Open Journal of Nephrology
Vol.3 No.1(2013), Article ID:29218,10 pages DOI:10.4236/ojneph.2013.31009
Biomarkers in Acute Kidney Injury
1Division of Nephrology, Department of Medicine, Taipei Medical University-Shuang Ho Hospital, Taipei Medical University, Chinese Taipei
2Department of Pediatrics, Taoyuan Armed Forces General Hospital, Chinese Taipei
3Department of Pediatrics, Tri-Service General Hospital, National Defense Medical Center, Chinese Taipei
4Department of Medicine, Cardinal Tien Hospital, School of Medicine, Fu Jen Catholic University, New Taipei City, Chinese Taipei
5Division of Nephrology, Department of Medicine, Tri-Service General Hospital, National Defense Medical Center, Chinese Taipei
Email: #kuochenglu@gmail.com
Received December 23, 2012; revised January 20, 2013; accepted March 13, 2013
Keywords: Acute kidney injury; Critical care centers; Biomarkers; Renal replacement therapy
ABSTRACT
Acute kidney injury (AKI) is one of the popular topics of discussions due to increasing development of biomarkers recently. The disease progression and prognosis may be determined by these biomarkers detected in blood and urine specimens. Since acute kidney injury is associated with a broad spectrum of disease conditions, prevention and early detection of AKI becomes very important in those clinical settings. Early measurements of AKI biomarkers predict subsequent development of intrinsic AKI, dialysis requirement, duration of intensive care unit stay and finally affect mortality. We, here, discuss the acute kidney injury in different clinical situations and associated natures of biomarkers, which may help us guide to prevent and treat AKI more effectively.
1. Introduction
Acute kidney injury (AKI) is one of the major causes of morbidity and mortality encounter in hospitalized patients, especially intensive care centers. Early detection may improve the hospital stay, patients’ prognosis and reduce expensive medical costs. Nowadays, different types of bio-markers for acute kidney injury are emerging to play a role in accurate diagnosis, early detection, monitoring therapy and predicting the prognosis. This review discusses the clinical use of biomarkers in acute kidney injury.
2. Definition and Classification of Acute Kidney Injury (AKI)
Acute kidney injury (AKI), a preferred nomenclature to previously termed acute renal failure, refers to a spectrum of disease ranging from a minimal elevation in serum creatinine to anuric renal failure, clinically manifested by changes in blood chemistry and fluid disturbances.
In 2002, the Acute Dialysis Quality Initiative (ADQI) group proposed a definition for AKI stems from criteria for three grades of increasing severity (Risk of acute renal failure, Injury to the kidney, Failure of kidney function) and two outcome classes (Loss of kidney function and End-stage kidney disease) (RIFLE classification) [1,2].
Chertow and colleagues noticed that a rise in serum creatinine of just ≥0.3 mg/dl had a four-fold higher multivariable-adjusted risk of death [3]. The Acute Kidney Injury Network (AKIN) group modified the AKI definition based on RIFLE criteria in 2005 [4]. This new staging system classified the patients with a change in serum creatinine (SCr) concentration ≥ 0.3 mg/dl (≥26.4 μmol/l) within 48 hours as AKIN stage 1, whereas patients receiving renal replacement therapy are included in AKIN Stage 3. RIFLE-Risk classified as Stage 1, RIFLE-Injury and Failure as Stages 2 and 3, respectively; and the two outcome classes RIFLE-Loss and RIFLE-End stage kidney disease has been removed [4] (Table 1).
3. Acute Kidney Injury (AKI) in Different Situations
3.1. Community-Acquired AKI
Acute renal injury occurred at the time of hospital admission is regarded as community-acquired AKI and
Table 1. Diagnostic criteria for acute kidney injury depend on acute kidney injury (AKIN) network.
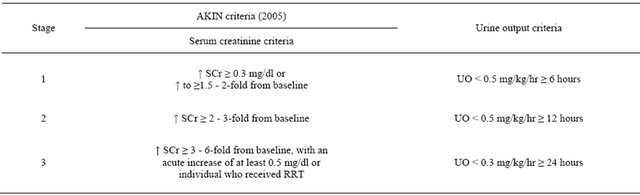
Scr: serum creatinine; UO: urine output [4].
found to be present in about 1% of hospital admissions [5]. The causes of community-acquired AKI also may include pre-renal azotemia, intrinsic renal failure, and post-renal obstruction. The risk factors for developing community-acquired AKI include, old age > 75 yrs, patients with underlying chronic kidney disease (CKD, eGFR < 60 mls/min/1.73m2), cardiac failure, atherosclerotic peripheral vascular disease, liver disease, diabetes mellitus and patients taking nephrotoxic medications, alternative medical therapies, etc. Other disorders include exercise or heat-stroke related rhabdomyolysis, crushsyndrome from natural disasters such as earthquakes, may also contribute to community-acquired AKI [5].
3.2. Hospital-Acquired AKI
AKI is present in about 7% of all hospitalized patients [6], and more than 30% of critically ill patients experienced AKI [7,8]. The incidence of AKI greatly increased in the hospital settings with improved critical care, increase diagnostic and procedural techniques, and improved management of other organ failures. The risk factors for hospital acquired AKI are sepsis, elder age, pre-existing kidney dysfunction. Since the widespread use of primary percutaneous coronary intervention protocols and intravenous contrast related investigations in intensive care unit, contrast related renal injuries are becomingly known as important risk factor for AKI. Other causes of AKI intensive care units may also include pre-renal azotemia, ischemia, organ failures, toxins, obstruction, etc. The severe AKI in ICU is now considered as a part of a multiple system organ failure (MSOF) complex and its mortality varies over 50-80% in ICU [9, 10]. The natural course of AKI has not been benign as previously thought, and >20% of ICU dialysis survivors progress to CKD and ESRD within 3 - 5 years. In a cohort study, the RIFLE criteria—risk, injury, and failure— found to be associated with inpatient mortality rates of 8.8%, 11.4%, and 26.3%, respectively [11]. Pathophysiology of AKI The mechanism of acute kidney injury depends on the severity of insults which vary from increased risk, progressive damage to decreased glomerular filtration rate (GFR), kidney failure and death. Abolishment of renal auto-regulation occurs in ischemic renal injury due to imbalance between vasoconstrictors and vasodilators. Diminished renal perfusion may cause elevated of endothelial injury markers, inflammatory mediators like TNFa, IL-18, etc. with reduced nitric oxide derivatives with resultant endothelial injury. High levels of vasoconstrictors with persistent hypoxia may lead to cellular damage and cell death with reduction in GFR. Necrosis and desquamation of tubular cells result in tubular obstruction, which may further reduce GFR. Local inflammatory mediators may lead to interstitial inflammation, small vessel obstruction and finally, local ischemia [12,13]. Direct nephrotoxic effects may result in decreased GFR in cases of toxic AKI.
A better understanding of the clinical continuum of AKI is needed for better diagnostic and therapeutic measures. The systemic inflammatory response syndrome [14], insulin resistance with hyperglycemia associated injuries [15], and associated oxidative stress may take part in the development of ischemic and toxic AKI in critically ill patients. Since kidney injury itself may also generate oxidative stress, increased oxidative stress biomarkers are found to be elevated [16,17]. Biomarkers of structural injury elevated earlier than those for functional injury during the AKI event. Overall, biomarkers, especially urinary biomarkers of AKI will facilitate earlier diagnosis, provide specific preventative and therapeutic strategies and with the overall improve in outcomes.
4. Types of Biomarkers
An ideal biomarker of AKI would fulfill the following:
• Increase in the urine or blood within minutes or hours after a renal insult;
• Remains elevated as long as the renal injury persists;
• Correlates quantitatively with the extent of renal injury;
• Decreases proportionally with the renal recovery status.
Urinary biomarkers are regarded more non-invasive, easy to measure, easily obtainable, and clinically earlier detection than blood biomarkers [18,19]. Injury to different segment regions of nephrons may excrete different specific urinary biomarkers. Detection of high-molecular weight protein, like albumin, immunoglobulin, and transferrin in the urine may be associated with glomerular injury. Low-molecular weight proteinuria (e.g. α-1 microglobulin, β-1 microglobulin and retinol binding proteins) [20,21], brush border antigens [20,22,23], urinary enzymes [20,24] and other urinary proteins [25-28] are associated with damage to renal tubules. However, sufficient validation is needed to use these markers for the screening and differentiating the site of injury clinically.
Depend on the time of appearance after AKI, the urinary biomarkers may be classified into biomarkers of structural injury, and those of functional injury. Structural injury biomarkers are those appearing in the urine immediately after tubular cell apoptosis, and include KIM-1, NGAL, NAG, IL-18, and clusterin. Functional injury biomarkers are delayed markers for injury which may indicate global renal dysfunction, and constitutes cystatin C, total protein, albumin and β-2 microglobulin (Table 2).
5. Biomarkers under Evaluation in Humans
Recently, more than 20 protein biomarkers have been intensively reviewed in human and animal models of AKI to identify which ones may signal AKI prior to a rise in serum creatinine. Since the gold standard kidney biopsies are not clinically practicable in AKI patients as outcome measures, rise in serum creatinine is still used as clinical outcome. A potential biomarker should increase rapidly in the settings where the timing of renal injury is known (e.g. after cardiopulmonary bypass (CPB) or immediate post-transplant period). The validity of biomarker is assessed most commonly by receiver operating characteristic (ROC) curve, which plots 1 minus specificity of the biomarker on the x-axis against the sensitivity of the biomarker on the y-axis. The area under curve (AUC) is generated from these plots, and an AUC of 1 represents a perfect biomarker and AUC of 0.5 reflects complete lack of accuracy.
5.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Neutrophil gelatinase-associated lipocalin (NGAL) is the one of the most consistant biomarker found during AKI. It is a 25-kDa polypeptide covalently bound to gelatinase from human neutrophils, and predominantly found in proliferating nuclear antigen-positive proximal tubule cells. It is markedly up-regulated in early post-ischemic mouse and rat kidneys [29]. The urinary NGAL protein has been demonstrated to predict the occurrence of ischemic AKI in pediatric and adult patients after cardiac surgery [30,31]. In a prospective study done on pediatric patients undergone cardiopulmonary bypass (CPB) by Mishra et al. [30], the urinary NGAL was increased 100 fold and detected within 2 hours of CPB, which precede 50% increase in SCr by 1 - 3 days. In contrast, urinary NGAL did not rise in those patients who did not develop AKI. The area under the receiver operating characteristic curve (AUC-ROC) was 0.99 at 2 hours and 1.00 at 4 hours after CPB [30]. Plasma NGAL was also found to predict morbidity and mortality in pediatric patients who undergo cardiac surgery [32]. Urinary NGAL also was found to be elevated within 1 hour after cardiac surgery in adult patients and revealed the AUC-ROC of 0.74 at 3 hours and 0.8 at 18 hours [31]. In a small prospective study of pediatric and adult kidney transplant patients, urinary NGAL is demonstrated as an excellent predictive marker (AUC-ROC 0.9 at day 0 after transplantation) for the development of delayed graft dysfunction and renal replacement therapy [33]. Furthermore, a recent study demonstrated that a single urine NGAL measurement in the emergency department could better predict the need for Nephrology consultations, intensive care unit admission, dialysis initiation, or mortality, etc. than could an elevated serum creatinine [34].
5.2. Kidney Injury Molecule-1 (KIM-1)
KIM-1 is an orphan trans-membrane receptor of unknown function. It is undetectable in normal kidney tissue or urine, and is markedly increased in ischemic and nephrotoxic proximal tubule epithelial cell injury, and in renal cell carcinoma [35-37]. In a small cross-sectional study, Han et al. [38] demonstrated the elevation of urinary KIM-1 levels within 12 hours after an initial ischemic insult and much earlier than granular casts appearance in established AKI. Higher urinary KIM-1 was also associated with adverse outcomes in these established AKI patients. Urinary KIM-1 was elevated and found to have an AUC-ROC of 0.57 at 2 hours, 0.83 at 12 hours, and 0.78 at 24 hours after pediatric cardiac surgery in a case-control study [39] using the same cohort in the NGAL study previously described [30]. High urinary KIM-1 may also predict the graft loss in renal transplant patients independent of other common risk factors like creatinine clearance, proteinuria, and donor age [40]. Urinary KIM-1 was also proved in some studies to distinguish AKI from CKD and normal [41,42].
Table 2. Biomarkers of acute kidney injury.

*Available in both serum and urine; DRAF: delayed renal allograft function; RCC: renal cell carcinoma; AKI: Acute kidney injury; IL: interleukin; TNF: tumor necrosis factor.
5.3. Interleukin (IL)-18
Interleukin (IL)-18 is a pro-inflammatory cytokine which involve in mediating inflammation process during ischemic, sepsis and nephrotoxic AKI [12]. Since IL-18 serves to recruit the neutrophils during ischemic injury, elevated urine IL-18 have been demonstrated in patients with ischemic acute tubular necrosis (ATN) [43]. In a crosssectional study, Parikh et al. demonstrated that markedly elevated day 0 post-transplantation urinary IL-18 concentration was found in patients with delayed allograft dysfunction (AUC 0.95) [43]. In a prospective study of 138 patients with acute respiratory distress syndrome, elevation of urinary IL-18 could predict AKI one day ahead of serum creatinine with an AUC-ROC 0.73, and also independently predicts mortality in this cohort group [44]. Urinary IL-18 was also found in elevated after CPB, and has an AUC-ROC of 0.61 at 4 hours, 0.75 at 12 hours, and 0.73 at 24 hours following CPB to predict AKI [45]. In the critically ill patients, the urinary IL-18 level was also regarded as an independent predictor of mortality.
5.4. Cystatin C
Cystatin C is a 13kD cysteine protease inhibitor protein that is produced by all nucleated cells into plasma, and freely filtered from glomerulus, completely reabsorbed and not secreted in the tubules. It is less influenced by factors other than glomerular filtration rate (e.g. age, gender, race, or muscle mass) [44]. Several studies have demonstrated that a change in serum and urine Cystatin C is more sensitive than a change in Serum creatinine in predicting a change in glomerular filtration [46-48].
In a prospective study of 85 critically ill patients at high risk to develop AKI, a 50% increase in serum cystatin C was noted one to two days before serum creatinine with an AUC of 0.97 and 0.82 respectively [49]. Furthermore, serum cystatin C also predicts the risk of AKI-associated cardiovascular morbidity and mortality in critically ill patients [50]. In older patients, increased serum cystatin C is also found to be a stronger predictor for the risk of death and cardiovascular events [50,51]. Furthermore, serum cystatin C levels > 1.0 mg/L may also predict cardiovascular events and mortality in participants with serum creatinine-based eGFR > 60 ml/min per 1.73 m2 [52,53]. Urine cystatin C has also been studied and some studies found that urine Cystatin C performed better for AKI prediction than did serum Cystatin C [54,55]. In addition, increased urinary cystatin C and α1-microglobulin may be early predictors of an unfavorable clinical outcome in ATN, reflected by the requirement for RRT. Severity prediction with these markers could assist in improving the outcome of ATN [55]. Cystatin C also is extensively studied in chronic kidney disease patients. Since serum cystatin C has higher sensitivity and higher negative predictive value in determination of reduced GFR than serum creatinine, many studies examine the serum cystatin C to use clinically in GFR determination [56]. A recent cross-sectional study found that the combined creatinine-cystatin C equation to estimate GFR better than either of these markers alone in chronic kidney disease patients [57]. Although these findings are encouraging, additional studies are further needed for clinical use of serum and urine cystatin-C in acute and chronic renal disease conditions.
6. Biomarker Combinations in Different Clinical Conditions
The ultimate diagnostic goal in AKI is to distinguish pre-renal azotemia, renal AKI (ATN), obstruction, Urinary traction infections, and underlying CKD, etc. Studies are underway to distinguish the various types of AKI by using biomarkers. For example, urinary KIM-1 could distinguish ischemic ATN from pre-renal azotemia and CKD in one study [41], and KIM-1 also found to distinguish AKI from CKD and normal with an AUC of 0.94 [42].
Biomarker combination may be required in improving determination and differentiating the AKI. The sequential appearance of AKI biomarkers has been noted during serial measurements of multiple urinary biomarkers after pediatric cardiac surgery, with urine NGAL peaks at 2 hrs followed by the IL-18 peak at 12 hrs. Urinary IL-18 has a lower AUC (0.74 at 12 hrs) than NGAL (0.99 at 2 hrs) [31,58]. This sequential increase resembles troponin I, CPK and LDH of acute myocardial injury, which may help us to determine the time since injury in AKI. Although NGAL alone can predict AKI in the early hours, the combination of both urinary markers may predict the borderline cases in later periods after surgery. Several biomarker combinations may also predict AKI after adult cardiac surgery (Figure 1) [59]. These biomarker combinations have been tested in only small studies and limited clinical situations. Multicenter studies are needed to determine which biomarker combination best predict and determine outcome in AKI.
7. Proposed Biomarker Strategies for Renal Replacement Therapy (RRT) Initiation in AKI
Another hope for biomarkers in AKI is to determine the time to start renal replacement therapy (RRT). Although the results are encouraging for some biomarkers, there still need to create the biomarker-based strategies for RRT initiation. Since wide practice variation in timing of initiating RRT and heterogeneity of AKI in various situations,
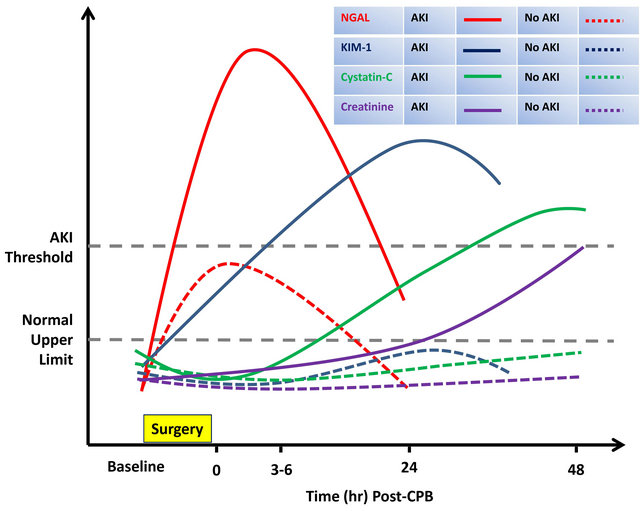
Figure 1. The comparism for the predicted time course of change in urine or serum biomarker levels in AKI and non-AKI patients after cardiac surgery. Patterns of change represent ideal circumstances, which have not been consistently demonstrated in clinical studies. AKI: acute kidney injury; CPB: cardiopulmonary bypass; creatinine: serum creatinine; cystatin-C: serum cystatin-C; KIM-1: urinary kidney injury molecule-1; NGAL: urinary neutrophil gelatinase-associated lipocalin. Modified from [59].
the decision making in initiation of RRT remains subjective in most clinical settings.
An opinion-based clinical algorithm was drawn using RIFLE/AKIN criteria, and several patient-specific factors to decide when to start RRT in critically ill adults [60]. Recently, the well accepted metabolic derangements need for RRT include plasma BUN > 100 mg/dl, hyperkalemia > 6 mEq/L with EKG abnormalities, hypermagnesemia > 8 mEq/L, severe metabolic acidosis pH < 7.15, diuretic resistant fluid overload [61-65]. Some studies demonstrate better renal outcome in patients who start RRT while in RIFLE-Risk or Injury than those who started RRT in RIFLE-Failure status [66,67]. Clinically, it is possible for some RIFLE-Risk and Injury cases to recover spontaneously before complications develop and in these cases RRT may not benefit. And on the other hand, if we could easily categorize these patients in RISK-Risk and Injury cases with severe and sustained renal injury, we can start RRT to provide renal support and to prevent AKI-related complications.
Some studies suggest novel biomarkers like NGAL, Cystatin-C, NAG, KIM-1, and α1-microglobulin can distinguish patients in whom RRT will be needed [55, 68]. A potential use of biomarkers integrated into the clinical decision algorithms to initiate RRT is recently proposed by Cruz et al. (Figure 2) [69]. In this algorithm, two cut-offs are needed, one represents a high likelihood of needing RRT, and another indicates RRT is very improbable. The first cut-off would identify those AKI patients in whom spontaneous renal recovery is not likely. The second cut-off distinguishes those AKI patients with probable spontaneous renal recovery. These cut-off values may be absolute value or relative change from baseline value, and vary with different clinical settings. Biomarkers are repeatedly measured during conservative therapy to monitor and reassess the disease progress. However, more studies are needed to use these biomarkers effectively in clinical AKI patients.
8. Limitations of Biomarker Use in Clinical Acute Kidney Injury
The heterogeneity of AKI especially in intensive care unit needs more than one biomarker to obtain sufficient sensitivity and specificity for AKI screening. An analysis of multiple biomarkers may need in additional studies before biomarkers may be used in routine clinical practice. Recently, none of the promising biomarkers have been systematically evaluated in the various clinical settings

Figure 2. Biomarker-based strategy for renal replacement therapy in AKI patients. AKI: acute kidney injury; BM: biomarker; RRT: renal replacement therapy; AKIN: Acute Kidney Injury Network. Modified from [69].
of AKI. Additionally, no cutoff value that is predictive of AKI has been available nowadays. The role of these biomarkers in association with other comorbidities including sepsis, etc. is not accomplished.
9. Conclusion
Bomarkers of AKI such as NGAL, KIM-1, IL-18 and Cystatin C are now becoming greatest interest among different AKI clinical settings. Early measurements of AKI biomarkers predict subsequent development of intrinsic AKI, dialysis requirement, ICU stay, days of hospital stay and finally affects mortality. Future studies should evaluate biomarker outcomes independent of serum creatinine, and should consider biomarkers as entry criteria for AKI therapeutic protocols. Such an advance would finally find out the gold standard biomarker for AKI, as in case of troponin I for potential myocardial ischemia.
REFERENCES
- R. Bellomo, C. Ronco, J. A. Kellum, R. L. Mehta and P. Palevsky, “Acute Renal Failure—Definition, Outcome Measures, Animal Models, Fluid Therapy and Information Technology Needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group,” Critical Care, Vol. 8, No. 4, 2004, pp. R204-R212. doi:10.1186/cc2872
- J. A. Kellum, N. Levin, C. Bouman and N. Lameire, “Developing a Consensus Classification System for Acute Renal Failure,” Current Opinion in Critical Care, Vol. 8, No. 6, 2002, pp. 509-514. doi:10.1097/00075198-200212000-00005
- G. M. Chertow, E. Burdick, M. Honour, J. V. Bonventre and D. W. Bates, “Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients,” Journal of the American Society of Nephrology, Vol. 16, No. 11, 2005, pp. 3365-3370. doi:10.1681/ASN.2004090740
- R. L. Mehta, J. A. Kellum, S. V. Shah, B. A. Molitoris, C. Ronco, D. G. Warnock and A. Levin, “Acute Kidney Injury Network: Report of an Initiative to Improve Outcomes in Acute Kidney Injury,” Critical Care, Vol. 11, No. 2, 2007, p. R31. doi:10.1186/cc5713
- J. Kaufman, M. Dhakal, B. Patel and R. Hamburger, “Community-Acquired Acute Renal Failure,” American Journal of Kidney Diseases, Vol. 17, No. 2, 1991, pp. 191-198.
- K. Nash, A. Hafeez and S. Hou, “Hospital-Acquired Renal Insufficiency,” American Journal of Kidney Diseases, Vol. 39, No. 5, 2002, pp. 930-936. doi:10.1053/ajkd.2002.32766
- R. L. Mehta, M. T. Pascual, S. Soroko, et al., “Spectrum of Acute Renal Failure in the Intensive Care Unit: The PICARD Experience,” Kidney International, Vol. 66, No. 4, 2004, pp. 1613-1621. doi:10.1111/j.1523-1755.2004.00927.x
- M. Ostermann and R.W. Chang, “Acute Kidney Injury in the Intensive Care Unit According to RIFLE,” Critical Care Medicine, Vol. 35, No. 8, 2007, pp. 1837-1843. doi:10.1097/01.CCM.0000277041.13090.0A
- F. Liano, C. Felipe, M. T. Tenorio, M. Rivera, V. Abraira, J. M. Saez-de-Urturi, J. Ocana, C. Fuentes and S. Severiano, “Long-Term Outcome of Acute Tubular Necrosis: A Contribution to Its Natural History,” Kidney International, Vol. 71, No. 7, 2007, pp. 679-686. doi:10.1038/sj.ki.5002086
- S. M. Bagshaw, K. B. Laupland, C. J. Doig, G. Mortis, G. H. Fick, M. Mucenski, T. Godinez-Luna, L. W. Svenson and T. Rosenal, “Prognosis for Long-Term Survival and Renal Recovery in Critically Ill Patients with Severe Acute Renal Failure: A Population-Based Study,” Critical Care, Vol. 9, No. 6, 2005, pp. R700-R709. doi:10.1186/cc3879
- E. A. Hoste, G. Clermont, A. Kersten, R. Venkataraman, D. C. Angus, D. De Bacquer and J. A. Kellum, “RIFLE Criteria for Acute Kidney Injury Are Associated with Hospital Mortality in Critically Ill Patients: A Cohort Analysis,” Critical Care, Vol. 10, No. 3, 2006, p. R73. doi:10.1186/cc4915
- J. V. Bonventre and J. M. Weinberg, “Recent Advances in the Pathophysiology of Ischemic Acute Renal Failure,” Journal of the American Society of Nephrology, Vol. 14, No. 8, 2003, pp. 2199-2210. doi:10.1097/01.ASN.0000079785.13922.F6
- R. W. Schrier, W. Wang, B. Poole and A. Mitra, “Acute Renal Failure: Definitions, Diagnosis, Pathogenesis, and Therapy,” The Journal of Clinical Investigation, Vol. 114, No. 1, 2004, pp. 5-14.
- M. S. Rangel-Frausto, D. Pittet, M. Costigan, T. Hwang, C. S. Davis and R. P. Wenzel, “The Natural History of the Systemic Inflammatory Response Syndrome (SIRS). A Prospective Study,” Journal of the American Medical Association, Vol. 273, No. 2, 1995, pp. 117-123. doi:10.1001/jama.1995.03520260039030
- G. Van den Berghe, A. Wilmer, G. Hermans, et al., “Intensive Insulin Therapy in the Medical ICU,” The New England Journal of Medicine, Vol. 354, No. 5, 2006, pp. 449-461. doi:10.1056/NEJMoa052521
- J. Himmelfarb, E. McMonagle, S. Freedman, et al., “Oxidative Stress Is Increased in Critically Ill Patients with Acute Renal Failure,” Journal of the American Society of Nephrology, Vol. 15, No. 9, 2004, pp. 2449-2456. doi:10.1097/01.ASN.0000138232.68452.3B
- G. H. Metnitz, M. Fischer, C. Bartens, H. Steltzer, T. Lang and W. Druml, “Impact of Acute Renal Failure on Antioxidant Status in Multiple Organ Failure,” Acta Anaesthesiologica Scandinavica, Vol. 44, No. 3, 2000, pp. 236-240. doi:10.1034/j.1399-6576.2000.440304.x
- P. Devarajan, “Emerging Biomarkers of Acute Kidney Injury,” Contributions to Nephrology, Vol. 156, 2007, pp. 203-212. doi:10.1159/000102085
- P. Devarajan, “Emerging Urinary Biomarkers in the Diagnosis of Acute Kidney Injury,” Expert Opinion on Medical Diagnostics, Vol. 2, No. 4, 2008, pp. 387-398.
- N. E. Tolkoff-Rubin, R. H. Rubin and J. V. Bonventre, “Noninvasive Renal Diagnostic Studies,” Clinics in Laboratory Medicine, Vol. 8, No. 3, 1988, pp. 507-526.
- C. Bazzi, C. Petrini, V. Rizza, et al., “Urinary Excretion of IgG and Alpha(1)-Microglobulin Predicts Clinical Course Better than Extent of Proteinuria in Membranous Nephropathy,” American Journal of Kidney Diseases, Vol. 38, No. 2, 2001, pp. 240-248. doi:10.1053/ajkd.2001.26080
- N. Taniguchi, M. Tanaka, C. Kishihara, et al., “Determination of Carbonic Anhydrase C and Beta 2-Microglobulin by Radioimmunoassay in Urine of Heavy-MetalExposed Subjects and Patients with Renal Tubular Acidosis,” Environmental Research, Vol. 20, No. 1, 1979, pp. 154-161. doi:10.1016/0013-9351(79)90094-X
- A. Mutti, S. Lucertini, P. Valcavi, et al., “Urinary Excretion of Brush-Border Antigen Revealed by Monoclonal Antibody: Early Indicator of Toxic Nephropathy,” Lancet, Vol. 2, No. 8461, 1985, pp. 914-917. doi:10.1016/S0140-6736(85)90850-5
- J. Boldt, T. Brenner, J. Lang, B. Kumle and F. Isgro, “Kidney-Specific Proteins in Elderly Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass,” Anesthesia and Analgesia, Vol. 97, No. 6, 2003, pp. 1582- 1589. doi:10.1213/01.ANE.0000090146.02929.2E
- K. Sugimura, T. Goto, K. Tsuchida, Y. Takemoto, T. Kim and T. Kishimoto, “Production and Activation of Hepatocyte Growth Factor in Acute Renal Failure,” Renal Failure, Vol. 23, No. 3-4, 2001, pp. 597-603. doi:10.1081/JDI-100104741
- F. Mariano, G. Guida, D. Donati, et al., “Production of Platelet-Activating Factor in Patients with Sepsis-Associated Acute Renal Failure,” Nephrology, Dialysis, Transplantation, Vol. 14, No. 5, 1999, pp. 1150-1157. doi:10.1093/ndt/14.5.1150
- H. Zhou, T. Pisitkun, A. Aponte, et al., “Exosomal Fetuin-A Identified by Proteomics: A Novel Urinary Biomarker for Detecting Acute Kidney Injury,” Kidney International, Vol. 70, No. 10, 2006, pp. 1847-1857. doi:10.1038/sj.ki.5001874
- Y. Muramatsu, M. Tsujie, Y. Kohda, et al., “Early Detection of Cysteine Rich Protein 61 (CYR61, CCN1) in Urine Following Renal Ischemic Reperfusion Injury,” Kidney International, Vol. 62, No. 5, 2002, pp. 1601- 1610. doi:10.1046/j.1523-1755.2002.00633.x
- J. Mishra, Q. Ma, A. Prada, et al., “Identification of Neutrophil Gelatinase-Associated Lipocalin as a Novel Early Urinary Biomarker for Ischemic Renal Injury,” Journal of the American Society of Nephrology, Vol. 14, No. 10, 2003, pp. 2534-2543. doi:10.1097/01.ASN.0000088027.54400.C6
- J. Mishra, C. Dent, R. Tarabishi, et al., “Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Biomarker for Acute Renal Injury after Cardiac Surgery,” Lancet, Vol. 365, No. 9466, 2005, pp. 1231-1238. doi:10.1016/S0140-6736(05)74811-X
- G. Wagener, M. Jan, M. Kim, et al., “Association between Increases in Urinary Neutrophil Gelatinase-Associated Lipocalin and Acute Renal Dysfunction after Adult cardiac Surgery,” Anesthesiology, Vol. 105, No. 3, 2006, pp. 485-491. doi:10.1097/00000542-200609000-00011
- C. L. Dent, Q. Ma, S. Dastrala, et al., “Plasma Neutrophil Gelatinase-Associated Lipocalin Predicts Acute Kidney Injury, Morbidity and Mortality after Pediatric Cardiac Surgery: A Prospective Uncontrolled Cohort Study,” Critical Care, Vol. 11, No. 6, 2007, p. R127. doi:10.1186/cc6192
- C. R. Parikh, A. Jani, J. Mishra, et al., “Urine NGAL and IL-18 Are Predictive Biomarkers for Delayed Graft Function Following Kidney Transplantation,” American Journal of Transplantation, Vol. 6, No. 7, 2006, pp. 1639- 1645. doi:10.1111/j.1600-6143.2006.01352.x
- T. L. Nickolas, M. J. O’Rourke, J. Yang, et al., “Sensitivity and Specificity of a Single Emergency Department Measurement of Urinary Neutrophil Gelatinase-Associated Lipocalin for Diagnosing Acute Kidney Injury,” Annals of Internal Medicine, Vol. 148, No. 11, 2008, pp. 810-819.
- T. Ichimura, J. V. Bonventre, V. Bailly, et al., “Kidney Injury Molecule-1 (KIM-1), a Putative Epithelial Cell Adhesion Molecule Containing a Novel Immunoglobulin Domain, Is Up-Regulated in Renal Cells after Injury,” The Journal of Biological Chemistry, Vol. 273, No. 7, 1998, pp. 4135-4142. doi:10.1074/jbc.273.7.4135
- T. Ichimura, C. C. Hung, S. A. Yang, J. L. Stevens and J. V. Bonventre, “Kidney Injury Molecule-1: A Tissue and Urinary Biomarker for Nephrotoxicant-Induced Renal Injury,” American Journal of Physiology Renal Physiology, Vol. 286, No. 3, 2004, pp. F552-F563. doi:10.1152/ajprenal.00285.2002
- W. K. Han, A. Alinani, C. L. Wu, et al., “Human Kidney Injury Molecule-1 Is a Tissue and Urinary Tumor Marker of Renal Cell Carcinoma,” Journal of the American Society of Nephrology, Vol. 16, No. 4, 2005, pp. 1126-1134.
- W. K. Han, V. Bailly, R. Abichandani, R. Thadhani and J. V. Bonventre, “Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury,” Kidney International, Vol. 62, No. 1, 2002, pp. 237-244. doi:10.1046/j.1523-1755.2002.00433.x
- W. K. Han, S. S. Waikar, A. Johnson, et al., “Urinary Biomarkers in the Early Diagnosis of Acute Kidney Injury,” Kidney International, Vol. 73, No. 7, 2008, pp. 863-869. doi:10.1038/sj.ki.5002715
- M. M. van Timmeren, V. S. Vaidya, R. M. van Ree, et al., “High Urinary Excretion of Kidney Injury Molecule-1 Is an Independent Predictor of Graft Loss in Renal Transplant Recipients,” Transplantation, Vol. 84, No. 12, 2007, pp. 1625-1630. doi:10.1097/01.tp.0000295982.78039.ef
- W. K. Han, V. Bailly, R. Abichandani, R. Thadhani and J. V. Bonventre, “Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury,” Kidney International, Vol. 62, No. 1, 2002, pp. 237-244. doi:10.1046/j.1523-1755.2002.00433.x
- M. H. Rosner, “Urinary Biomarkers for the Detection of Renal Injury,” Advances in Clinical Chemistry, Vol. 49, 2009, pp. 73-97. doi:10.1016/S0065-2423(09)49004-8
- C. R. Parikh, A. Jani, V. Y. Melnikov, S. Faubel and C. L. Edelstein, “Urinary Interleukin-18 Is a Marker of Human Acute Tubular Necrosis,” American Journal of Kidney Diseases, Vol. 43, No. 3, 2004, pp. 405-414. doi:10.1053/j.ajkd.2003.10.040
- C. R. Parikh, E. Abraham, M. Ancukiewicz and C. L. Edelstein, “Urine IL-18 Is an Early Diagnostic Marker for Acute Kidney Injury and Predicts Mortality in the Intensive Care Unit,” Journal of the American Society of Nephrology, Vol. 16, No. 10, 2005, pp. 3046-3052. doi:10.1681/ASN.2005030236
- C. R. Parikh, J. Mishra, H. Thiessen-Philbrook, et al., “Urinary IL-18 Is an Early Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery,” Kidney International, Vol. 70, No. 1, 2006, pp. 199-203. doi:10.1038/sj.ki.5001527
- E. Coll, A. Botey, L. Alvarez, et al., “Serum Cystatin C as a New Marker for Noninvasive Estimation of Glomerular Filtration Rate and as a Marker for Early Renal Impairment,” American Journal of Kidney Diseases, Vol. 36, No. 1, 2000, pp. 29-34. doi:10.1053/ajkd.2000.8237
- V. R. Dharnidharka, C. Kwon and G. Stevens, “Serum Cystatin C Is Superior to Serum Creatinine as a Marker of Kidney Function: A Meta-Analysis,” American Journal of Kidney Diseases, Vol. 40, No. 2, 2002, pp. 221-226. doi:10.1053/ajkd.2002.34487
- S. Song, M. Meyer, T. R. Turk, et al., “Serum Cystatin C in Mouse Models: A Reliable and Precise Marker for Renal Function and Superior to Serum Creatinine,” Nephrology, Dialysis, Transplantation, Vol. 24, No. 4, 2009, pp. 1157-1161. doi:10.1093/ndt/gfn626
- S. Herget-Rosenthal, G. Marggraf, J. Husing, et al., “Early Detection of Acute Renal Failure by Serum Cystatin C,” Kidney International, Vol. 66, No. 3, 2004, pp. 1115-1122. doi:10.1111/j.1523-1755.2004.00861.x
- M. G. Shlipak, M. J. Sarnak, R. Katz, et al., “Cystatin C and the Risk of Death and Cardiovascular Events among Elderly Persons,” The New England Journal of Medicine, Vol. 352, No. 20, 2005, pp. 2049-2060. doi:10.1056/NEJMoa043161
- M. J. Sarnak, R. Katz, C. O. Stehman-Breen, et al., “Cystatin C Concentration as a Risk Factor for Heart Failure in Older Adults,” Annals of Internal Medicine, Vol. 142, No. 7, 2005, pp. 497-505.
- M. G. Shlipak, L. F. Fried, C. Crump, et al., “Cardiovascular Disease Risk Status in Elderly Persons with Renal Insufficiency,” Kidney International, Vol. 62, No. 3, 2002, pp. 997-1004. doi:10.1046/j.1523-1755.2002.00522.x
- M. G. Shlipak, R. Katz, M. J. Sarnak, et al., “Cystatin C and Prognosis for Cardiovascular and Kidney Outcomes in Elderly Persons without Chronic Kidney Disease,” Annals of Internal Medicine, Vol. 145, No. 4, 2006, pp. 237-246.
- J. L. Koyner, M. R. Bennett, E. M. Worcester, et al., “Urinary Cystatin C as an Early Biomarker of Acute Kidney Injury Following Adult Cardiothoracic Surgery,” Kidney International, Vol. 74, No. 8, 2008, pp. 1059- 1069. doi:10.1038/ki.2008.341
- S. Herget-Rosenthal, D. Poppen, J. Husing, et al., “Prognostic Value of Tubular Proteinuria and Enzymuria in Nonoliguric Acute Tubular Necrosis,” Clinical Chemistry, Vol. 50, No. 3, 2004, pp. 552-558. doi:10.1373/clinchem.2003.027763
- S. Herget-Rosenthal, S. Trabold, F. Pietruck, M. Holtmann, T. Philipp and A. Kribben, “Cystatin C: Efficacy as Screening Test for Reduced Glomerular Filtration Rate,” American Journal of Nephrology, Vol. 20, No. 2, 2000, pp. 97-102. doi:10.1159/000013564
- L. A. Inker, C. H. Schmid, H. Tighiouart, et al., “Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C,” The New England Journal of Medicine, Vol. 367, No. 1, 2012, pp. 20-29. doi:10.1056/NEJMoa1114248
- C. R. Parikh, J. Mishra, H. Thiessen-Philbrook, et al., “Urinary IL-18 Is an Early Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery,” Kidney International, Vol. 70, No. 1, 2006, pp. 199-203. doi:10.1038/sj.ki.5001527
- D. R. McIlroy, G. Wagener and H. T. Lee, “Biomarkers of Acute Kidney Injury: An Evolving Domain,” Anesthesiology, Vol. 112, No. 4, 2010, pp. 998-1004. doi:10.1097/ALN.0b013e3181cded3f
- [61] S. M. Bagshaw, D. N. Cruz, R. T. Gibney and C. Ronco, “A Proposed Algorithm for Initiation of Renal Replacement Therapy in Adult Critically Ill Patients,” Critical Care, Vol. 13, No. 6, 2009, p. 317. doi:10.1186/cc8037
- [62] N. Lameire, “Pathophysiology of Acute Renal Failure in Sepsis,” Acta Clinica Belgica, Vol. 59, No. 4, 2004, pp. 199-208.
- [63] N. Lameire, W. Van Biesen and R. Vanholder, “Acute Renal Failure,” Lancet, Vol. 380, No. 9857, 2012, p. 1904.
- [64] J. A. Foland, J. D. Fortenberry, B. L. Warshaw, et al., “Fluid Overload before Continuous Hemofiltration and Survival in Critically Ill Children: A Retrospective Analysis,” Critical Care Medicine, Vol. 32, No. 8, 2004, pp. 1771-1776. doi:10.1097/01.CCM.0000132897.52737.49
- [65] P. Bent, H. K. Tan, R. Bellomo, et al., “Early and Intensive Continuous Hemofiltration for Severe Renal Failure after Cardiac Surgery,” The Annals of Thoracic Surgery, Vol. 71, No. 3, 2001, pp. 832-837. doi:10.1016/S0003-4975(00)02177-9
- [66] R. P. Dellinger, J. M. Carlet, H. Masur, et al., “Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock,” Critical Care Medicine, Vol. 32, No. 3, 2004, pp. 858-873. doi:10.1097/01.CCM.0000117317.18092.E4
- [67] M. Bell, E. Liljestam, F. Granath, J. Fryckstedt, A. Ekbom and C. R. Martling, “Optimal Follow-Up Time after Continuous Renal Replacement Therapy in Actual Renal Failure Patients Stratified with the RIFLE Criteria,” Nephrology, Dialysis, Transplantation, Vol. 20, No. 2, 2005, pp. 354-360. doi:10.1093/ndt/gfh581
- [68] C. C. Shiao, V. C. Wu, W. Y. Li, et al., “Late Initiation of Renal Replacement Therapy Is Associated with Worse Outcomes in Acute Kidney Injury after Major Abdominal Surgery,” Critical Care, Vol. 13, No. 5, 2009, p. R171. doi:10.1186/cc8147
- [69] V. S. Vaidya, S. S. Waikar, M. A. Ferguson, et al., “Urinary Biomarkers for Sensitive and Specific Detection of Acute Kidney Injury in Humans,” Clinical and Translational Science, Vol. 1, No. 3, 2008, pp. 200-208. doi:10.1111/j.1752-8062.2008.00053.x
- [70] D. N. Cruz, H. R. de Geus and S. M. Bagshaw, “Biomarker Strategies to Predict Need for Renal Replacement Therapy in Acute Kidney Injury,” Seminars in Dialysis, Vol. 24, No. 2, 2011, pp. 124-131. doi:10.1111/j.1525-139X.2011.00830.x
NOTES
*These authors equally contributed to this work.
#Corresponding author.

