Advances in Microbiology
Vol.3 No.8A(2013), Article ID:41297,9 pages DOI:10.4236/aim.2013.38A009
Diversity of Filamentous Fungi of Area from Brazilian Caatinga and High-Level Tannase Production Using Mango (Mangifera indica L.) and Surinam Cherry (Eugenia uniflora L.) Leaves under SSF
1Department of Mycology, Federal University of Pernambuco, Recife, Brazil
2Academic Unit of Garanhuns, Federal Rural University of Pernambuco, Garanhuns, Brazil
Email: *cristina.motta@ufpe.br
Copyright © 2013 Roberta Cruz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 2, 2013; revised November 2, 2013; accepted November 9, 2013
Keywords: Caatinga; Filamentous Fungi; Tannase; Solid-State Fermentation; Mangifera indica L.
ABSTRACT
Tannase is a biotechnologically important enzyme that can be produced during fungal fermentation of organic matter. The Caatinga is an exclusive Brazilian ecosystem that has been largely unexplored by science, particularly its filamentous fungal diversity. This study evaluated the diversity of filamentous fungi in the Caatinga soils of Pernambuco, Brazil, and their potential for tannase production by solid-state fermentation (SSF) of mango (Mangifera indica L.) and Surinam cherry (Eugenia uniflora L.) leaves. A total of 4711 isolates were obtained, 2090 during the rainy seasonand 2621 during the dry season. The isolates belonged to 18 genera and 66 species, with Aspergillus and Penicillium having the highest species richness. The dry season had a higher diversity index. Aspergillus was the dominant genus, and A. flavus, A. sclerotiorum, and A. ochraceus the most abundant species. A representative of each species was tested for tannase production using dried mango and Surinam cherry leaves as substrates; the leaves contained 14.28 and 7.0 g/L tannin, respectively. Most fungal species produced tannase, but the highest yields were obtained when mango leaves were used as substrate for Penicillium restrictum (accession URM 6044), Aspergillus flavofurcatus (URM 6142), and A. stromatoides (URM 6609), which produced 104.16, 87.51, and 81.83 U/mL tannase, respectively. These yields exceeded previously published reports. Filamentous fungi from Caatinga soils have great potential for producing tannase by SSF, and low-cost mango leaves make excellent substrate.
1. Introduction
The Caatinga is an ecosystem unique to Brazil that is home to many endemic species of plants, animals, and microorganisms. It has a semi-arid climate, with high temperatures and low rainfall, between 200 and 800 mm per year [1]. The soils, with rare exceptions, are very shallow, not flooded, mineralogically rich, stony, and with poor water-holding capacity [2]. Most rainfall in the Caatinga (50% - 70%) is concentrated in three consecutive months, with long periods of drought common [1].
The National Park Catimbau (Catimbau Valley) is an important region of the Caatinga of Pernambuco, Brazil, considered the second largest archaeological park in Brazil. This fully-protected conservation area was created in 2002 [3]. The park is an excellent resource for both archaeological and ecological studies, especially for studies of filamentous fungi of biotechnological interest, where unusual or new to science species are found.
Filamentous fungi comprise a diverse group of microscopic forms that are widely distributed in nature [4]. In fact, the vast majority of fungal species probably spend some part of their life cycles as soil filaments and play key roles in cycling organic matter in soil ecosystems [5].
In addition to their essential role in decomposition, some species of fungi have broad biotechnological potential and are widely used to produce economically-important enzymes [6]. Tannase is one such enzyme [7].
Tannase (tannin acyl hydrolase EC3.1.1.20) hydrolyzes ester linkages and side-hydrolyzable tannins, such as tannic acid, gallic acid, and glucose. Its extracellular production by bacteria, yeasts and filamentous fungi can be induced in the presence of tannic acid. The genera Aspergillus and Penicillium are excellent producers of this enzyme [8,9]. Tannase has wide utility in the beverage (especially in juice and beer production), cosmetics, pharmaceutical, and chemical industries [10].
Due to the importance of microbial tannase in various industries, production processes are necessary, which will minimize costs, increase yield, and contribute directly to environmental balance. For example, many agro-industrial wastes are commonly discarded into the environment untreated [11]. In this context, solid-state fermentation (SSF) by fungi presents an excellent alternative for producing tannase while simultaneously reducing waste [7]. Because filamentous fungi grow in nature in solid substrates, such as wood, roots, stems, and leaves of plants, in the absence of free water [12].
Successful production of tannase by SSF has been reported using palm kernel cake, bran tamarind seeds, and jambul leaves (Syzygium cumini) as carbon sources [12, 13]. This study is the first to assess the diversity of filamentous fungi isolated from Caatinga soils of Northeastern of Brazil and their biotechnological potential for SSF production of tannase using mango leaves (Mangifera indica L.) and Surinam cherry (Eugenia uniflora L.) as low-cost substrates.
2. Materials and Methods
2.1. Study Area and Sample Collection
Six soil samples were collected at the Catimbau National Park, Buíque, Pernambuco, Brazil (S08˚04'25", W37˚15'52"). Three sets of samples were collected in the dry months of June, July, and August, 2009, when rainfall was 0.0, 11.0, and 0.0 mm, respectively, while three were taken during the rainy season in February, March, and April, 2010, when rainfall was 109.0, 120.0, and 236.0 mm, respectively. At each time, ten soil samples were collected in each of three 4 × 25 m transects at a depth of 0 - 20 cm. The ten samples from each transect were combined to give a single composite sample. A total of 18 samples were obtained (six months × three transects each). Soil temperature was measured using a digital thermometer (UT325 Contemp-São Paulo, Brazil). All samples were transported at room temperature in sterilized thin polyethylene plastic bags to the research laboratory of the URM (University of Recife Mycology) culture collection [14].
2.2. Isolation and Purification
Fungi were isolated using suspension methods [15]. All 18 composite soil samples were suspended in sterile distilled water and successively diluted to a final concentration 1:10,000 g/mL. Each dilution was inoculated into five Petri dishes containing Sabouraud agar medium supplemented with 50 mg/L chloramphenicol (SA-C) and five Petri dishes containing dichloran agar medium with rose bengal supplemented with 50 mg/L chloramphenicol. Overall, 180 Petri dishes of Caatinga soil extracts were obtained. The plates were incubated at 28˚C (±2˚C) for 72 h [14].
To purify fungal isolates, fragments of fungal colonies were transferred to Petri dishes containing SA-C medium. After confirmation of purity, the fungal cultures were maintained on malt extract agar medium (MEA) or on potato dextrose agar medium at 25˚C (±2˚C) for later identification based on specific literature [16-25].
2.3. Soil Analysis
The pH was measured by mixing the soil with water (1:2.5 ratio). The available concentrations of aluminum (Al), calcium (Ca), and magnesium (Mg) were extracted from the soil using 1 M KCl solution in the ratio 1:10 and quantified by titration. Potassium (K), sodium (Na), and phosphorus (P) were extracted with Mehlich solution in a 1:1 (soil:solution) ratio. The K and Na levels were determined by flame photometry (Analyser 910, São Paulo, Brazil), and the P level using a spectrophotometer (Spectrum, SP-1105, São Paulo, Brazil) at 725 nm. The potential acidity (H+Al) was extracted with calcium acetate and quantified by titration [26].
2.4. Comparison of Filamentous Fungal Diversity between the Dry and Rainy Seasons
The diversity of filamentous fungi in the rainy and dry seasons was compared using the Shannon-Wiener index [27] calculated with the program NTSYSpc v. 2.21 [28].
2.5. Principle Component Analysis (PCA)
The original matrix of biotic and abiotic Caatinga was reduced to species that occurred at less than 70% frequency. Then the data were standardized so that parameters with different units could be compared. The Pearson product-moment correlation coefficient between parameters was calculated and used to order the factors to extract eigenvectors and eigenvalues. We projected the first two factors in two-dimensional space, using factors measured outside the sampling sites. The principle component analysis (PCA) was conducted in NTSYSpc. 2.21 [28].
2.6. Solid Substrates
Leaves of mango (Mangifera indica L.) and Surinam cherry (Eugenia uniflora L.) were collected in the Atlantic Forest region in the city of Jaboatão of Guararapes, Pernambuco. The leaves were rinsed with distilled water and dried in an oven at 55˚C for 72 h. The dry material was finely ground in a food processor (Maggiore Pro 1458001, Mallory-Ceará, Brazil) to produce particles of approximately 50 µm, then stored in a dark container at room temperature until the time of fermentation [29].
2.7. Microorganisms
A representative of each isolated species was used in the fermentations. The strains were grown and maintained on MEA slants at 30˚C. Cultures were preserved at 4˚C for short-term storage.
2.8. Inoculum Preparation
Each species isolated, grown for 7 days on MEA was prepared a spore suspension in 10 ml of sterile distilled water with 1% Tween 80. The spores were transferred with the aid of platinum loop. After homogenization, the spores present in the suspension were counted with the aid of a Neubauer chamber [13].
2.9. Moistening Medium and Preparation of SSF Medium for Inoculation
Five grams of each substrate were added separately in Erlenmeyer flasks type. They were then autoclaved at 121˚C for 20 minutes. The medium used to moisten the SSF was prepared salt solution with 0.5% w/v NH4NO3, 0.1% w/v and MgSO4∙7H2O 0.1% w/v NaCl and autoclaved at 121˚C for 20 minutes. In each Erlenmeyer flask was added 5 ml of salt solution and 1 ml of the spore suspension. The contents were mixed and then incubated at 30˚C for 96 hours [13].
2.10. Tannin Estimation
Tannin content was estimated following the protein precipitation method [30]. Dried leaves were ground into 50 µm particles in methanol and kept overnight at 4˚C. One mL of extract was mixed with 3 mL of BSA solution and kept for 15 min at room temperature. The tubes were centrifuged at 5000 g for 10 min, the supernatant was discarded, and the pellet was dissolved in 3 mL of SDStriethanolamine solution. One milliliter of FeCl3 solution was added and tubes were kept for 15 min at room temperature for color stabilization. Color was read at 530 nm against a blank.
2.11. Enzyme Extraction
After fermentation, in each Erlenmeyer flask were inserted 50 mL of distilled water containing 0.01% Tween 80, previously sterilized. Then the flasks were shaken on shaker (Tecnal TE421, São Paulo, Brazil) at 150 rpm for 10 min to complete mixing of the contents. Then the crude extract was obtained by filtration using Whatman filter paper number 1. The filtrate was collected in flasks and preserved at 4˚C for later analysis [13].
2.12. Enzyme Assay
Tannase activity was estimated through the formation of a chromogen between gallic acid (released by the action of tannase on methyl gallate) and rhodanine (2-thio-4- ketothiazolidine), in which pink color developed was read at 520 nm using a spectrophotometer (HitachiU5100). One unit of tannase activity was defined to the amount of enzyme required to liberate one micromole of gallic acid perminute under defined reaction conditions. Enzyme yield the expressed was units/mL/min [31].
3. Results
We obtained 4711 fungal isolates, 2090 in the rainy season and 2621 in the dry season. The isolates represented 18 genera, including 13 Ascomycota and five Zygomycota, and a total of 66 species. The ascomycotes were: Acremonium (1 specie), Aspergillus (23), Chaetomium (1), Curvularia (1), Eupenicillium (1), Fusarium (3), Gliomastix (1), Neocomospora (1), Neosarthorya (1), Papulaspora (1), Penicillium (26), Scopulariopsis (1), and Talaromyces (1). The zygomycotes were: Absidia (1), Gongronella (1), Mortierella (1), Rhizopus (1), and Syncephalastrum (1). A representative of each species was incorporated into the Catalogue of Micoteca URM Culture Collection (WDCM604) of the Federal University of Pernambuco, Recife, Brazil (Table 1).
During the rainy season, the average soil temperature was 26˚C and the dry period, 40˚C. According to the Shannon-Wiener index, the overall diversity of filamentous fungi in the Caatinga was high. However, the dry period had higher diversity (3078 bits∙ind−1) than the rainy season (2486 bits∙ind−1) (Figure 1). The rainy season had greater species richness (53 taxa) than the dry (33) (Table 1).
The genera with the highest species richness were Penicillium and Aspergillus. Although more species (26) of Penicillium were present, their populations had few individuals, 99 in the rainy season and 281 in the dry season. Aspergillus, with 23 species, had larger populations and was the dominant genus, with 1727 individuals
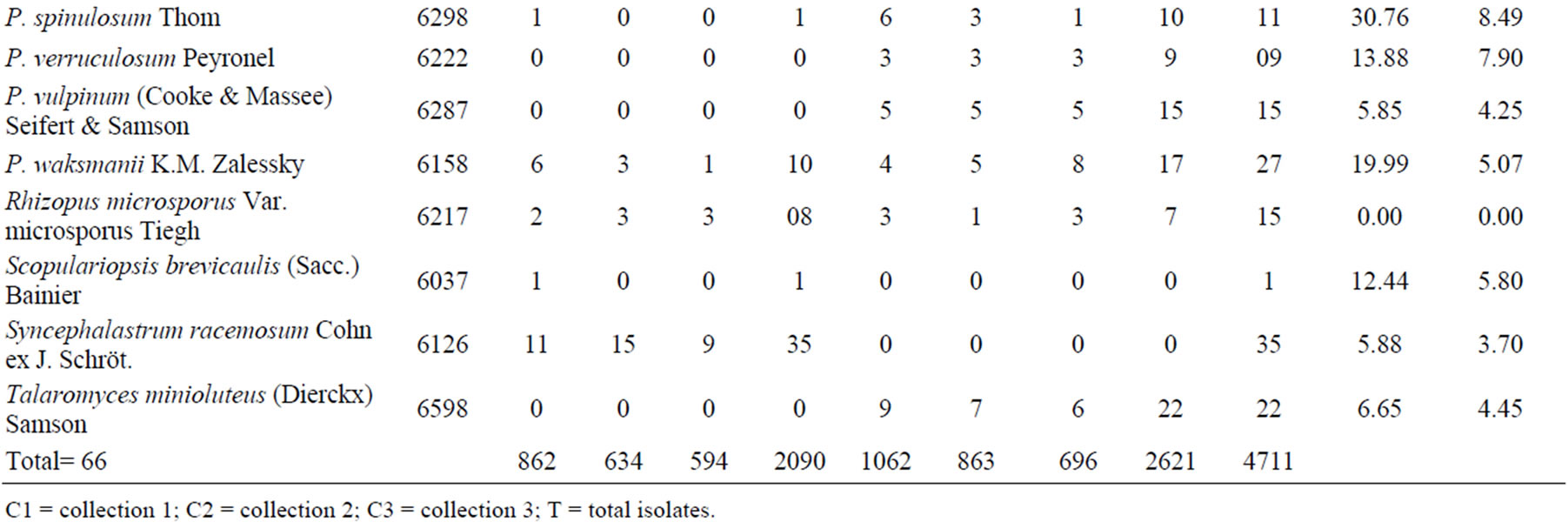
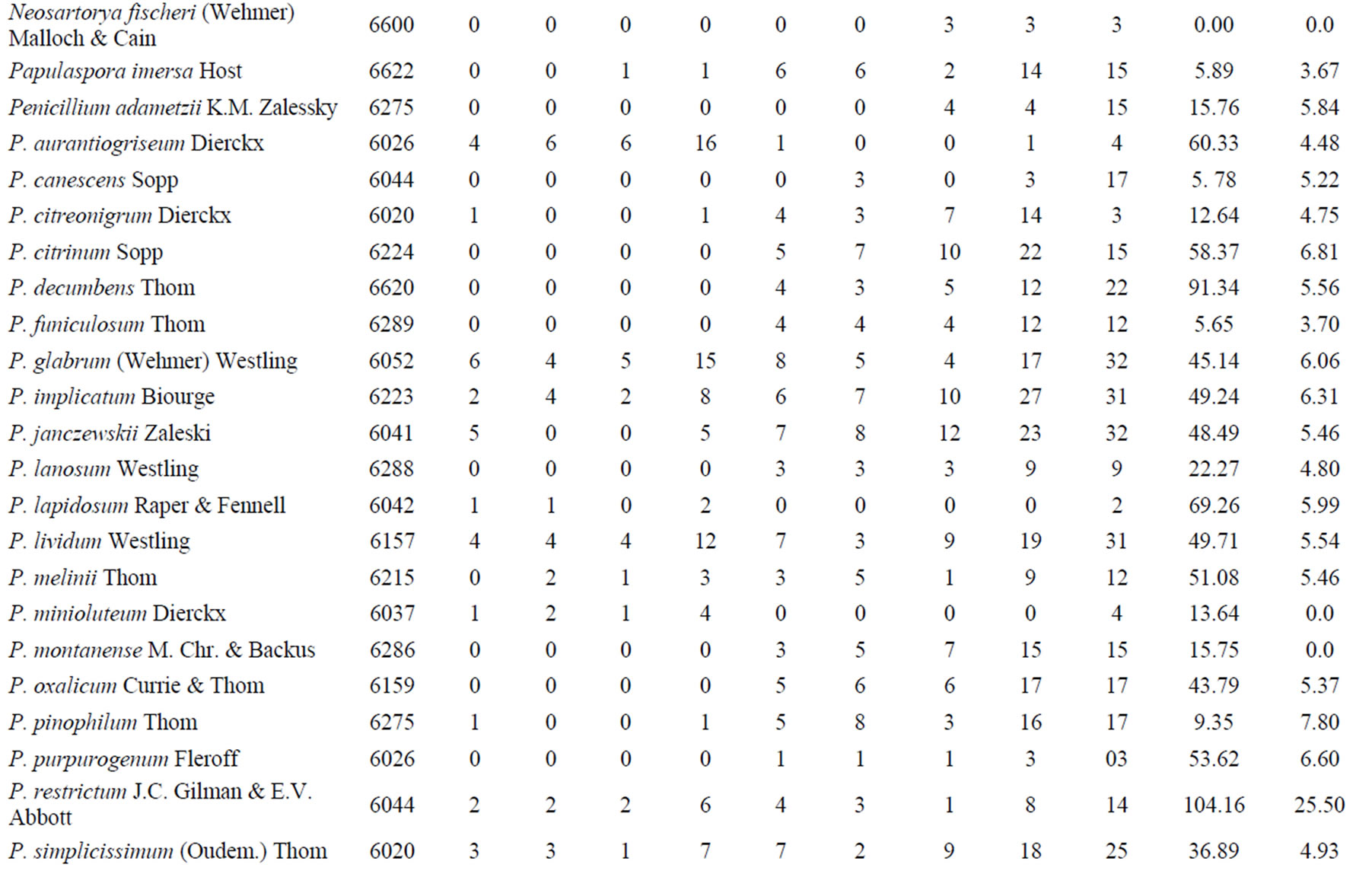


Table 1. Number of isolates of filamentous fungi species collected during the rainy and dry season in the Caatinga area and activity of Tannase species.
C1 = collection 1; C2 = collection 2; C3 = collection 3; T = total isolates.
in the rainy season and 2082 in dry season. Aspergillus flavus, Asp. sclerotiorum, and Asp. ochraceus were the most the abundant species (Table 1).
The physico-chemical analyses of the soil samples revealed a pH range of 4.0 - 7.9 among the 18 collections. There was variation in the Al concentration (0.1 - 0.5 cmolc∙dm−3), Ca (0.60 - 9.60 cmolc∙dm−3), H+Al (0.8 - 2.10 cmolc∙dm−3), K (0.12 - 0.86 cmolc∙kg−1), Mg (0.7 - 2.90 cmolc∙kg−1), Na (0.25 - 0.50 cmolc∙kg−1) and P (3.70 - 280.19 cmolc∙kg−1) (Table 2).
The first three factors of the PCA performed with species having more than 70% frequency of occurrence explained 68.47% of the data variation; these factors were
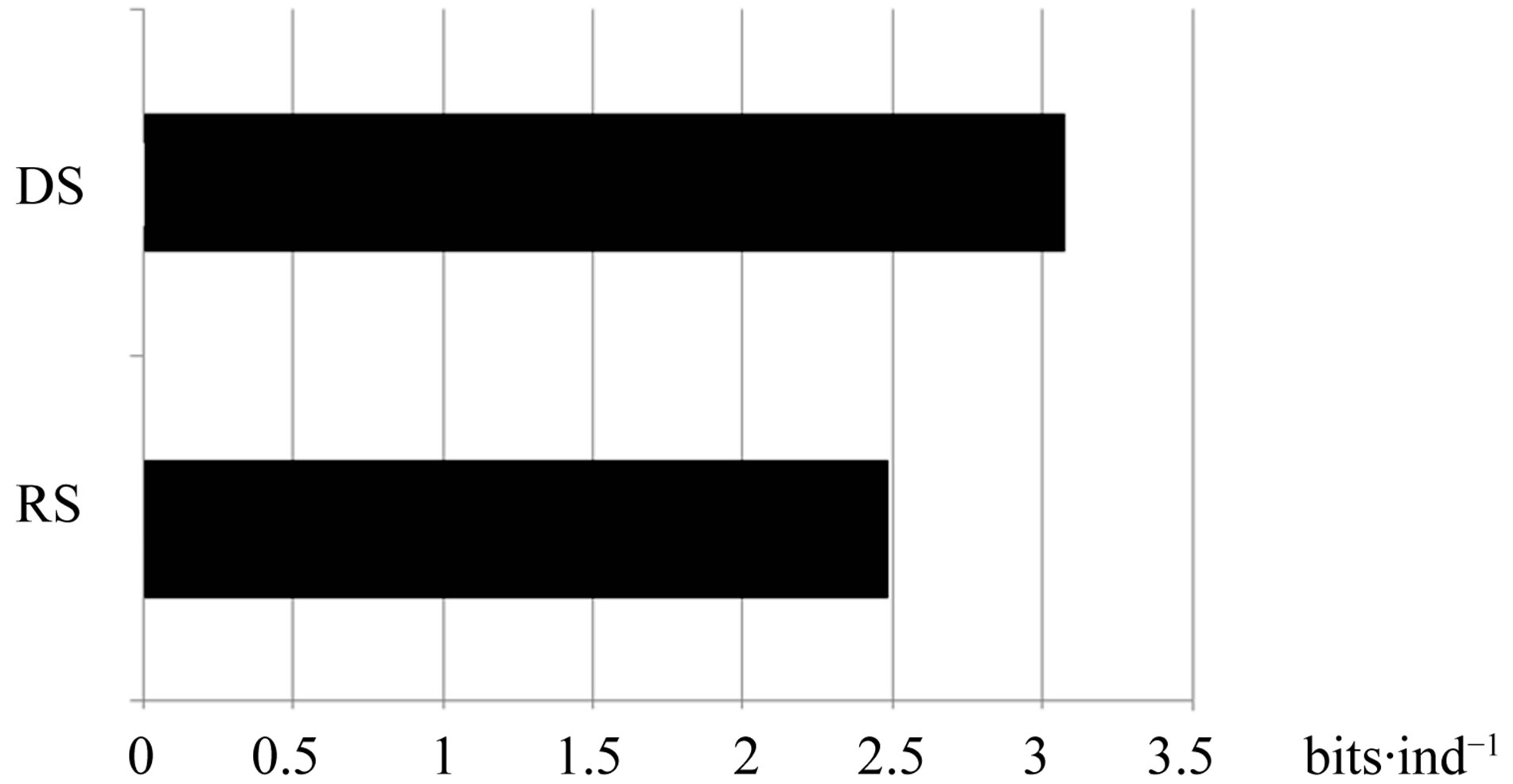
Figure 1. Diversity (bits∙ind−1) of filamentous fungi species in the Caatinga in the rainy season (RS) and dry season (DS). Statistical analysis based on Shannon index.
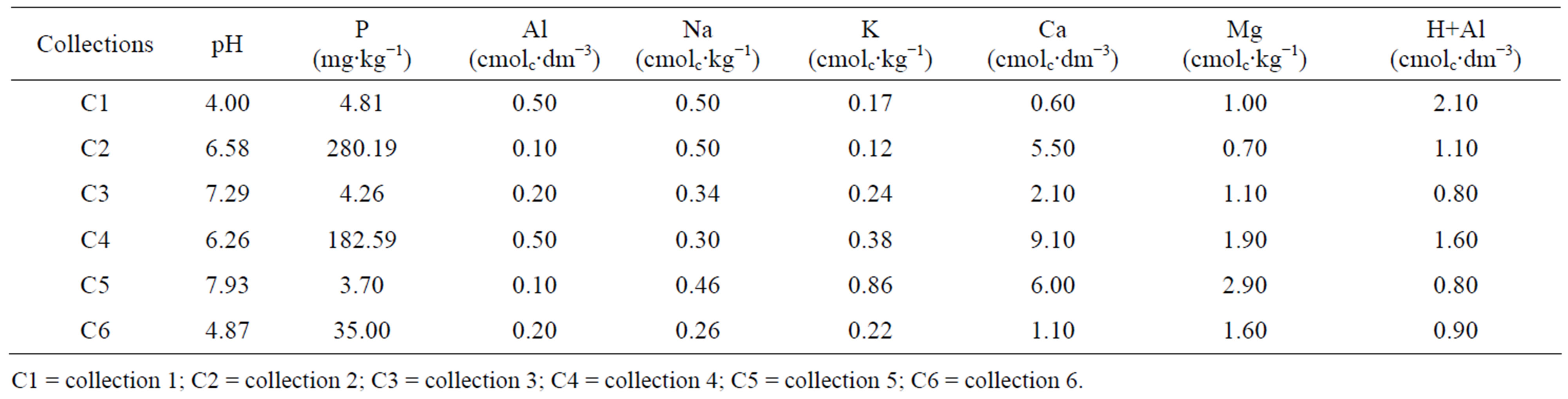
Table 2. Analysis of abiotic factors of soil samples from the area of Caatinga (Catimbau Valley, Buíque-Pernambuco, Brazil).
C1 = collection 1; C2 = collection 2; C3 = collection 3; C4 = collection 4; C5 = collection 5; C6 = collection 6.
associated with the main abiotic soil parameters. The first factor explained 31.43% of the variation in the data, the second 21.59%, and the third 15.44% (Figure 2). In the first factor, temperature, Al content, and the species Penicillium implicatum, P. janczewski, P. simplicissimum and P. waksmanii were directly correlated with one another. These parameters were inversely correlated with Mg content and Asp. ochraceus. In the second factor, Acremonium terricola, Asp. parasiticus, Asp. niger, and P. glabrum were inversely related to Asp. flavus, Rhizopus microsporus, and Ca and H+Al levels. In the third factor, the levels of P and Na presented directly correlated to P. lividum, which were inversely correlated with Asp. sclerotiorum, pH, and K (Figure 2).
Dried mango and Surinam cherry leaves contained 14.28 and 7.0 g/L tannins, respectively. Higher production of tannase was obtained when mango leaves were used as the substrate for filamentous fungi in SSF. Penicillium restrictum, Asp. flavofurcatus, and Asp. stromatoides were the best producers, generating 104.16, 87.51, and 81.83 U/mL, respectively. Seven species did not produce tannase: Chaetomium cupreum, Gliomastix murorum, Gongronellabutleri, Mortierella ramanniana, Neocomospora vasinfecta, Neosartorya fischeri, and R. microsporus. When Surinam cherry leaves were used as substrate, the tannase production was quite low, with maximum activity (25.50 U/mL) also expressed by P. restrictum. Twelve species did not produce tannase: Asp. sclerotiorum, Asp. Stromatoides, Asp. sulphureus, C. cupreum, Eupenicillium shaerii, Fusarium redolens, F. solani, Gl. murorum, Gon. butleri, M. ramanniana, Neoc. vasinfecta, Neos. fischeri, P. minioluteum, P. montanense, and R. microsporus (Table 1).
4. Discussion
The semiarid Caatinga, compared with other Brazilian formations, has many extreme meteorological characteristics: high solar radiation, the highest average annual temperature, few clouds, the lowest relative humidities, high potential evapotranspiration, and, in particular, lower and extremely seasonal rainfall in most of the area that occurs during a very short period of the year [32]. According to Cavalcanti et al. [4], such environmental characteristics may favor the development of xerophilic filamentous fungi in these semiarid soils.
According Werneck [33], the Caatinga biome has received little scientific research attention relative to other tropical forests, although the Caatinga biome has high diversity that was not previously recognized. However, there remains much to be studied, especially with regard to soil fungi. This study corroborated the estimates of Werneck [33], as revealed by the high diversity of filamentous fungi in Catimbau Valley soil, especially in the dry season.
In semi-arid region of Xingó, Bahia, Brazil, Cavalcanti et al. [4] evaluated the diversity of filamentous fungi in soil municipalities. The authors isolated and identified 96 taxa and found that the two most representative genera were Penicillium and Aspergillus, with 31 and 18 species, respectively. The results obtained in this study corroborate those found by Cavalcanti et al. [4], where Penicillium and Aspergillus had the most species, with 26 and 23, respectively.
Although present in dry soils, most species of Penicillium prefer soils with available water [22]. According to Klich [34], who studied the biogeography of Aspergillus in samples of soil and leaf litter, noted that this genus occurs more frequently in desert environments, supporting our findings of large populations of Aspergillus Catimbau Valley soils.
Studies of the diversity of filamentous fungi in Caatinga soils are scarce. In 2006, Santiago and Souza-Motta [35] assessed the diversity of Zygomycota present in Caatinga soils in Bahia, Brazil and identified seven species, Absidia blakesleeana Lendner, Abs. cylindrosporaHagem, Abs. hialospora (Saito) Lendn., Cunninghamella elegans Lendner, R. microsporus V. Thieghen, R. oryzae Went. & Prinsen Geerl., and Syncephalastrum racemosum (Cohn.) Schroet. Only five Zygomycota were found in this study, Abs. cylindropora, Gon. butleri, M. ramanniana var. angulispora, R. microsporus var. microsporus and Syn. racemosum.
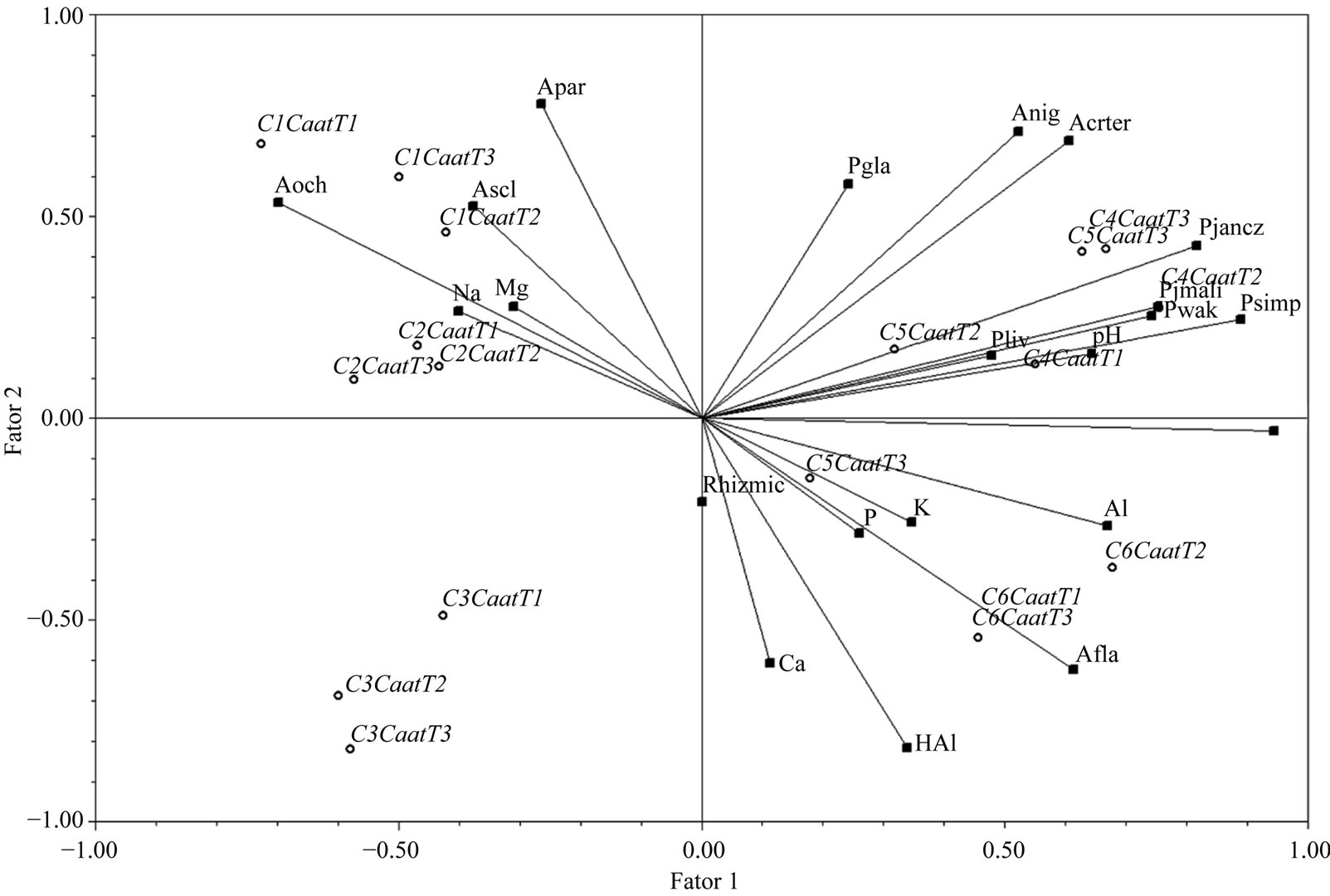
Figure 2. Two-dimensional projection of the first two factors of the principal component analysis of Caatinga area studied. (Fator 1) = Factor 1; (Fator 2) = Factor 2.
The PCA revealed the correlations, both positive and negative, between some species abundances and some physical parameters. Aspergillus flavus, which is common in soils [20] was the dominant species in the Caatinga biome study area. The population levels of Asp. flavus and R. microsporus var. microsporus were inversely correlated with those of Acremonium terricola, Asp. Parasiticus, Asp. niger, and P. glabrum, indicating that these species compete with each other, and with levels of Ca and H+Al, indicating that these abiotic parameters directly influence the competition.
The populations of P. implicatum, P. janczewski, P. simplicissimum, and P. waksmanii tended to increase with soil temperature and Al levels, while the population of Asp. ochraceus and Mg levels tended to decrease. The abundance of Aspergillus sclerotiorum, the second largest population, was directly correlated to higher pH and K level and inversely correlated to P and Na levels and the abundance of P. lividum. Aspergillus ochraceus was the third most abundant species in the soil and therefore dominant to and competing directly with P. implicatum, P. janczewski, P. simplicissimum, and P. waksmanii probably by outcompeting them for soil nutrients.
In addition to the high fungal diversity in the study area, some species had substantial potential to produce tannase at activity levels higher than those previously reported in the literature. Kumar et al. [12] evaluated the production of tannase by different fungal species in SSF using leaves from jujube (Zyzyphus mauritiana), jambul (Syzygium cumini), aamla (Phyllanthus emblica), and jowari (Sorghum vulgare). The authors observed a maximum yield of 69 U/g dry tissue after 96 h incubation at 30˚C for Asp. ruber fermenting leaves of jambul. Those results were far below those found in the present study, which was observed maximum production of 104.16 U/mL tannase by P. restrictum URM when mango leaves were used as substrate, indicating that mango leaves make promising substrates for SSF production of fungal tannase.
Manjit et al. [36] evaluated tannase production by SSF using Asp. fumigatus isolated from tannery effluents. As substrate, leaves from amla, jujube, jambul, Syzygium sp., and kikar (Acacia nilotica), typical Indian plants that acted as inducers of enzyme production. After optimization of production the authors observed maximum yield of tannase was 174.32 U/g at 25˚C after 96 h of incubation at pH 5.0. In this study, P. restrictum URM 6044 produced 104.16 U/mL under unoptimized conditions; presumably this value could be considerably improved after optimization.
In 2012, Selwal & Selwal [7] evaluated tannase production by a strain of P. atramentosum from tannery effluents in SSF using amla, jujube, jambul, Syzygium sp., and kikar leaves. The authors observed maximum tannase yields of 170.75 and 165.56 U/g dry tissue from jambul and kikar leaves, respectively, incubated at 28˚C for 96 h. The form of showed of the results of this study are different from literature, as presented in U/mL, numerically lower results are to be compared with these data. The mango leaves fermented showed maximum production of 104.16 U/mL tannase by P. restrictum URM 6044 in a preliminary screening appear much more promising for producing tannase.
5. Conclusion
The results of this study are very promising for the production of tannase, because the filamentous fungi isolated from Catimbau Valley soil produced high levels of the enzyme using low-cost mango leaves as substrate. Penicillium restrictum URM 6044, Asp. flavofurcatus URM 6142, and Asp. stromatoides URM 6609 are recommended for use in subsequent studies to optimize tannase production by SSF of mango leaves.
6. Acknowledgements
The authors thank to the Foundation for Science and Technology of the State of Pernambuco (FACEPE) for financial support, as well as Dr. Sigrid Newman (UFPE), Paulo Freitas and Researcher Dr. Francisco Rangel (CETENE) for all support during the fungal analyses and identification.
REFERENCES
- I. R. Leal, J. M. C. Silva, M. Tabarelli and T. E. Lacher Jr., “Mudando o Curso da Conservacão da Biodiversidade na Caatinga do Nordeste do Brasil,” Megadiversidade, Vol. 1, No. 1, 2005, pp. 139-146.
- J. J. A. Alves, M. A. Araújo and S. S. Nascimento, “Degradação da Caatinga: Uma Investigação Ecogeográfica,” Caminhos de Geografia, Vol. 9, 2009, pp. 143-155.
- L. Geise, R. Paresque, S. Harley, L. T. Shirai, D. Astúa and G. Marroig, “Non-Volantmammals, Parque Nacional do Catimbau, Vale do Catimbau, Buíque, State of Pernambuco, Brazil, with Karyologic Data,” Check List, Vol. 6, No. 1, 2010, pp. 180-186.
- M. A. Q. Cavalcanti, L. G. Oliveira, M. J. S. Fernandes and D. M. Lima, “Fungos Filamentosos Isolados do Solo em Municípios na Região Xingó, Brasil,” Acta Botanica Brasilica, Vol. 20, No. 4, 2006, pp. 831-837. http://dx.doi.org/10.1590/S0102-33062006000400008
- P. Bridge and B. Spooner, “Soilfungi: Diversity and Detection,” Plant Soil, Vol. 232, No. 1-2, 2001, pp. 147-154. http://dx.doi.org/10.1023/A:1010346305799
- P. Puangsombat, U. Sangwanit and D. Marod, “Diversity of Soil Fungi in Different Land Use Types in Tha KumHuai Raeng Forest Reserve, Trat Province,” Natural Sciences, Vol. 44, 2010, pp. 1162-1175.
- M. K. Selwal and K. K. Selwal, “High-Level Tannase Production by Penicillium atramentosum KM Using Agro Residues under Submerged Fermentation,” Annals of Microbiology,” Vol. 62, No. 1, 2012, pp. 139-148. http://dx.doi.org/10.1007/s13213-011-0238-1
- A. M. Costa, W. X. Ribeiro, E. Kato, A. R. G. Monteiro and R. M. Peralta, “Production of Tannase by Aspergillus tamarii in Submerged Cultures,” Brazilian Archives of Biology and Technology, Vol. 51, No. 2, 2008, pp. 399- 404. http://dx.doi.org/10.1590/S1516-89132008000200021
- A. B. El-tanash, A. A. Sherief and A. Nour, “Gallic Acid Production by Tannase of Aspergillus awamori Using Response Surface Methodology,” Innovative Romanian Food Biotechnology, Vol. 10, 2012, pp. 9-17.
- P. D. Belur and G. Mugeraya, “Microbial Production of Tannase: State of the Art,” Research Journal of Microbiology, Vol. 6, 2011, pp. 25-40.
- G. A. S. Pinto, E. S. Brito, A. M. R. Andrade, S. L. P. Fraga and R. B. Teixeira, “Fermentação em Estado Sólido: Uma Alternativa para o Aproveitamento e Valorização de Resíduos Agroindustriais Tropicais,” EMBRAPA Comunicado Técnico Online, Vol. 102, 2005, pp. 1-5.
- R. Kumar, J. Sharma and R. Singh, “Production of Tannase from Aspergillus ruber under Solid-State Fermentation Using Jamun (Syzygium cumini) Leaves,” Microbiology Research, Vol. 162, No. 4, 2007, pp. 384-390.
- A. Sabu, A. Pandey, M. J. Daud and G. Szakacs, “Tamarind Seed Powder and Palm Kernel Cake: Two Novel Agro Residues for the Production of Tannase under Solid State Fermentation by Aspergillus niger ATCC 16620,” Bioresource Technology, Vol. 96, No. 11, 2005, pp. 1223-1228. http://dx.doi.org/10.1016/j.biortech.2004.11.002
- R. Cruz, C. Santos, J. S. Lima, K. A. Moreira and C. M. Souza-Motta, “Diversity of Penicillium in Soil of Caatinga and Atlantic Forest Areas of Pernambuco, Brazil: An Ecological Approach,” Nova Hedwigia, Vol. 97, No. 3-4, 2013, pp. 543-556. http://dx.doi.org/10.1127/0029-5035/2013/0127
- F. E. Clark, “Agar-Plate Method for Total Microbial Count,” In: C. A. Black, D. D. Evans, J. L. White, L. E. Ensminger, F. E. Clark and R. C. Dinaver, Eds., Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, Madson, New York, 1965, pp. 1460-1466.
- H. L. Barnett and B. B. Hunter, “Illustrated Genera of Imperfect Fungi,” MacMillan Publishing Company, New York, 1987.
- K. H. Domsch, W. Gams and T. H. Anderson, “Compendium of Soil Fungi,” Eching: IHW-189, Verlag, 2007.
- M. B. Ellis, “Dematiaceous Hyphomycetes,” Commonwealth Mycological Institute, Kew, 1971.
- M. B. Ellis, “More Dematiaceous Hyphomycetes,” Commonwealth Mycological Institute, Kew, 1976.
- M. A. Klich, “Identification of Common Aspergillus Species,” Centraal Bureau vöör Schimmelcultures, Utrecht, 2002.
- M. A. Klich and J. I. Pitt, “A Laboratory Guide to Common Aspergillus Species and Their Teleomorphs,” CSIRO Division of Food Research, North Ryde, 1988.
- J. I. Pitt, “A Laboratory Guide to Common Penicillium Species,” Commonwealth Scientific and Industrial Research Organization, North Wales, 1991.
- K. B. Raper and C. Thom, “A Manual of the Penicillia,” Williams and Wilkins Company, Baltimore, 1949.
- R. A. Samson and J. C. Frisvad, “Penicillium Subgenus Penicillium: New Taxonomics Schemes, Mycotoxins and Other Extrolites,” Studies in Mycology, Vol. 49, 2004, pp. 1-260.
- B. C. Sutton, “The Coelomycetes: Fungi Imperfecti with Pycnidia, Acervuli and Stromata,” Commonwealth Mycological Institute, Kew, 1980.
- EMBRAPA, “Manual de Análises Químicas de Solos Plantas e Fertilizantes,” Embrapa Informações Tecnol- ógicas, Embrapa, Brasília, 2009.
- C. E. Shannon and W. Weaver, “The Mathematical Theory of Commnunication,” Bell System Technical Journal, Vol. 27, 1948, pp. 379-423. http://dx.doi.org/10.1002/j.1538-7305.1948.tb01338.x
- F. J. Rohlf and D. L. Fisher, “Test for Hierarchical Structure in Random Data Sets,” Systematic Zoology, Vol. 17, No. 4, 1968, pp. 407-412. http://dx.doi.org/10.2307/2412038
- B. Treviño-Cueto, M. Luis, J. C. Contreras-Esquivel, R. Rodrigues, A. Aguilera and C. N. Agilar, “Gallic Acid and Tannase Accumulation during Fungal Solid State Culture of a Tannin-Rich Desert Plant (Larrea tridentate Cov.),” Bioresource Technology, Vol. 98, No. 3, 2007, pp. 721-724. http://dx.doi.org/10.1016/j.biortech.2006.02.015
- A. E. Hagerman and L. G. Butler, “Protein Precipitation Method for the Quantitative Determination of Tannins,” Journal of Agricultural and Food Chemistry, Vol. 26, No. 4, 1978, pp. 809-812. http://dx.doi.org/10.1021/jf60218a027
- S. Sharma, T. K. Bhat and R. K. Dawra, “A Spectrophotometric Method for Assay of Tannase Using Rhodanine,” Analytical Biochemistry, Vol. 279, No. 1, 2000, pp. 85-89. http://dx.doi.org/10.1006/abio.1999.4405
- G. Oliveira, M. B. Araújo, T. F. Rangel, D. Alagador and J. A. F. Diniz-Filho, “Conserving the Brazilian Semiarid (Caatinga) Biome under Climate Change,” Biodiversity Conservation, Vol. 21, No. 11, 2012, pp. 2913-2926. http://dx.doi.org/10.1007/s10531-012-0346-7
- F. P. Werneck, “The Diversification of eastern South American Open Vegetation Biomes: Historical Biogeography and Perspectives,” Quaternary Science Reviews, Vol. 30, No. 13-14, 2011, pp. 1630-1648. http://dx.doi.org/10.1016/j.quascirev.2011.03.009
- M. A. Klich, “Biogeography of Aspergillus Species in Soil and Litter,” Mycologia, Vol. 94, No. 1, 2002, pp. 21-27. http://dx.doi.org/10.2307/3761842
- A. L. C. M. A. Santiago and C. M. Souza-Motta, “Mucorales Isolados do Solo de Mineração de Cobre e Produção de Amilase e Inulinase,” Acta Botanica Brasilica, Vol. 20, No. 3, 2006, pp. 641-647. http://dx.doi.org/10.1590/S0102-33062006000300014
- Manjit, A. Yadav, N. K. Aggarwal, K. Kumar and A. Kumar, “Tannase Production by Aspergillus fumigatus MA under Solid-State Fermentation,” World Journal of Microbiology and Biotechnology, Vol. 24, No. 12, 2008, pp. 3023-3030. http://dx.doi.org/10.1007/s11274-008-9847-7
NOTES
*Corresponding author.

