Open Journal of Nephrology
Vol. 2 No. 4 (2012) , Article ID: 25501 , 4 pages DOI:10.4236/ojneph.2012.24017
Tubulointerstitial Nephritis Complicated with Primary Sjögren’s Syndrome under Treatment for Type 2 Diabetes Mellitus
1Department of Diabetes, Metabolism and Kidney Disease, Edogawa Hospital, Tokyo, Japan
2Department of Internal Medicine, Edogawa Hospital, Tokyo, Japan
3Department of Neurology, Edogawa Hospital, Tokyo, Japan
Email: *antoku@edogawa.or.jp
Received November 15, 2012; revised December 15, 2012; accepted December 22, 2012
Keywords: Tubulointerstitial Nephritis; Sjögren’s Syndrome; Type 2 Diabetes Mellitus
ABSTRACT
Tubulointerstitial nephritis complicated by primary Sjögren’s syndrome in a patient under treatment for type 2 diabetes mellitus was diagnosed in the early stage of the disease by renal biopsy. The symptoms of primary Sjögren’s syndrome, such as thirst and polydipsia, were masked by the characteristic symptoms of type 2 diabetes mellitus. An association between sicca symptoms and diabetes mellitus (types 1 and 2) has been previously reported. Hence, it is possible that there are common underlying immunological mechanisms between primary Sjögren’s syndrome and diabetes mellitus of both types. Intervention with steroids in a timely manner appears to have prevented or slowed the progression of renal impairment.
1. Introduction
Sjögren’s syndrome (SjS) is an autoimmune disorder that is predominantly characterized by the lymphocytic infiltration of exocrine glands, leading to keratoconjunctivitis sicca and xerostomia. SjS may present as a primary disorder or as a condition secondary to other autoimmune and chronic inflammatory disorders such as rheumatoid arthritis or systemic lupus erythematosus. SjS can also manifest itself through extraglandular morbidities such as pyrexia, arthritis, lymphadenopathy, and/or nephropathy [1,2]. Here, we report a case of tubulointerstitial nephritis associated with primary SjS in which the symptoms of thirst and polydipsia were masked by the characteristic symptoms of type 2 diabetes mellitus (DM). Decline of renal function was considered to be prevented or slowed by early diagnosis and intervention with glucocorticoid administration.
2. Case Report
The case was a 68-year-old man with independent activities of daily living. His has background history of hypertension, hyperuricemia and osteoarthritis of the knees. He has never been pointed out hyperglycemia in his regular health check. He had no family history of DM or connective tissue disorders. There was no history of smoking or drinking. He visited our hospital in April 2008 because of thirst, polydipsia, and malaise since March 2008. Upon presentation, the random blood glucose level was 36.3 mmol/L and hemoglobin A1c (HbA1c) was 10.1%. Although alpha-glucosidase inhibitor and sulfonylurea were prescribed, he was admitted to our hospital in the end of April 2008 because of no symptomatic improvement and for introduction of intensive insulin therapy. On admission, he was alert and orientated. His height, weight, and body mass index were 175 cm, 57 kg, and 18.6 kg/m2, respectively. Vital signs were as follows: body temperature, 36.8˚C; pulse rate, 58 beats per min (regular); blood pressure, 128/65 mmHg. No cervical lymphadenopathy was noted. There were several treated decayed teeth. The first and second heart sound was audible, with no murmurs. The lungs were clear on auscultation. The abdomen was flat and soft. The liver, spleen, and kidneys were not palpable. There was no peripheral edema or joint swelling. The patellar and Achilles tendon reflexes were reduced bilaterally. The vibratory sensation of the feet was blunted.
There was an obvious decline in renal function, i.e., blood urea nitrogen was 12.1 mmol/L and creatinine was 124.6 μmol/L (estimated glomerular filtration rate was 39.7 mL/min/1.73 m2). Urinalysis was positive for glucose (4+), but was negative for protein and occult blood. Twenty-four-hour urine protein excretion was 0.15 g/day, which was within the reference range. Random urine albumin corrected by creatinine was 22.4 mg/g creatinine, which was also unremarkable. Serum total protein was high (98 g/L) with a normal albumin level of 44 g/L. Polyclonal hypergammaglobulinemia (IgG 31.9 g/L; IgA 11.8 g/L; IgM 1.1 g/L) was confirmed by serum protein electrophoresis. Bence-Jones protein was not detected. Serum C-reactive protein was negative. Screening for autoimmune disorders was positive for rheumatoid factor (85.3 IU/mL), antinuclear antibody (1:320, speckled pattern), anti-SS-A/Ro antibody (≥500 U/mL), and anti-SSB/La antibody (416 U/mL). Vasculitis screening was negative for PR3-ANCA and MPO-ANCA. Glycemic control was considered to be unsatisfactory because the fasting plasma glucose level was still high (26.0 mmol/L) and glycoalbumin level was 47.8%. Anti-GAD antibody titer was <0.3 U/mL, but endogenous insulin secretion was diminished, as shown by the 24-hour urine C-peptide immunoreactivity, which was 6.1 µg/day. Abdominal ultrasonography and computed tomography revealed no morphological abnormalities in the kidneys, pancreas or liver.
Schirmer’s test revealed a reduction in tear production (right eye 4 mm/5min; left eye 3 mm/5min). The corneal fluorescein test was also positive, which is consistent with keratoconjunctivitis sicca. No diabetic changes were found in the ocular fundi. The labial salivary gland biopsy specimens showed massive mononuclear infiltrations around the ductules of the minor salivary glands, which was consistent with sialadenitis (Figure 1). Along with the elevation of the anti-SS-A/Ro and anti-SS-B/La antibodies, 3 of 4 diagnostic criteria, proposed by the Ministry of Health, Labour and Welfare of Japan in 2004, were matched, confirming diagnosis of primary SjS [1].
Renal biopsy was performed for the renal insufficiency upon suspicion of etiologies other than diabetic nephropathy. There were no diabetic changes in the glome-
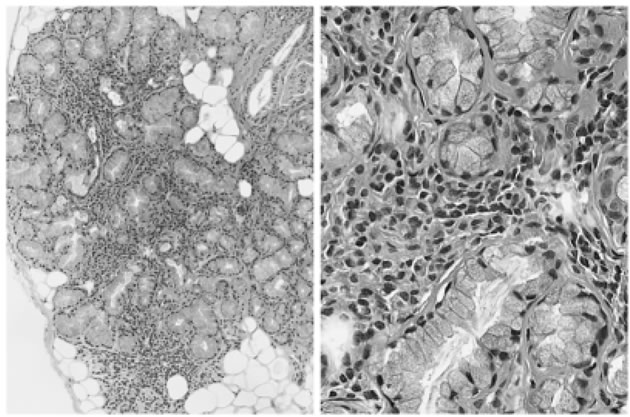
Figure 1. Hematoxylin and eosin stain of the labial salivary gland biopsy specimen shows mononuclear infiltration around the ductules of the minor salivary glands, which was consistent with sialadenitis (left ×100; right ×400).
ruli. Significant interstitial mononuclear infiltrates and mild tubular changes were observed, which was consistent with partial fibrosis in the renal interstitium (Figure 2).
Control of blood glucose levels improved after treatment with oral hypoglycemic agents was switched to intensive insulin therapy. This led to maintenance of HbA1c levels at approximately 6.0% - 7.0% and glycoalbumin levels at approximately 18.0% - 21.0%. Because symptoms such as thirst and polydipsia remained unchanged even after the introduction of intensive insulin therapy, prednisolone (40 mg po daily) was introduced with careful attention to blood glucose levels. Deterioration of renal function appeared to be prevented, and the serum creatinine levels were stabilized during 106 - 124 μmol/L (Figure 3). Urinalysis was negative for protein during the entire course of the disease and treatment. Thirst and polydipsia gradually improved. Prednisolone has been stepped down to 4 mg po daily in August 2012.
3. Discussion
This case was diagnosed as tubulointerstitial nephritis
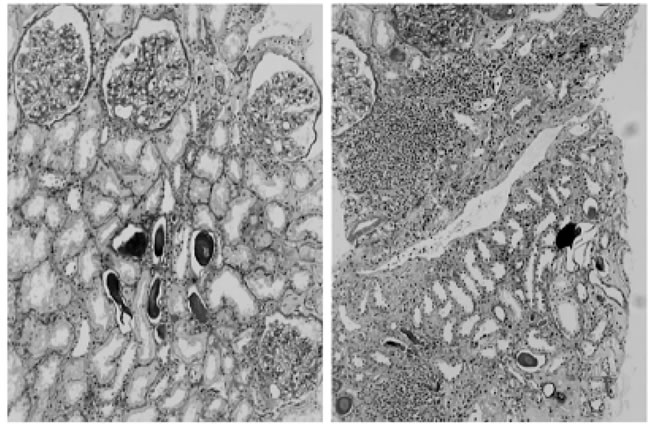
Figure 2. Periodic acid schiff PAS stain of the renal biopsy specimen shows no diabetic changes in the glomeruli (left ×100) and significant interstitial mononuclear infiltration, which is representative of interstitial nephritis. Mild tubular changes were also seen (right ×100).
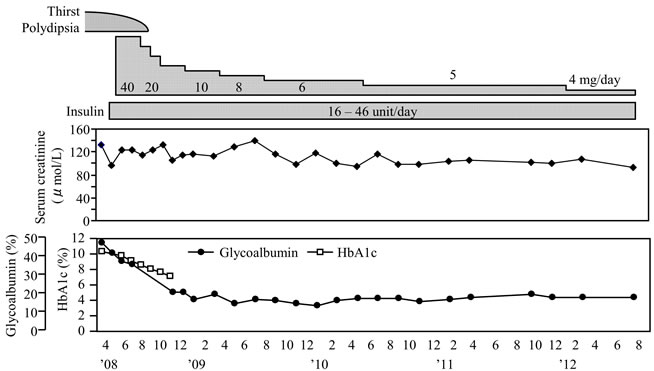
Figure 3. Clinical course of the patient.
of primary SjS, in which the characteristic symptoms (thirst and polydipsia) had overlapped with those of type 2 DM. Pathologically primary SjS demonstrates infiltration of the salivary (predominantly parotid) and lacrimal glands by lymphocytes and plasma cells, leading to the presentation of “sicca syndrome.” This immune process is also known to involve a variety of extraglandular organs [2]. Tubulointerstitial nephritis is well known to be a renal complication of primary SjS. Its incidence has been reported to vary from 2 to 67% [3-6]. As tubulointerstitial nephritis progresses, irreversible changes such as extensive fibrosis of the renal interstitium and atrophy of the renal tubules may ensue. The glomeruli usually appear normal under a light microscope, but membranoproliferative or membranous glomerulonephritis rarely develops after immune complex deposition [4]. The degree of renal impairment is normally benign, resulting in mild elevation of serum creatinine and subtle changes in urinalysis. Progression to end-stage renal disease is reported to be rare [7,8]. Steroids are believed to have potential to prevent or decelerate deterioration of renal function caused by tubulointerstitial nephritis of primary SjS. This is, however, the case only if steroid treatment is initiated before the progression of an irreversible process, such as extensive interstitial fibrosis and/or tubular atrophy.
Although DM is not a common complication of SjS, an association between the two disorders has been discussed previously [9-12]. Ikai et al. reviewed 11 Japanese patients who simultaneously suffered from type 1 DM, Hashimoto’s thyroiditis and SjS. Their research suggested possibility of a common underlying immunological pathology (i.e. HLA) in the disease process [9]. Insulin-therapy-resistant DM caused by autoantibodies to insulin receptors was reported to coincide with SjS [10]. Pal reviewed 43 patients with type 1 DM and 57 patients with type 2 DM for keratoconjunctivitis sicca. In these cases, Schirmer’s test was positive in 6/43 (13.9%) cases of type 1 DM, and in 22/57 (38.6%) cases of type 2 DM [11]. Binder et al. reported that 56/102 (55%) patients with type 1 DM showed sicca symptoms with a positive test for anti-SS-A/Ro antibodies [12]. Anti-SS-A/Ro antibody less specific than anti-SS-B/La antibody in terms of diagnosis of primary SjS is one of the anti-non-histone antinuclear antibodies. It is found in several autoimmune/ connective tissue diseases such as systemic lupus erythematosus, scleroderma, polymyositis/dermatomyositis and so forth. Our case was considered to be type 2 DM based on the pattern of onset and the fact that the antiGAD antibody test was negative. Between SjS and type 2 DM as well as type 1 DM, there may be a common underlying pathogenesis yet to be studied (i.e. HLA, common antigenicity and autoantibodies).
In the present case, tubulointerstitial nephritis of primary SjS, which had been masked by the characteristic symptoms of type 2 DM, was diagnosed by renal biopsy in an early stage of the disease. The introduction of steroid therapy in a timely manner appears to have prevented the progression of renal impairment.
REFERENCES
- T. Fujibayashi, S. Sugai, N. Miyasaka, Y. Hayashi and K. Tsubota, “Revised Japanese Criteria for Sjögren’s Syndrome (1999): Availability and Validity,” Modern Rheumatology, Vol. 14, No. 6, 2004, pp. 425-434. doi:10.1007/s10165-004-0338-x
- K. Asmussen, V. Andersen, G. Bendixen, M. Schiødt and P. Oxholm, “A New Model for Classification of Disease Manifestations in Primary Sjögren’s Syndrome: Evaluation in a Retrospective Long-Term Study,” Journal of Internal Medicine, Vol. 239, No. 6, 1996, pp. 467-474. doi:10.1046/j.1365-2796.1996.418817000.x
- W. H. Tu, M. A. Shearn, J. C. Lee and J. Hopper Jr, “Interstitial Nephritis in Sjögren’s Syndrome,” Annals of Internal Medicine, Vol. 69, No. 6, 1968, pp. 1163-1170.
- H. M. Moutsopoulos, J. E. Balow, T. J. Lawley, N. I. Stahl, T. T. Antonovych and T. M, Chused, “Immune Complex Glomerulonephritis in Sicca Syndrome,” The American Journal of Medicine, Vol. 64, No. 6, 1978, pp. 955-960. doi:10.1016/0002-9343(78)90449-7
- M. Pertovaara, M. Korpela and A. Pasternack, “Factors Predictive of Renal Involvement in Patients with Primary Sjögren’s Syndrome,” Clinical Nephrology, Vol. 56, No. 1, 2001, pp. 10-18.
- N. Bossini, S. Savoldi, F. Franceschini, et al., “Clinical and Morphological Features of Kidney Involvement in Primary Sjögren’s Syndrome,” Nephrology Dialysis Transplantation, Vol. 16, No. 12, 2001, pp. 2328-2336. doi:10.1093/ndt/16.12.2328
- S. Masouridi, A. G. Tzioufas, J. P. Ioannidis, F. N. Skopouli and H. M. Moutsopoulos, “Clinically Significant and Biopsy-Documented Renal Involvement in Primary Sjögren’s Syndrome,” Medicine (Baltimore), Vol. 79, No. 4, 2000, pp. 241-249. doi:10.1097/00005792-200007000-00005
- S. Kubo, K. Hiroshige, A. Osajima, M. Takasugi and A. Kuroiwa, “Autopsy Findings of Primary Sjögren’s Syndrome with End-Stage Renal Failure,” Nephron, Vol. 65, No. 3, 1993, p. 485. doi:10.1159/000187542
- T. Ikai T, Y. Kanezaki, T. Shimada, et al., “Slowly Progressive Type I Diabetes Seen in a Case with Polyglandular Autoimmune Syndrome Complicated by Sjögren’s Syndrome and Chronic Thyroiditis,” Tokushima Red cross Hospital Medical Journal, Vol. 13, No. 2008, pp. 37-42, (in Japanese, abstract in English).
- O. Kinoshita, K. Kubo and S. Kusama, “A Case of Insulin Resistant Diabetes Mellitus Due to Insulin-Receptor Autoantibodies with Sjögren’s Syndrome,” Nippon Naika Gakkai Zasshi (The Journal of The Japanese Society of Internal Medicine), Vol. 77, No. 10, 1988, pp. 1572-1575. doi:10.2169/naika.77.1572
- B. Pal, “Sjögren’s Syndrome and Diabetes Mellitus,” British Journal of Rheumatology, Vol. 29, No. 2, 1990, pp. 157-158. doi:10.1093/rheumatology/29.2.157
- A. Binder, P. J. Maddison, P. Skinner, A. Kurtz and D. A. Isenberg, “Sjögren’s Syndrome: Association with Type-1 Diabetes Mellitus,” British Journal of Rheumatology, Vol. 28, No. 6, 1989, pp. 518-520. doi:10.1093/rheumatology/28.6.518
NOTES
*Corresponding author.

