Open Journal of Veterinary Medicine
Vol.3 No.1(2013), Article ID:29112,6 pages DOI:10.4236/ojvm.2013.31009
Diurnal Rhythm of Antioxidant Biomarkers in Equines
1Departamento de Medicina Veterinária, Universidade Federal Rural de Pernambuco, Recife, Brazil
2Núcleo de Pesquisa Equina, Departamento de Zootecnia, Universidade Federal Rural de Pernambuco, Recife, Brazil
3Faculdade de Medicina Veterinária, Universidade Estadual do Ceará, Fortaleza, Brazil
Email: *erika.vet.pe@gmail.com
Received December 13, 2012; revised January 14, 2013; accepted February 16, 2013
Keywords: Glutathione Peroxidase; Uric Acid; Antioxidants; Diurnal Rhythm
ABSTRACT
The aim of the present study was to test the hypothesis that glutathione peroxidase, uric acid, and a number of hematological biomarkers do not vary in adult mares, over a 24-hour period. Seven adult (age 10 ± 6 yrs; weight 370 ± 30 kg) Arabian Purebred mares were used. Blood samples were collected every two hours, except during the period after-meals, when samples were collected every 30 minutes, totaling four samples in two hours. These samples were used to analyze glutathione peroxidase (GPx), uric acid (UA), glucose (GLU), total plasma protein (TPP), red blood cells (RBC), hemoglobin (HB), hematocrit (HT), red cell distribution width (RDW), white blood cells (WBC) and lymphocytes (LYM). One-way repeated measures analysis of variance (ANOVA) was used to determine significant differences. Tukey’s test was used for multiple comparisons between the averages. Ρ values < 0.05 were considered statistically significant. Antioxidant biomarkers GPx and UA, as well as hematological biomarkers TPP, RBC, HB and HT exhibited a diurnal rhythm that was not affected by food ingestion, while RDW-SD, RDW-CV, WBC and LYM did not present a statistically significant change in the same period.
1. Introduction
The antioxidant system consists of both enzymatic and non-enzymatic components which work coordinately to detoxify the body of reactive oxygen species [1]. Enzy matic antioxidant defense includes superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT). Non-enzymatic antioxidants are represented by ascorbic acid (Vitamin C), α-tocopherol (Vitamin E), glutathione (GSH), carotenoids, flavonoids [2,3] and uric acid [4,5].
Oxidant agents are normally produced as a result of normal cellular metabolism [6] during mitochondrial electron transport of oxygen in aerobic respiration [7]. Reactive oxygen species participate of physiological events like aging [8], and then, it is known how to be involved in the etiology of many diseases like cancer [7].
During exposure to oxidant agents, reduced glutathione (GSH) is oxidized faster than hemoglobin and others erythrocyte constituents, protecting them from oxidative degradation [9]. Reduced glutathione is used by glutahione peroxidase (GPx) to detoxify hydrogen peroxide and oxidized glutathione (GSSG) is formed as a result. Glutathione reductase is necessary to convert GSSG to GSH, which also contributes to the detoxification of hydrogen peroxide [10]. The enzyme glutathione peroxidase (GPx) uses GSH to convert H2O2 into H2O. This reaction results in glutathione oxidized (GSSG) [11,12].
Diurnal rhythms are approximate 24-hour cycles in the behavioral, physiological, and biochemical processes of organisms [13]. A 24-hour assessment of the antioxidant biomarkers in equine blood samples provides important biochemical data which will be used to determine health status, performance-related indices and/or illnesses. The aim of the present study was to test the hypothesis that glutathione peroxidase and a number of hematological biomarkers do not vary in adult resting mares over a 24- hour period.
2. Material and Methods
2.1. Ethical
The Universidade Federal Rural de Pernambuco Institutional Animal Care and Use Committee approved all methods and procedures used in this study (protocol # 62/2007-CTA/DZ).
2.2. Animals
Seven clinically healthy purebred Arabian mares (~10 years old, ~370 kg) were used in the present study. Before starting this experiment, mares were maintained together at pasture in the Equine Research Center of the Universidade Federal Rural de Pernambuco, Recife-PE (8˚1'10"S; 34˚57"14"O) for 30 days, receiving food-concentrated 700 g [crude protein (min.) 10% protein, ethereal extract (min.) 18% fat, digestible energy 4.37 Mcal/ kg] plus 1.500 g [crude protein (min.) %, ethereal extract (min.) %, digestible energy (min.) E], as well as water and mineral salt ad libitum on a daily basis.
2.3. Sample Timing
Venous blood was collected from the jugular using needles and vacuum tubes with previously refrigerated heparin. Samples were taken every two hours over a 24- hour period, except during the period after-meals, in which samples were taken every 30 minutes, totaling four samples in two hours.
During the sample collection, animals were kept in pickets and no exercise was induced to avoid any interference in the results.
2.4. Sample Analysis
Blood samples were placed immediately in refriggered heparin tubes in an isothermal box with ice and transported to the Applied Biotechnology Laboratory of Animal Production (BIOPA) within 5 - 10 min to analyze the glucose (GLU), total plasma protein (TPP), red blood cells (RBC), hemoglobin (HB), hematocrit (HT), red cell distribution width (RDW), white blood cells (WBC) and lymphocytes (LYM). Whole blood (heparin) was kept in a refrigerator (4˚C) for further analysis of the GPx within 24 hours of collection, as required for the Ransel kit. Plasma aliquots were stored at −20˚C until further uric acid analysis (about 3 weeks post-sampling).
An aliquot of blood from heparin tubes was centrifuged (15 min at 1200 g) to obtain plasma, which was immediately used to measure plasma glucose (Accucheck Advantage II) and total protein (refractometry). hemoglobin (HB), hematocrit (HT), white blood cells (WBC), lymphocytes (LYM), Red Blood Cell Distribution width (RDW-SD and RDW-CV) were assessed with an automatic cell counter, Sysmex Corp (pocH-100iv Diff). GPx activity was determined in whole blood by spectrophotometry at 340 nm, using a commercial Ransel 505® kit (Randox Laboratories Limited, UK). Plasmatic uric acid was also determined by spectrophotometry using a commercial kit (Doles, BR).
Methods that have been developed for the measurement of the antioxidant activity of fluids are all essentially inhibition methods: a free radical species is generated; there is an end point by which the presence of the radical is detected and the antioxidant activity of the added sample inhibits the end point by scavenging the free radical [14]. The Ransel 505® Kit, used in the present study, utilizes cumene hydroperoxide to generate hydrogen peroxide. Therefore, glutathione peroxidase is measured by the observation of both the initial and final inhibition point over a three minute period of the spectrophotometer reading.
2.5. Statistical Analysis
Results are expressed as means ± standard error of the mean (SEM). The results were analyzed using repeated measures one-way ANOVA. Post-hoc tests were performed using Tukey’s test. The significance level was set at P < 0.05 [15]. If no significant difference was found between times, the data were averaged, unless otherwise stated. SigmaStat 3.0 for Windows 7 was used for all analysis.
3. Results and Discussion
The results of the present study indicated that there is a diurnal rhythm for the antioxidant biomarkers glutathione peroxidase and uric acid (Figure 1) as well as for the biomarkers glucose and plasma total protein (Table 1).
The hematological biomarkers red blood cells, hemoglobin and hematocrit exhibited variations (Table 2).
 (a)
(a) (a)
(a)
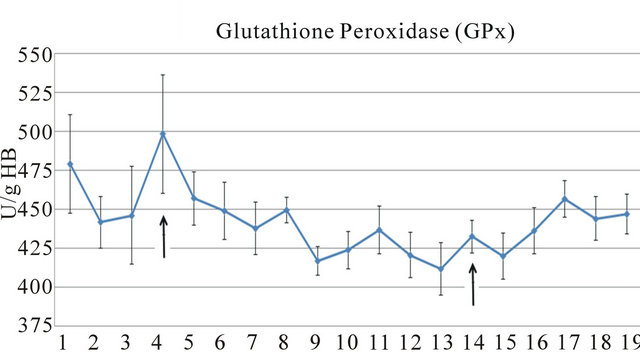
Figure 1. Alterations in levels of glutathione peroxidase (GPx) (a), and uric acid (UA) (b) over 24-hour period in mares. Arrows indicate the feeding periods.
Table 1. Change in concentration of glucose and total plasma protein along 24-hour period in mares.

Observations: #Feeding; Mean values with same superscript letters in a given column are not significantly different, whereas those with different superscript letters are significantly (P < 0.05) different as judged by Tukey’s test.
Table 2. Alterations in red blood cells, hemoglobin and hematocrit over a 24-hour period in mares.
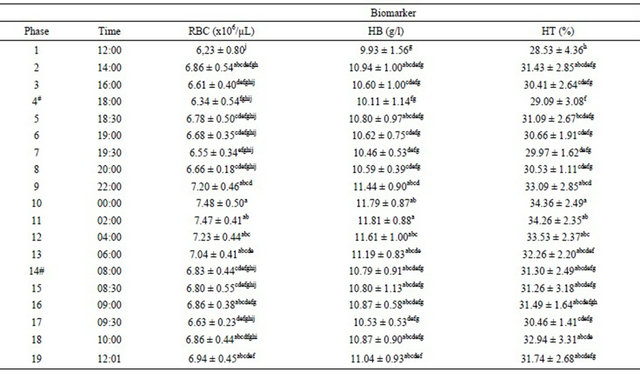
#Feeding; All values are Mean ± s.d.; Mean values with same superscript letters in a given column are not significantly different, whereas those with different superscript letters are significantly (P < 0.05) different as judged by Tukey’s test.
White blood cells, lymphocytes and Red Cell Distribution Width (SD/CV) did not vary throughout the test period.
The activity of erythrocyte glutathione peroxidase varied over the 24-hour period (Figure 1). This is the first time that 24-hour period of this important antioxidant biomarker has been demonstrated in mares. The data of the present study can be used in subsequent studies of GPx in equines. The maximum concentration of GPx was observed at 18:00 h (498.5 ± 100.66 IU/g Hb) and the minimum values occurred after 22:00 h (417.0 ± 24.19 IU/g Hb), extending to 8:30 (420.1 ± 39.72 IU/g Hb).
Smarsh et al. (2010) [16] tested various nutraceuticals in exercising horses and concluded that a single dose did not produce an effect on either oxidative stress or antioxidant status. Selenium supplementation affected mare and foal plasma, muscle and colostrum Se concentrations, but not GPx activity [17]. In the present study, the glutathione peroxidase did not vary after concentrate supplementation.
The lower activity of erythrocyte glutathione peroxidase in the nocturne period with less muscular activity, and in the diurnal period with higher muscular activity, was related to normal behavior. This finding suggests that the activity of this antioxidant biomarker is not influenced by the amount of muscular activity in this equine category. Martin et al. (2010) [13] observed that while horse activity and behavior may be greatly influenced by, and in some cases driven by, external environmental factors including interaction with humans.
However, this activity is significantly influenced by endogenous circadian rhythms (rhythms which represent the output of cellular autonomous circadian oscillators, i.e., 24-h cellular clocks) that exist both centrally (brain) and peripherally.
Moffarts et al. (2004) [18] used the same kit (Ransel) as the present study to investigate GPx in healthy Standardbred horses and found lower values (around half) of this antioxidant biomarker. This result could be partly explained by the different times of samples processing (8 h × 24 h), the breed studied or a different workload.
Previous studies have demonstrated a depletion of equine plasma glutathione peroxidase after exercise [19- 21] and after foaling [4]. Another study showed that training and exercise intensity significantly influenced blood antioxidant markers in healthy Standardbred horses [18].
Uric acid peaked 30 minutes after the first concentrated food supply (phase 5), but no statistically significant difference (P > 0.05) was found between that phase and other stages or after the second meal (phase 14). The plasma levels of uric acid were not influenced by feeding in the present study.
In phases 9 and 18, lower plasmatic uric acid levels were observed, with a 12-hour interval between these phases. It has long been known that the kidney is responsible for more than 70% of urate excretion to promote homeostasis [22]. The alternation between the lifting phase and the peak and decline in uric acid values over twelve hours can be related to the physiological regulation of this antioxidant biomarker to maintain homeostasis.
The concentrate was given to mares immediately after phases 4 and 14, and new samplings were taken every 30 minutes for a total of two hours. Plasma glucose increased after meals over the two hours, as previously described in the literature [23-25].
There was no change in TPP concentrations after meals. The intake of nutrients by concentrate food ingestion did not affect TPP, thereby corroborating the results of Greppi et al. (1996) [24], nor did it affect the other parameters analyzed, with the exception of glucose. Lower values of TPP were observed in phases 7 and 8 (19:30 and 20:00). On the other hand, higher values were recorded at 06:00 (phase 13). Wanderley et al. (2010) [26] studied Mangalarga-Marchador horses before, during and after exercise and observed possible alterations in plasma volume and dehydration, which were probably associated with increases in TPP concentration. This result could indicate that TPP circadian rhythm follows physiological hydric regulation.
Hematological values were not affected by food supplementation (Table 2). Higher hematological values (Table 2) were recorded previously in a studied with athletic horses in a jumping test and at rest [27]. The lower values found in the present study could be associated to the low levels of exercise performed by the mares. RBC, HB, and HT exhibited lower values in phase 1. However, this did not occur at the same time period on the next day, in the final phase [22]. Lumsden et al. [28] related that differences in hematological values could be determined by many environmental factors, management techniques and the equine category. Hematocrit and hemoglobin exhibited the highest values during the dark period, which is similar to the other results [24].
No alterations were found in RDW-SD, RDW-CV. Electronic hematological counters provided additional information about the cell volume in routine blood tests. This equipment calculates the erythrocyte diameter distribution, called Red Blood Cell Distribution Width, whose value reflects the degree of heterogeneity between the red blood cells through a quantitative analysis [29, 30].
No alterations were found in the number of lymphocytes over the 24-hour period. Calamari et al. [31] demonstrated that this number was not affected by antioxidant treatment in white blood cells.
The values of glutathione peroxidase, uric acid, glucose and total plasma protein revealed statistically similar results when comparing their initial and final values (phases 1 and 19) for each biomarker. On the other hand, statistically different results were found between the initial and final values of the hematological biomarkers.
The complexity of regulating the circadian of antioxidant biomarkers and hematological biomarkers certainly cannot be completely demonstrated in a single study and in a certain environmental condition. However, this study may help to clarify the circadian rhythm of horses, in conjunction with other previous and future studies [13,24,32-34].
4. Conclusion
The antioxidant biomarkers glutathione peroxidase and uric acid exhibited a diurnal variation. This variation was also found for plasma total protein and in hematological biomarkers, red blood cells, hemoglobin and hematocrit. However, white blood cells, lymphocytes and Red Cell Distribution Width (RDW-SD and RDW-CV) did not vary over time. The results of the present study suggest that the time of blood collection may be significant when interpreting the hematological and biochemical parameters of a clinical investigation.
5. Acknowledgements
The authors would like to thank Guabi Nutrição Animal for providing food for horses maintenance.
REFERENCES
- R. A. Jacob and B. J. Burri, “Oxidative Damage and Defense,” The American Journal of Clinical Nutrition, Vol. 63, No. 6, 1996, pp. 985S-990S.
- M. Valko, D. Leibfritz, J. Moncol, et al., “Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease,” The International Journal of Biochemistry & Cell Biology, Vol. 39, No. 1, 2007, pp. 44-84. doi:10.1016/j.biocel.2006.07.001
- M. Belviranli and H. Gökbel, “Acute Exercise Induced Oxidative Stress and Antioxidant Changes,” European Journal of Medical Genetics, Vol. 3, No. 3, 2006, pp. 126-131.
- R. Górecka, M. W. Kleczkowski, W. Klucinski, et al., “Changes in Antioxidant Components in Blood of Mares during Pregnancy and after Foaling,” Bulletin of the Veterinary Institute in Puławy, Vol. 46, No. 2, 2002, pp. 301- 305.
- N. Kirschvink, B. De Moffarts and P. Lekeux, “The Oxidant/Antioxidant Equilibrium in Horses,” The Veterinary Journal, Vol. 177, No. 2, 2008, pp. 178-191. doi:10.1016/j.tvjl.2007.07.033
- M. Valko, C. J. Rhodes, J. Moncol, et al., “Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer,” Chemico-Biological Interactions, Vol. 160, No. 1, 2006, pp. 1-40. doi:10.1016/j.cbi.2005.12.009
- R. Sapariya and P. Bhatt, “Significance of Oxidative Stress and the Role of Antioxidants in Cancer,” Research Journal of Pharmacology, Vol. 5, No. 5, 2011, pp. 59-67.
- T. C. Squier, “Oxidative Stress and Protein Aggregation during Biological Aging,” Experimental Gerontology, Vol. 36, No. 9, 2001, pp. 1539-1550. doi:10.1016/S0531-5565(01)00139-5
- H. S. Jacob and J. H. Jandl, “Effect of Sulfhydryl Inhibition on Red Blood Cells,” The Journal of Biological Chemistry, Vol. 241, No. 18, 1966, pp. 4243-4250.
- M. L. Urso and P. M. Clarkson, “Oxidative Stress, Exercise, and Antioxidant Supplementation,” Toxicology, Vol. 189, No. 1-2, 2003, pp. 41-54. doi:10.1016/S0300-483X(03)00151-3
- B. Halliwell, “Free Radicals and Antioxidants: A Personal View,” Nutrition Reviews, Vol. 52, No. 8, 1994, pp. 253-265. doi:10.1111/j.1753-4887.1994.tb01453.x
- M. Bacila, “Bioquímica Veterinária,” 2nd Edition, Robe, São Paulo, 2003.
- A. M. Martin, J. A. Elliott, P. Duffy, et al., “Circadian Regulation of Locomotor Activity and Skeletal Muscle Gene Expression in the Horse,” Journal of Applied Physiology, Vol. 109, No. 5, 2010, pp. 1328-1336. doi:10.1152/japplphysiol.01327.2009
- C. Rice-Evans and N. J. Miller, “Total Antioxidant Status in Plasma and Body Fluids,” Methods in Enzymology, Vol. 234, No. 24, 1994, pp. 279-293. doi:10.1016/0076-6879(94)34095-1
- I. B. M. Sampaio, “Estatística Aplicada à Experimentação Animal,” 3rd Edition, Editora Fundação de Ensino e Pesquisa em Medicina Veterinária e Zootecnia, 2007.
- D. N. Smarsh, N. Liburt, J. Streltsova, et al., “Oxidative Stress and Antioxidant Status in Intensely Exercising Horses Administered Nutraceutical Extracts,” Equine Veterinary Journal, Vol. 42, No. S38, 2010, pp. 317-322. doi:10.1111/j.2042-3306.2010.00182.x
- B. J. Karren, J. F. Thorson, C. A. Cavinder, et al., “Effect of Selenium Supplementation and Plane of Nutrition on Mares and Their Foals: Selenium Concentration and Glutathione Peroxidase,” Journal of Animal Science, Vol. 88, No. 3, 2010, pp. 991-997. doi:10.2527/jas.2008-1743
- B. Moffarts, N. Kirschvink, T. Art, et al., “Impact of Training and Exercise Intensity on Blood Antioxidant Markers in Healthy Standardbred Horses,” Equine and Comparative Exercise Physiology, Vol. 1, No. 3, 2004, pp. 211-220.
- C. A. Williams, D. S. Kronfeld, M. T. Hess, et al., “Antioxidant Supplementation and Subsequent Oxidative Stress of Horses during an 80-Km Endurance Race,” Journal of Animal Science, Vol. 82, No. 2, 2004, pp. 588-594.
- B. J. Hargreaves, D. S. Kronfeld, J. N. Waldron, et al., “Antioxidant Status of Horses during Two 80-Km Endurance Races,” Journal of Nutrition, Vol. 132, No. 6, 2002, pp. 1781S-1783S.
- M. Janiak, M. Suska, W. Dudzin’Ska, et al., “Blood Glutathione Status and Activity of Glutathione-Metabolizing Antioxidant Enzymes in Erythrocytes of Young Trotters in Basic Training,” Journal of Animal Physiology and Animal Nutrition, Vol. 94, No. 2, 2010, pp. 137-145. doi:10.1111/j.1439-0396.2008.00889.x
- M. S. Lipkowitz, “Regulation of Uric Acid Excretion by the Kidney,” Current Rheumatology Reports, Vol. 14, No. 2, 2012, pp. 179-188.
- C. L. Depew, J. R. D. L. Thompson, J. M. Fernandez, et al., “Changes in Concentration of Hormones, Metabolites, and Amino Acids in Plasma of Adult Horses Relative to Overnight Feed Deprivation Followed by a Pellet-Hay Meal Feet at Noon,” Journal of Animal Science, Vol. 72, No. 6, 1994, pp. 1530-1539.
- G. F. Greppi, L. Casini, D. Gatta, et al., “Daily Fluctuations of Haematology and Blood Biochemistry in Horses Fed Varying Levels of Protein,” Equine Veterinary Journal, Vol. 28, No. 5, 1996, pp. 350-353. doi:10.1111/j.2042-3306.1996.tb03104.x
- A. O. Gobesso, M. Etchichury and H. Tosi, “Resposta Plasmática de Glicose e Insulina em Equinos Alimentados com Diferentes Fontes de Amido,” Brazilian Journal of Veterinary Research and Animal Science, Vol. 46, No. 4, 2009, pp. 324-331.
- E. K. Wanderley, H. C. Manso-Filho, H. E. C. C. Manso, et al., “Metabolic Changes in Four-Beat Gaited Horses after Field Marcha Simulation,” Equine Veterinary Journal, Vol. 42, No. S38, 2010, pp. 105-109. doi:10.1111/j.2042-3306.2010.00288.x
- D. C. R. Dias, J. S. Rocha, F. M. Mello, et al., “Influência do Exercício Sobre o Hemograma, Enzimas Marcadoras de Lesão Muscular e Índice de Peroxidação de Biomoléculas em Equinos Submetidos à Atividade de Salto,” Revista Brasileira de Ciência Veterinária, Vol. 18, No. 1, 2011, pp. 36-42.
- J. H. Lumsden, R. Rowe, K. Mullen, “Hematology and Biochemistry Reference Values for the Light Horse,” Canadian Journal of Comparative Medicine, Vol. 44, No. 1, 1980, pp. 32-42.
- G. Weiser, C. Kohn and A. Vachon, “Erythrocyte Volume Distribution Analysis and Hematologic Changes in Two Horses with Immune-Mediated Hemolytic Anemia,” Veterinary Pathology, Vol. 20, No. 4, 1983, pp. 424-433. doi:10.1177/030098588302000405
- M. R. S. Balarin, R. S. Lopes, A. Kohayagawa, et al., “Valores da Amplitude de Distribuição do Tamanho dos Eritrócitos (RDW) em Equinos Puro Sangue Inglês (PSI) Submetidos a Exercício de Diferentes Intensidades,” Brazilian Journal of Veterinary Research and Animal Science, Vol. 43, 2006, pp. 637-641.
- L. Calamari, F. Abeni and G. Bertin, “Metabolic and Hematological Profiles in Mature Horses Supplemented with Different Selenium Sources and Doses,” Journal of Animal Science, Vol. 88, No. 2, 2010, pp. 650-659. doi:10.2527/jas.2009-1855
- L. C. Golland, D. L. Evans, G. M. Stone, et al., “Maximal Exercise Transiently Disrupts Hormonal Secretory Patterns Standardbred Geldings,” Equine Veterinary Journal, Vol. 30, No. S30, 1999, pp. 581-585. doi:10.1111/j.2042-3306.1999.tb05288.x
- O. M. Lepage, L. Descoteaux, M. Marcoux, et al., “Circadian Rhythms of Osteocalcin in Equine Serum. Correlation with Alkaline Phosphatase, Calcium, Phosphate and Total Protein Levels,” Canadian Journal of Veterinary Research, Vol. 55, No. 1, 1991, pp. 5-10.
- G. K. Noble, E. Houghton, C. J. Roberts, et al., “Effect of Exercise, Training, Circadian Rhythm, Age, and Sex on Insulin-Like Growth Factor-1 in the Horse,” Journal of Animal Science, Vol. 85, No. 1, 2007, pp. 163-171. doi:10.2527/jas.2006-210
NOTES
*Corresponding author.

