilent XDB-C18, 5 μm, 4.6 mm × 250 mm). The mobile phase of acetonitrile 0.2% sodium 1-heptanesulfonate (20:80, v/v) was filtered through a 0.45 μm millipore filter and degassed using sonication (5 min). The column was operated at 30˚C. Flow rate was adjusted to 0.3 ml/min and the eluate was monitored at 330 nm.
2.6. Detection Limit and Recovery Percentage
The detection limit is defined as the concentration of STR in Japanese eel tissues, which can produce a HPLCUV signal that is three times greater than the signal-tonoise ratio.
2.7. Method Validation
The method was validated for plasma and muscle. The linearity, recovery, accuracy and precision were evaluated by analysis of the samples spiked with 50, 10, 5 and 1 μg/ml STR. Recovery was calculated by comparing the peak area of STR from processed samples with that from the STR standard in the mobile phase. The accuracy was determined by comparing the measured concentration with its true value. The limit of detection (LOD) of STR was defined as the STR concentration resulting in a peak height three times the signal noise.
2.8. Pharmacokinetics Analysis
Pharmacokinetic analysis was performed by the computer program 3P97 (version 1.0, edited by The Chinese Society of Mathematical Pharmacology, China). The model was selected on the basis of the residual sum of squares and the minimum Akaike’s information criterion (AIC) [8]. The area under the concentration-time curve (AUC) was calculated using the trapezoidal rule and was extrapolated to infinity [9]. The elimination characteristics of the drug from each tissue were estimated using linear regression analysis of the terminal phase of the elimination time curve [10]. The elimination rate constant (β) was the slope of linear regression equation on logtransferred STR concentration (ln C) against time, and the elimination half-life (t1/2β) was calculated from the equation t1/2β = 0.693/β for each tissue.
3. Results
3.1. Method Validation
Calibration curves showed a good linearity (r2 = 0.999) in the range of 0.1 to 100 μg/ml. The average interand intra-day precision (RSDs) were 0.9% - 2% and 1.1% - 3.1% for plasma and 0.5% - 3.9% and 0.7% - 4.0% for muscle. The STR accuracy for plasma ranged from 75% to 86% and mean recovery was 81%. The STR accuracy for muscle ranged from 73% to 81% and mean recovery was 77%. The limit of detection (LOD) of STR was determined as 0.05 μg/ml.
3.2. Pharmacokinetics Analysis in Serum
After i.m. administration, serum streptomycin concentration as a function of time is best described by an open two-compartment pharmacokinetic model (Figure 1). Pharmacokinetic data for streptomycin concentration in serum after i.m. administration of a single dose (50 mg/kg body weight) are presented in Table 1. STR was rapidly absorbed following administration. Plasma concentration reached 10.8 μg/ml at 0.167 h and the maximum plasma concentration (29.524 μg/ml) was reached at 0.218 h. After this the drug level declined rapidly and reached 0.24 μg/ml at 48 h. Correspondingly, the elimination half-life was 11.346 h. Other pharmacokinetic parameters were shown in Table 1.
The plasma concentration-time curve of STR after oral administration to fish at a single dose of 50 mg/kg (b.w.) is shown in Figure 2. The data of pharmacokinetics fitted to one-compartment open pharmacokinetic model, the pharmacokinetic parameters calculated from this model are given in Table 2. STR levels were recorded at the first sample point (0.167 h) after oral dosing, followed by an increasing profile and the drug reached the peak plasma concentration (0.35 μg/ml) at 11.96 h (Tmax), then it slowly decreased. The absorption half-life (T1/2ka) and elimination half-life (T1/2b) of STR were 5.529 h and 13.239 h respectively.
3.3. Residues of STR in Japanese Eel
The STR residue concentrations in muscle after intramuscular administration are listed in Table 3. The elimination of STR in muscle was rapid, the drug concentrations declined to 0.18 µg/g in muscle on 4 d and were undetectable in muscle on 5 d, the corresponding date after oral administration were 0.12 μg/ml in muscle on 3 d and were undetectable in muscle on 4 d.
4. Discussion
Drug residue problems have now become serious owing to the increasing and unreasonable use of antibiotic. Therefore, pharmacokinetics and residue elimination studies are important for establishing correct dosage regimes, promoting optimal use of the drug in question, and minimizing environmental impact. In the present study we determined the pharmacokinetics of STR in Japanese eel (Anguilla japonica) after a single dose (50 mg/kg) of intramuscular or oral administration in freshwater at 25˚C. To our best knowledge, this is the first study on the pharmacokinetics and tissue disposition of streptomycin in Japanese eel.
The V/F(c) is an accurate indication of the diffusion of the drug in the body, and AUC represents the extent of drug absorption and determines bioavailability. In the present study, the V/F(c) and AUC were about 11 and 7 times larger when administered i.m. (0.847 L/kg and 90.206 μg/ml·h) than administered orally (0.077 L/kg and 12.356 μg/ml·h). The time of plasma concentration above 2 μg/ml was approximately 12 h and 0 h following i.m. and oral administration (Table 1). Based on MIC data studied on bacteria from fish, 2 μg/ml STR showed high efficacy against most bacteria [11,12]. All these results
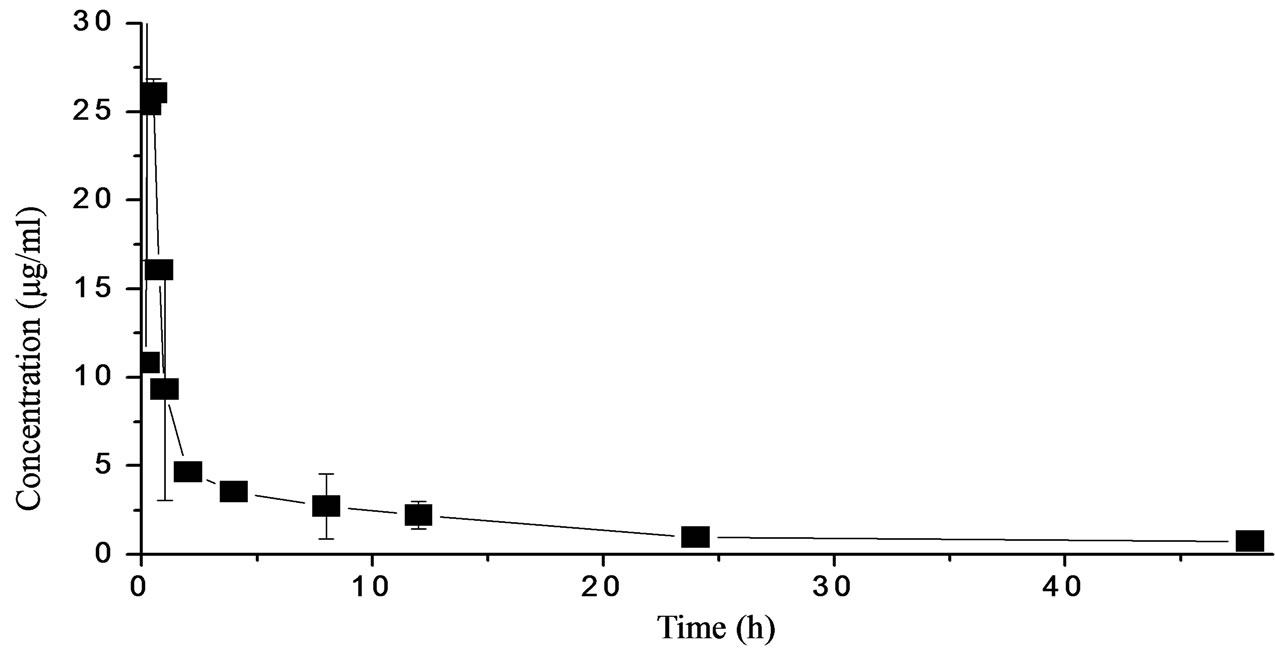
Figure 1. STR levels in plasma of Japanese eel following a single intramuscular administration (50 mg).
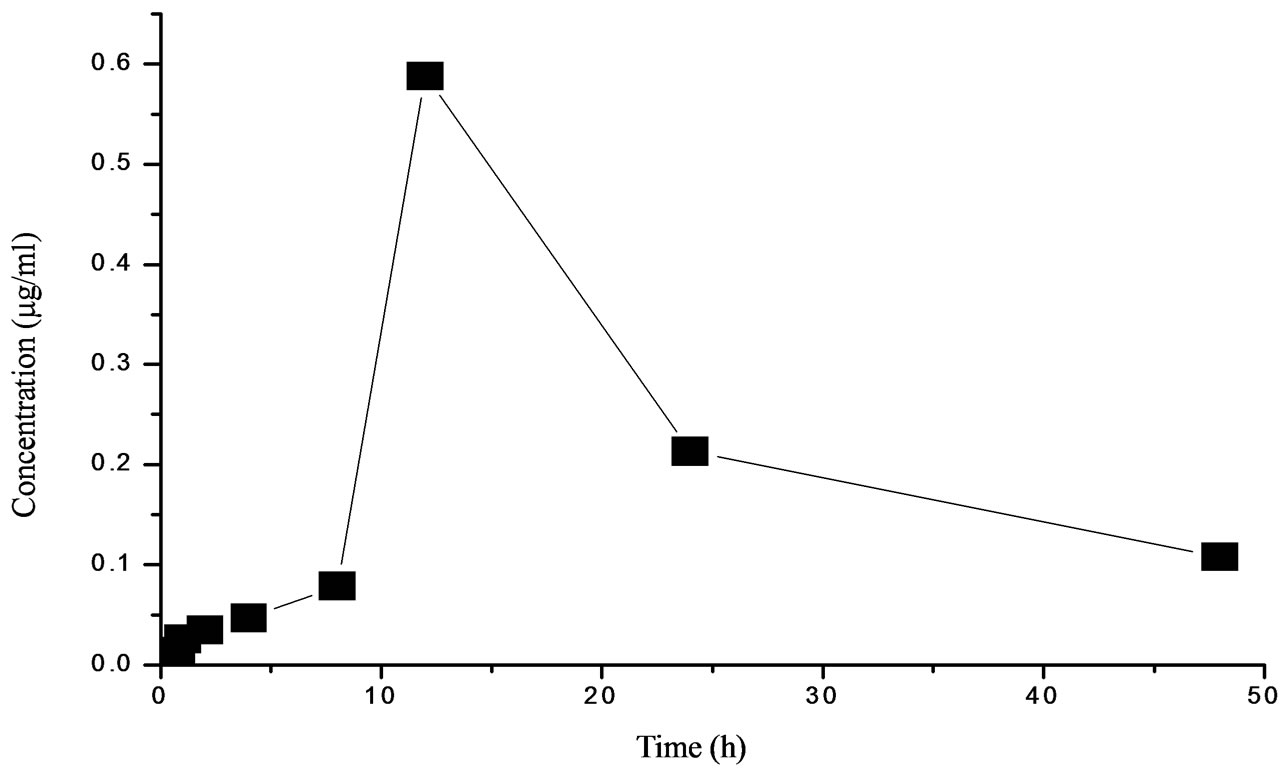
Figure 2. STR levels in plasma of Japanese eel following a single oral administration (50 mg·kg−1 b.w.).
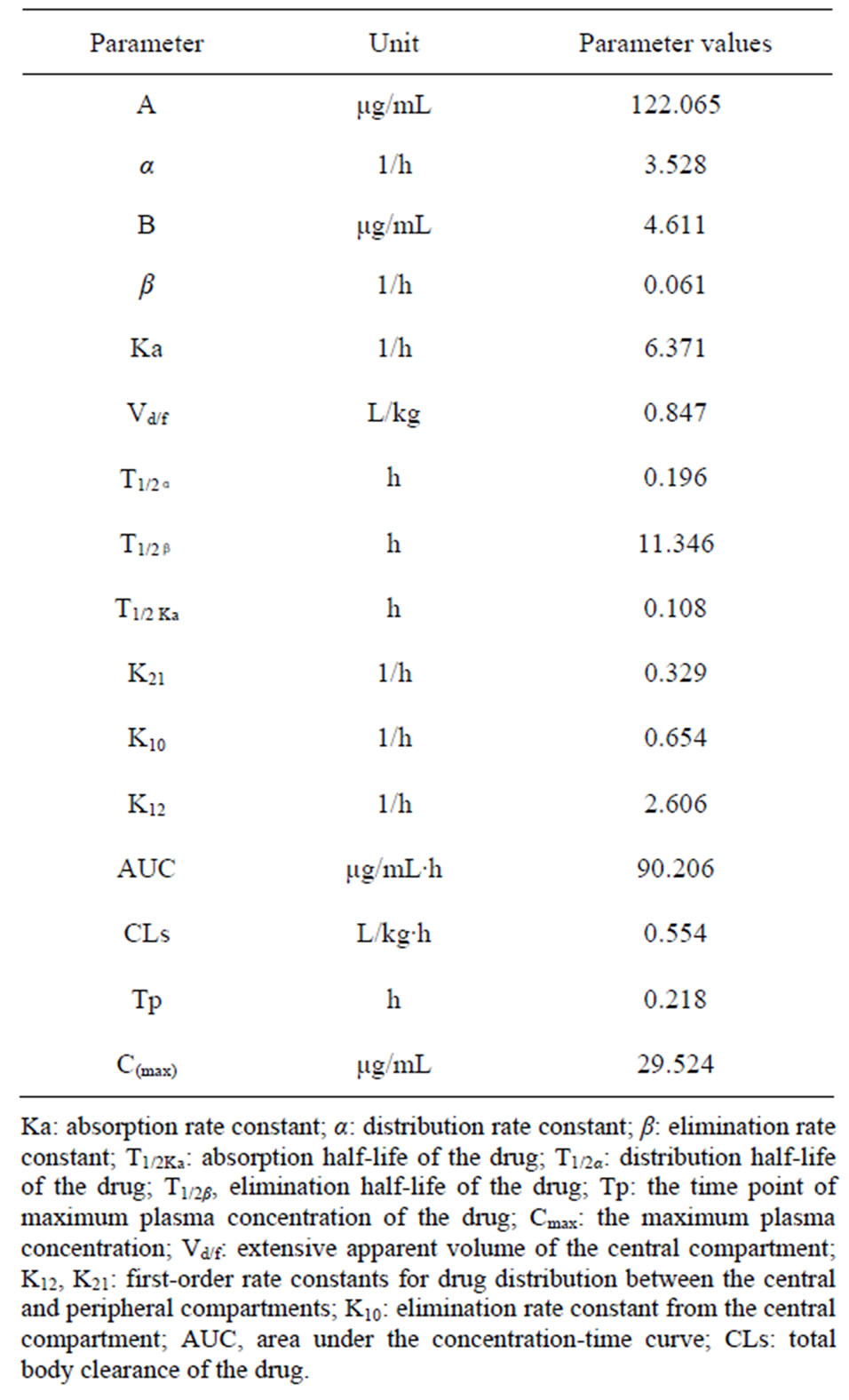
Table 1. Pharmacokinetic parameters for streptomycin sulfate of Japanese eel following a single intramuscular administration (50 mg·kg−1 b.w.).
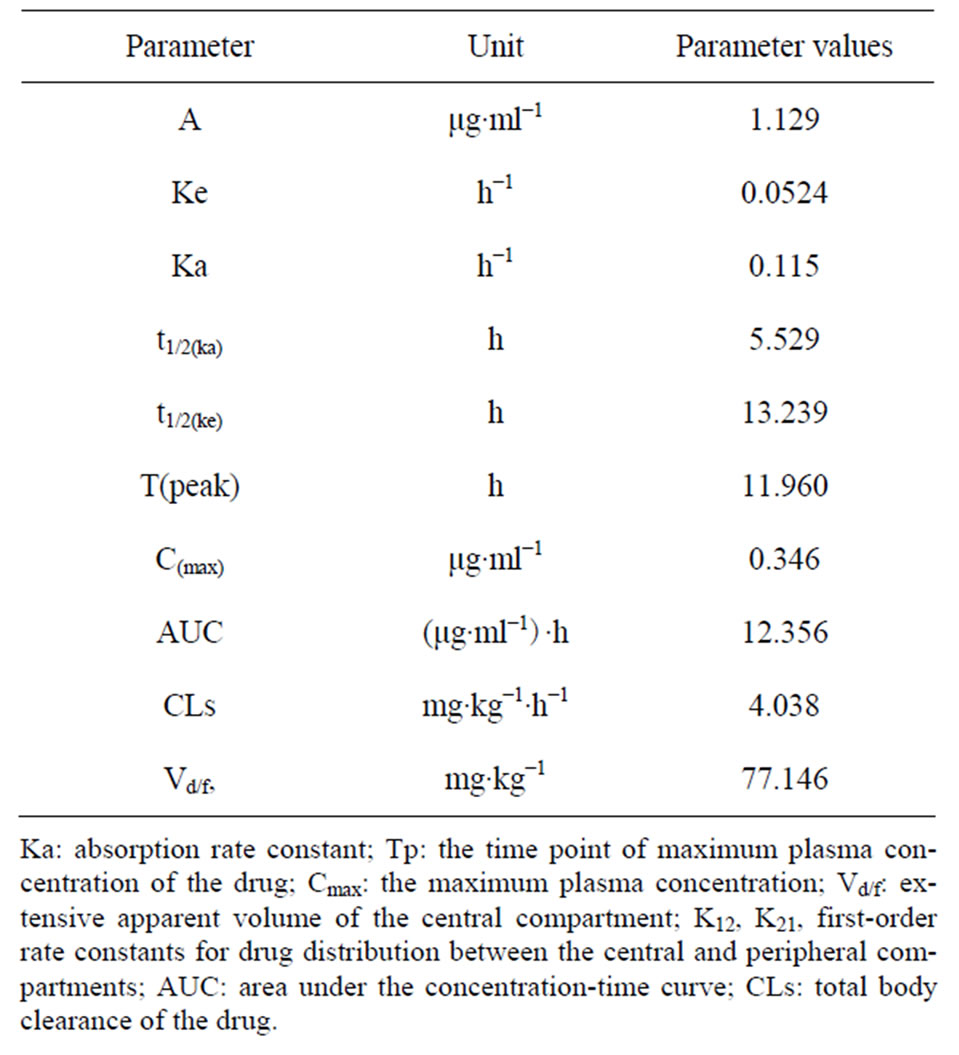
Table 2. Pharmacokinetic parameters for streptomycin sulfate of Japanese eel following a single oral administration (50 mg·kg−1 b.w.).
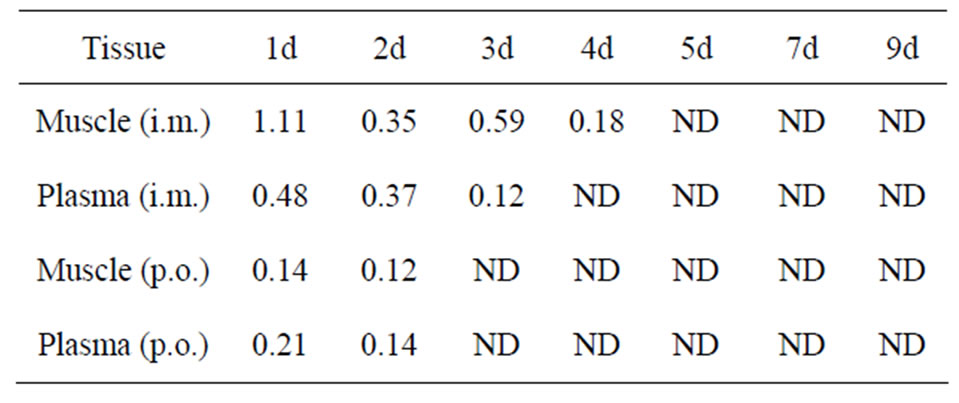
Table 3. Streptomycin sulfate concentrations in muscle and plasma following a single administration (50 mg·kg−1 b.w.).
indicated that i.m. administration is more pharmacologically advantageous than p.o. administration, which was in consistent with the results in other species of other studies [2,3].
STR was rapidly obsorbed following i.m. injection. The absorption half-life was 6 min and a mean peak serum concentration of 29.52 μg/ml was attained 30 min after drug administration. The values were considerably less than values previously reported for canels, 86 min and 16.8 min, respectively [13], and other animal species, including cattle (peak serum concentration 60 min [14, 15]) swine (peak serum concentration 60 min and absorption [16]), and dog (peak serum concentration 60 min [17]). The distribution half life, T1/2α, estimated in the present study for streptomycin following i.m. administration was about 0.2 h. This shows that the drug is rapidly distributed from the central compartment into the body tissues.
The elimination half life, T1/2β, was found to be 11.346 h. The reported tip of streptomycin was about 3.4 h in horse [5], and 2 - 3 h for normal adult human beings [7]. The above findings indicate that in fish and horse, the drug is eliminated comparatively more slowly than in man. The reason for the difference may be attributable to differences in species and pharmaceutical factors for administration.
In summary, the pharmacokinetic profile of STR shown by the present study demonstrates rapid absorption and slow elimination, and i.m. injection may be better than oral administration for therapy of common bacterial infections. Further work is needed to understand the pharmacokinetics of STR more clearly in Japanese eel and other species, and to obtain accurate and reasonable dosing regimen.
5. Acknowledgements
This work was supported by a grant from the National High Technology Research and Development Program of China (863 Program) (No. 2011AA10A216), as well as Special Fund for Agro-scientific Research in the Public Interest (No. 201203085), China.
REFERENCES
- R. Krieger, “Handbook of Pesticide Toxicology, Principles, Part 1,” Academic Press, San Diego, 2001.
- A. A. A. Hadi, I. A. Wasfi and A. K. Bsahir, “Pharmacokinetics of Streptomycin in Camels,” Journal of Veterinary Pharmacology and Therapeutics, Vol. 21, No. 6, 1998, pp. 494-496. doi:10.1046/j.1365-2885.1998.00171.x
- C. Jayachandran, M. K. Singh, S. D. Singh and N. C. Banerjee, “Pharmacokinetics of Streptomycin with Particular Reference to Its Distribution in Plasma Milk and Uterine Fluid of Shebuffaloes,” Veterinary Research Communications, Vol. 71, 1987, pp. 353-358.
- W. G. Venzke and C. R. Smith, “Streptomycin in Small Animal Medicine,” In: S. A. Waksrnan, Ed., Streptomycin: Nature and Practical Application, Willim & Wilkins Baltimore, Baltimore, 1949, p. 580.
- J. D. Baggot, D. N. Lone, R. J. Rose and J. Raus, “Pharmacokinetics of Some Aminoglycoside Antibiotics in the Horse,” Journal of Veterinary Pharmacology and Therapeutics, Vol. 4, No. 4, 198l, pp. 277-284.
- J. Y. Wang, W. J. Hu and Q. Y. Liu, “Studies on Pharmacokinetics of Streptomycin in Milk Goats,” Journal of Northwest Sci-Tech University of Agriculture and Forestry, Vol. 4, 1981, pp. 52-56.
- E. A. Jackson and D. McLeod, “Pharmacokinetics and Dosing of Anti-Microbial Agents in Renal Impairment,” American Journal of Hospital Pharmacy, Vol. 31, 1974, pp. 137-146.
- K. Yamaoka, Y. Nakagawa and T. Uno, “Application of Akaike’s Information Criterion (AIC) in the Evaluation of Linear Pharmacokinetic Equations,” Journal of Pharmacokinetics and Biopharmaceutics, Vol. 6, No. 2, 1978, pp. 165-175.
- T. Guo, “Modern Pharmacokinetics,” China Science and Technology Press, Beijing, 2005, pp. 1-26.
- D. A. Campbell, P. Pantazis and M. S. Kelly, “Impact and Residence Time of Oxytetracycline in the Sea Urchin, Psammechinus Miliaris, a Potential Aquaculture Species,” Aquacult, Vol. 202, 2001, pp. 73-87. doi:10.1016/S0044-8486(01)00600-7
- Y. C. Zhou, H. P. Fan, B. C. Liao and Z. Z. Zeng, “An Anti-Bacterial Formula Study for Aeromonas Hydrophila in Vitro,” Journal of Guangdong Ocean University, Vol. 30, No. 6, 2010, pp. 35-39.
- L. G. Song, N. Guang, B. Yi, C. H. Yang, R. L. Wan, X. Y. Zhang, Q. Shi and D. Zhang, “Analysis for Antimicrobial Susceptibility of Streptococcus Suis Isolated from Sichuan Province,” Chinese Journal of Veterinary Drug, Vol. 39, No. 10, 2005, pp. 1-5.
- A. Y. I. El-Gendi, M. G. A. El-Sayed, M. Atef and A. Z. Hussin, “Pharmacolinetic Interpretation of Some Antibiotics in Camels,” Archieves Internationales de Pharmacodynamic, Vol. 261, No. 2, 1983, pp. 186-195.
- P. B. Hannond, “Dihydrostreptomycin Dose-Serum Level Relationships in Cattle,” Journal of the American Veterinary Medical Association, Vol. 122, No. 912, 1953, pp. 203-206.
- R. H. Teske, L. D. Rolins and G. G. Carter, “Penicillin and Dihydrostreptomycin Serum Concentrations after Single and Repeated Doses to Feeder Steers,” Journal of the American Veterinary Medical Association, Vol. 160, No. 6, 1972, pp. 873-878.
- H. D. Mercer, H. F. Righter, G. Gordon and A. B. Carter, “Serum Concentration of Penicillin and Dihydrostreptomycin after Their Parenteral Administration in Swine,” Journal of the American Veterinary Medical Association, Vol. 160, 1971, pp. 61-65.
- W. G. Huber, “Streptomycin, Chloramphenicol, and Other Antibacterial Agents,” In: L. M. Jones, N. H. Boots, L. E. McDonald, Eds., Veterinary Pharmacology and Therapeutics, 4th Edition, Iowa State University Press, Ames, 1977, pp. 940-946.
NOTES
*Corresponding author.

