Open Journal of Pathology
Vol.3 No.2(2013), Article ID:30302,8 pages DOI:10.4236/ojpathology.2013.32018
Neutrophilia-Inducing Deferoxamine in Mice Infected with Staphylococcus aureus
![]()
1Department of Microbiology, College of Medicine, University of Tikrit, Tikrit, Iraq; 2Department of Microbiology, College Veterenary Medicine, University of Tikrit, Tikrit, Iraq.
Email: profaljebouri@yahoo.com
Copyright © 2013 Mohemid M. Al-Jebouri, Nihad A. Jafar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 8th, 2012; revised November 9th, 2012; accepted December 9th, 2012
Keywords: Neutrophilia; Deferoxamine; Gentamicin; S. aureus; Pathology
ABSTRACT
The ability to sequester iron is a primary defense mechanism against bacterial infection. Iron chelation therapy has been considered as a possible treatment for various infectious diseases. S. aureus isolated from neonatal septicemia were used to study the effect of deferoxamine and sub-minimal inhibitory concentration of gentamicine on some virulence factors of this isolates. Also an experimental sepsis was inducted in mice and treated with gentamicin and deferoxamine. The expression of virulence factor (alpha-hemolysin, beta-hemolysin, delta-hemolysin, coagulase, and DNase) by Staphylococcus aureus isolates was significantly decreased (p < 0.05) after exposure to DFO and/or gentamicin. The data of the present study showed that using of DFO led to significant decrease (p < 0.05) in the mortality rate of mice infected with S. aureus. In a murine model of S. aureus sepsis, deferoxamine treatment had an additional effect on survival and bacterial eradication from the organs of septicemic mice. In vitro exposure of S. aureus isolated to gentamicin and deferoxamine led to a decrease in the production of some virulence factors by these isolates.
1. Introduction
Because of the appearance of multiple drug resistant strains of microorganisms, new drugs to fight microorganisms are desperately needed. Iron chelation therapy has been considered as a possible treatment for various infectious diseases [1,2]. Bhimani found that human and bovine lactoferrins (Lf) showed weak in vitro antibacterial activity while Fe-saturated lactoferrin showed no activity. Lactoferrin-treated mice (1 mg, i.v.) when injected with 106 staphylococci, showed 30% - 50% reduction in kidney infections, and viable bacterial counts in the kidneys decreased 5 - 12-fold [3]. It has been known that Lf inhibits the growth of many bacteria, including S. aureus, by virtue of its ability to sequester iron and render it unavailable to microorganisms [4]. Also it was found that i.v. administration of HLF protect mice against experimental kidney infection [3]. Deferoxamine (DFO) is the most safe and effective iron chelator available today. The in vitro activity of DFO combined with cephalothin, gentamicin, cefotaxime, vancomycin, and fusidic acid was investigated against S. aureus. Generally, DFO acted synergistically with cephalothin, gentamicin, vancomycin, and fusidic acid, and in some cases synergy was demonstrated with DFO too [5]. Asbeck et al. study the effect of DFO on the antibacterial function of PMN. PMN were incubated for 20 hr. with various concentrations of DFO at 37˚C.They found that the uptake of radio-labeled S. aureus by PMN treated with DFO elevated from 10% to 20% higher than that of control PMN which didn’t pre-incubated with DFO. This effect was not observed when iron-saturated DFO was used [6].
2. Materials and Methods
2.1. Induction of Septicemia in Mice and Its Treatment with Deferoxamine and Gentamicin
2.1.1. Bacterial Strain
The clinical S. aureus isolated from neonatal septicemia case was used. The isolate was sub-cultured on blood agar at 37˚C overnight. Bacterial suspension was prepared by sodium chloride 0.9% solution and the concentration was adjusted by McFarland tube. The bacterial account needed for experimental sepsis was determined by a fore study. The fore study was begun with the inoculum dose of 1 × 107/ml bacterial suspension and gradually increased until experimental sepsis developed. Experimental sepsis was defined as the growth of S. aureus in two or more organs [7], and evidence of sepsis like hunched appearance, reduced spontaneous movement, lethargy, and disseminated intravascular coagulation (Figure 1) was noted. The inoculum dose of this study was estimated as 6 × 109 cfu/ml.
2.1.2. Study Design
Males of 6 - 8 weeks age mice were randomly divided into twelve groups according to dosage used; control group ( no DFO), DFO (0.625 mg) group, DFO (1.25 mg) group, DFO (2.5 mg) group; G, gentamicin (5 mg/kg) group, G (2.5 mg/kg) group, G (1.25 mg/kg) group, G (0.625 mg/kg) group, G (5 mg/kg) + DFO (0.6 mg) group, G (2.5 mg/kg) + DFO (0.6 mg) group, G (1.25 mg/kg) +DFO (0.6 mg) group, and G (0.625 mg/kg) + DFO (0.6 mg) group. Each group had 10 mice and were kept in different cages. Bacterial suspension was given intraperitoneally to the mice and when mice died, autopsy was done within one hour in aseptic conditions. If the mice were still alive at the end of seventh day, mice were sacrificed by cervical dislocation and autopsy was done. The samples were taken from lung, liver, heart, spleen, and kidney for microbiological and histopathological investigation. In each group, survival days were noted. Organs were kept in 2 ml of PBS, 0.1 ml quantities were transferred and cultured on blood agar overnight. The colonies on the agar were counted. The colonies more than 300 cfu in a plate noted as >300 cfu. Biopsies of organs forhistopathology were taken from 3 mice from each group. Tissue samples were stained with hematoxylin and eosin. The same pathologist examined tissue samples and he was unaware about the groups. Survivaldays, semi-quantitative bacterial count and histopathologic findings in the tissues of the treatment groups were compared with the control group.
2.2. Treatment
First dose of antibiotic was given at the sixth hour of bacterial inoculation. Gentamicin was given at different concentrations (5 mg/kg, 2.5 mg/kg, 1.25 mg/kg, and 0.625 mg/kg) intra-peritoneally every 12 hours for 7- days. 0.5 ml of saline containing DFO at different concentrations (0.6 mg, 1.25 mg, and 2.5 mg) were given intra-peritoneally 24 hr before bacterial challenge and proceed for five days. In control group, only bacteria suspension was given and no treatment was received.
2.3. Exposure of Staphylococcus aureus to Subinhibitory Concentration of Gentamicin
Muller-Hinton broth containing 1/2, 1/4 and 1/8 MIC gentamicin for each isolate of S. aureus were prepared in order to assess a virulence factor expression in the presence of these concentrations of drug. Each strain of S. aureus was inoculated to these media and incubated at 37˚C. After overnight incubation, doubling dilutions of culture supernatant centrifuged at 3000 rpm to remove bacteria, were made in diluent [phosphate-bufferedsaline (PBS), pH 7.1] and these used for quantitative measurements of virulence factor production [8].
2.4. Exposure of Staphylococcus aureus to Deferoxamine
Muller-Hinton broth containing 5 mg/ml DFO (Novartis Pharma, Basel, Switzerland) for each isolates of S. aureus were prepared. Each isolate of S. aureus was in oculated into these broths and incubated at 37˚C. After overnight incubation, doubling dilutions of culture supernatant, centrifuged at 3000 rpm to remove bacteria, were made in diluent [phosphate-buffered saline (PBS),
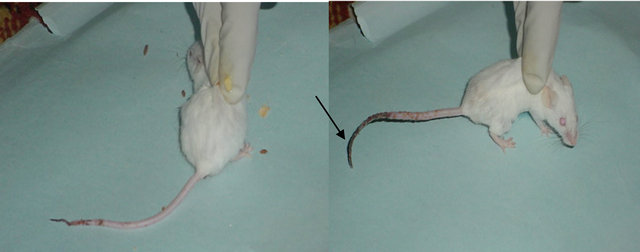
Figure 1. Disseminated intravascula coagulation in mice infected with Staphylococcus aureus isolated from septicemic neonates.
pH 7.1] and these used for quantitative measurements of virulence factor production [8].
2.5. Quantitative Measurements of Haemolysins
For the alpha haemolysin, doubling dilutions of culture supernatant for each isolate of S. aureus (original isolates and isolates exposed to SMIC of antibiotics and DFO), centrifuged at 3000 rpm to remove bacteria, were made in diluent [phosphate-bufferedsaline (PBS), pH 7.1] using microtiter plate and an equal volume of 2% v/v freshlywashed rabbit erythrocytes was added The plate was incubated at 37˚C for 60 min, at which time the highest dilution of culture supernatant causing haemolysis of 50% of the erythrocyte suspension was taken as the titer. The endpoint could be assessed accurately by measuring the number of intact RBCs using haemocytometer slide. A similar technique was used to measure beta-and delta-haemolysin, except that sheep erythrocytes were used as target cells for beta-haemolysin and human erythrocytes were used as target cells for delta-haemolysin [8].
2.6. Staphylococcal Coagulase Test
Each isolate of S. aureus (original isolates and isolates exposed to SMIC of antibiotics and DFO) was grown in nutrient broth (MAST DIAGNOSTICS, UK) with shaking for 16 hr, and culture supernatant was collected by centrifugation. Doubling dilutions of culture supernatant were made in diluent comprising PBS and 10% v/v nutrient broth, and an equal volume of 1/10 v/v citrated rabbit plasma was added. Clotting of the plasma after 4 hr of incubation at 37˚C indicated the presence of coagulase; the titre was taken as the highest dilution to produce a measurable clot [8].
2.7. DNase Test
Each isolate of S. aureus (original isolates and isolates exposed to SMIC of antibiotics and DFO) was grown for 24 h in brain-heart infusion broth. Serial dilutions of the culture supernatant were made in PBS and 20 µl aliquots were added to wells of 3 mm diameter cut in DNA agar (MAST DIAGNOSTICS, UK). The plates were incubated for 24 h and DNase activity was measured following the addition of 1 M HCl to the plate. Clear areas around the wells denoted enzyme activity; zone sizes were measured and the endpoint was taken as the highest dilution to produce ≥4 mm clearing [8].
2.8. Statistical Analysis
Statistical analyses were performed using the Chi-square test and One-way ANOVA test utilizing SPSS software. The level of significance was 0.95 with P-value < 0.05, and a high significance was 0.99 with p-value < 0.01.
3. Results
3.1. Effect of Deferoxamine on Virulence Factor Production by Staphylococcus aureus Isolates
The production of five virulence factor (alpha-hemolysin, beta-hemolysin, delta-hemolysin, coagulase, and DNase) by Staphylococcus aureus isolates were examined after exposure to DFO. The data showed that exposure of S. aureus isolates to DFO lead to significant decrease (p < 0.05 using One-way analysis) in the production of virulence factors by these isolates except for DNase test which did not show a difference before and after exposure to DFO (Table 1).
3.2. Effect of Gentamicin on Virulence Factor Production by Staphylococcus aureus Isolates
The expression of five virulence factors (alpha-hemolysin, beta-hemolysin, delta-hemolysin, coagulase, and DNase) by Staphylococcus aureus isolates were examined after growth with subinhibitory concentration of gentamicin. Gentamicin significantly (p < 0.05 using One-way analysis) inhibited alpha-haemolysin, betahaemolysin delta-hemolysin, coagulase, and DNase production by S. aureus when exposed to 1/2, 1/4 and 1/8 MIC of gentamicin compared with that in the absence of any drug (Table 2). For instance, coagulase titer for isoTable 1. Effect of deferoxamine on virulence factor production by Staphylococcus aureus isolates.
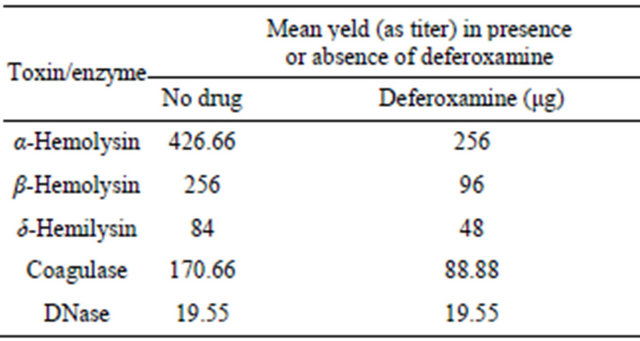
Table 2. Effect of Gentamicin on virulence factor production by Staphylococcus aureus isolates.
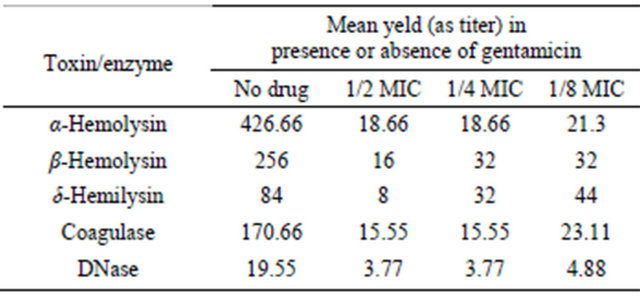
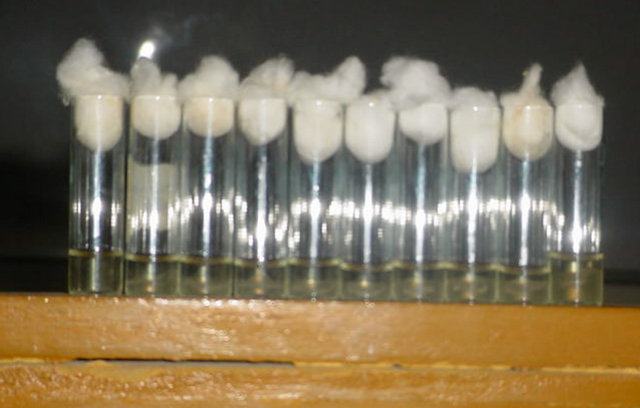
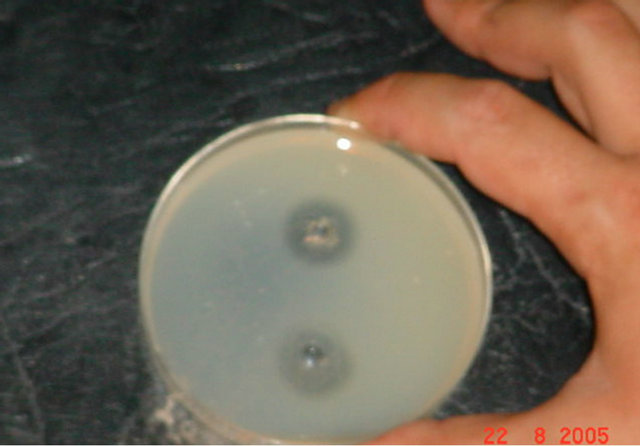
late No. 1 was 256 before exposure to gentamicin and it decreased to 16 after exposure to 1/2 MIC of gentamicin (Figures 2(a) and (b)). DNase titer for the same isolates was 16 before exposure to gentamicin and it decreased to 4 after exposure to 1/2 MIC of gentamicin (Figures 3 (a)-(c)).
3.3. Induction of Septicemia in Mice and Its Treatment with Deferoxamine, Gentamicin and Their Combinations
The data of the present study showed that using of the iron chelator (DFO) alone or in combination with subminimal inhibitory concentrations of gentamicin led to significant decrease (p < 0.05 using One-way analysis) in the mortality rate of mice infected with S. aureus (Table 3).
The present study also revealed that the bacteria were rapidly cleared off from the organs of septicemic mice in groups that received antibiotic, DFO, or their combinations. DFO received group, as with antibiotic received group, had significantly (p < 0.01 using One-way analysis) low bacterial count in the organ cultures compared with control group.
 (a)
(a) (b)
(b)
Figure 2. (a) Results of coagulase test for Staphylococcus aureus. Before and after Exposure to gentamicin (Titer 1/256). (b) Results of coagulase test for Staphylococcus aureus. Exposure to 1/2 MIC of gentamicin (Titer 1/16).
 (a)
(a)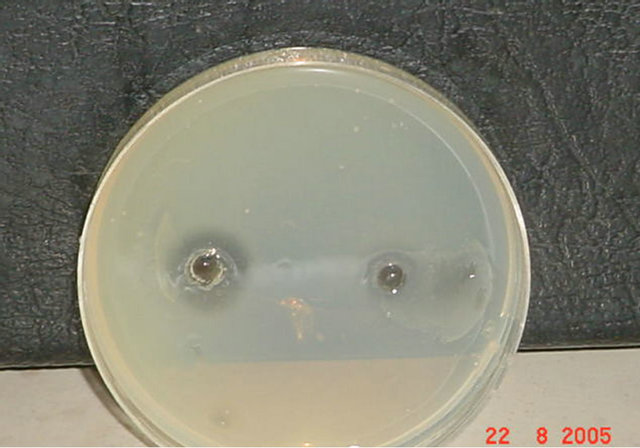 (b)
(b)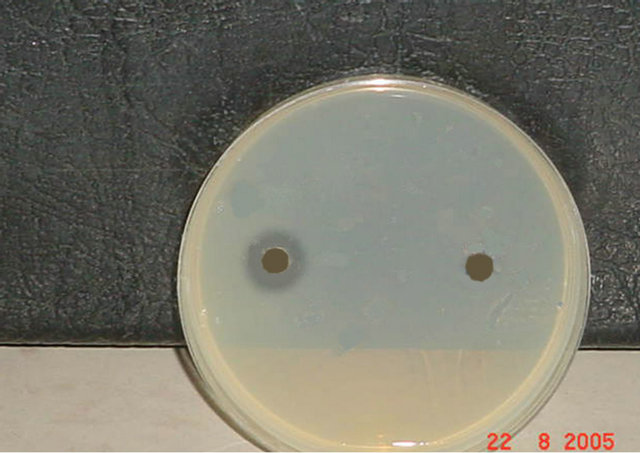 (c)
(c)
Figure 3. (a) Results of DNase Test for Staphylococcus aureus before and after exposure to gentamicin. Exposure to 1/2 MIC of gentamicin (Titer 1/4). (b) Results of DNase Test for Staphylococcus aureus before and after exposure to 1/2 MIC of gentamicin (Titer 1/8). (c) Results of DNase Test for Staphylococcus aureus before and after exposure to 1/2 MIC of gentamicin (Titer 1/16).
3.4. Histopathologic Changes Following S. aureus Sepsis in Mice
Tissue sections of the lung, heart, liver, spleen and kidney were collected from mice after induction of S. aureus sepsis in mice and their treatment with gentamicin, DFO, and their combinations. The examination of hematoxylin and eosin stained tissue sections revealed same pathoTable 3. Mortality among mice after induction of septicemia and its treatment with deferoxamine, gentamicin and their combinations.
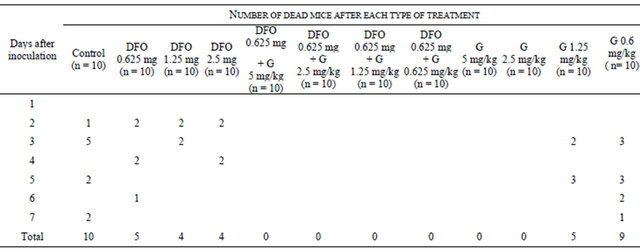
DFO: Deferoxamine, G: Gentamicin.
logical changes in the tissues especially in spleen and liver.
The data of the present study showed that S. aureus induced sepsis in mice and its treatment led to some pathological changes in mice organs especially spleen and liver. The main pathological changes in spleen include extramedulary hemopoiesis and dilatation of the sinusis which were found in all groups (Figure 4). Hemocederin pigmentation were found in groups that received DFO and gentamicin combinations.
In the liver, the main pathological changes include hydropic degeneration and cupfer cell hyperplesia (Figure 5), while there is no significant pathological changes in lung, kidney, and heart in different groups.
4. Discussion
The data showed that exposure of S. aureus isolates to DFO led to significant decrease in the production of virulence factors by these isolates except for DNase test which show no difference before and after exposure to DFO (Table 1). This result may be due to inactivation of onitase in S. aureus grown in the presence of DFO. Since, DFO lead to production of low iron pool in the surounding, and the 4Fe - 4S cluster of aconitase is in dynamic equilibrium with the surrounding iron pool, so that aconitase is rapidly demetallated when intracellular iron pools drop [9]. Demetallated aconitase is inactive enzyme, therefore, TCA cycle was inactivated. Inactivation prevented the post-exponential growth phase catabolism of acetate, resulting in premature entry into the stationary phase. This phenotype was accompanied by a significant reduction in the production of several virulence factors [10]. Somerville et al. determined that aconitase affects the synthesis of several S. aureus virulence factors and the expression of the global gene regulators RNA III and
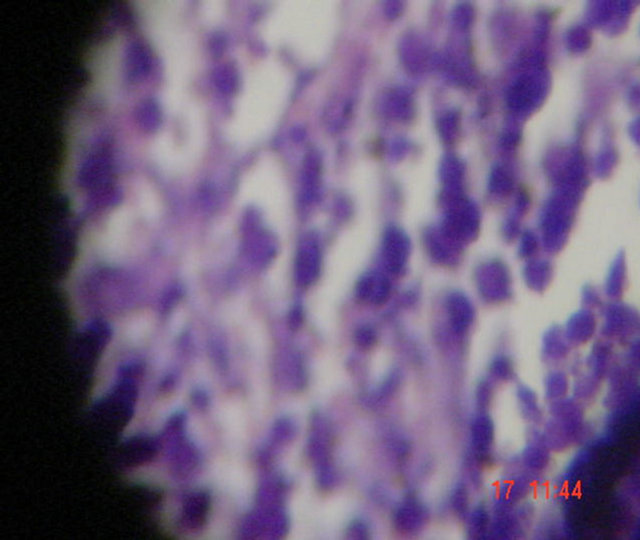
Collections of neutrophils with dilatation of sinuses
(a)

Neutrophils
(b)
Figure 4. Histopathologic changes in S. aureus-infected spleen of mice. Hematoxylin and Eosin-stained (a), (b) sections of infected tissues are shown.
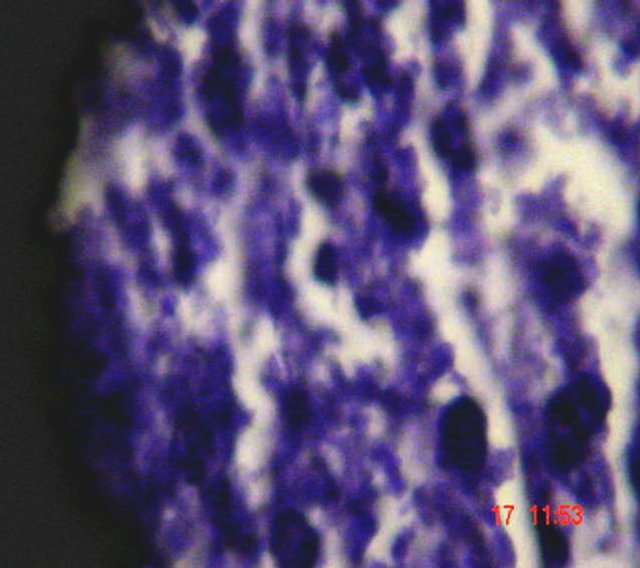
Hydropic degeneration
Figure 5. Histopathologic changes in S. aureus-infected liver of mice. Hematoxylin ad eosin-stained section of infected tissue are shown.
sarA [11].
The minimal inhibitory concentration of gentamicin was ranged between 0.5 - 8 μg/ml. According to European Committee on Antimicrobial Susceptibility Testing (EUCAST), MIC of ≤1 μg/ml interpretated as sensitive and MIC of >1 μg/ml interpretated as resistant [12]. Three out of 9 isolated tested were resistant to gentamicin.
It has long been known that the synthesis of many staphylococcal exoproteins, including virulence factors, is inhibited by subinhibitory concentrations of antibiotics whose mode of action is to block protein synthesis. In the present study, sub-MIC concentrations of gentamicin inhibited virulence factor expression by S. aureus isolates, as demonstrated by a significant decrease in five virulence factors (alpha-hemolysin, beta-hemolysin, delta-hemolysin, coagulase, and DNase)production by these isolates (Table 2). Similar result found by Gemmell and Ford [8] who estimated that Sub-MIC concentrations of linezolid led to a significant decrease in toxinand enzyme production by S. aureus and S. pyogenes. Also exposure to linezolid at concentrations below the MIC potentiated susceptibility of S. aureus and Streptococcus pyogenes to opsonophagocytosis by human neutrophils.
Although iron is required by both host and pathogen for survival and cellular replication, vertebrate animals are prone to tissue damage from exposure to excess iron. In order to protect them from this threat, a complex system has evolved to contain and detoxify this metal. This is known as the “iron withholding” defense system, which mainly serves to scavenge toxic quantities of iron and also remove iron from the circulation into metabolically inaccessible forms during acute infection [9]. The concept of reducing iron availability to pathogens was proposed as an approach to discovery of anti-infective drugs [13].
The data of the present study showed that using of the iron chelator DFO led to decrease in the mortality rate in mice infected with S. aureus (Table 3). Merali et al. [4] found that in a rat model of Pneumocystis carinii pneumonia, a 3-week infusion of DFO eliminated the trophozoite life cycle stage. It was mentioned somewhere that an anti pneumocyctis effect of DFO and they had assumed a mode of action by deprivation of nutritional iron; however, they found in another study that DFO penetrates P. carinii, causing irreversible damage, thus indicating a different mode of action [8]. Pradines et al. [1,14] found that iron chelator like a catecholate derived from spermidine, the N4-nonyl, N1, N8-bis(2,3-dihydroxybenzoyl) spermidine hydrobromide, FR160 (R = C9H19), was the most potent against the chloroquine-susceptible clone D6 and chloroquine-resistant clone W2 of P. falciparum. FR160 acted on parasites at considerably higher rates than desferrioxamine, and at all stages of parasite growth. Similar result found by Guillén et al. [13] who investigated the response to Staphylococcus aureus infection in transgenic mice carrying a functional human lactoferrin gene. They found that the transgenic mice cleared bacteria significantly better than congenic littermates, associated with a trend to reduce incidence of arthritis, septicemia, and mortality. They identified two pathways by which S. aureus clearance was enhanced. First, human lactoferrin directly inhibited the growth of S. aureus in vitro. Second, S. aureus-infected transgenic mice exhibited enhanced Th1 immune polarization. Because the Th1 response against S. aureus is protective [5], it seems likely that this enhancement is responsible for the improved bacterial clearance in the transgenic mice.
The nature of the T cell response to infectious agents or inflammatory stimuli has a major effect on the outcome of the disease. Th1 responses leading to macrophage activtion are protective during most intracellular infections [15]. Many factors influence the polarization of T cell response, including local iron availability. In our study, the decrease mortality rate among mice treated with DFO could be due to enhanced Th1 response. Since, DFO lead to decrease in iron availability leading to Th1 polarization [16-18].
As mentioned previously the in vitro exposure of S. aureus to DFO led to a significant decrease in the production of virulence factor by this bacterium (Table 1). These virulence factors are important in the pathogenesis of S. aureus, therefore, the decrease in the production of these virulence factors mean decrease in the development or severity of the disease caused by this microbe.
Moreover, the effect of DFO could be as a result of the followings: direct antibacterial activity, reduction of the free iron available to the infectious agent within the host. Th1 polarization of the immune system, increases the phagocytic function of PMN and decreases the virulence factor production by this microbe, which plays an important role in the pathogenesis of this microbe.
In current study, the pattern and degree of tissue damage varied from animal to animal. Some tissue sections displayed focal damage, while others showed a diffuse inflammatory response (acute inflammatory cells infiltration). Increased accumulation of inflammatory cells in the lung, spleen, and liver was observed (Figures 4-6). Metastic seeding of other organs with abscess formation is a common complication of S. aureus bacteremia, although, it was not observed clearly in the current study.
Tissue injury was more advanced in the liver, followed by spleen, then the lungs. Hepatomegaly and splenomegaly were the most common gross manifestations seen in the different mice groups. The histopathologic changes included leukocytes migrating away from vessels into the tissue parenchyma, accumulation of inflammatory cells in the interstitial spaces, and evidence of interstitial edema were noticed especially in the spleen and liver. The presence of a large number of dispersed leukocytes, as well as aggregates of inflammatory cells, in these tissues increased with time [9,10]. Furthermore, the assessment of the neutrophil superoxide, IL-8 and aldehyde in the present study showed an elevation in their amount and activity due to exposure of neutrophol to DFO. This finding might explain some of the claiming that DFO increases the activity of this type of leukocytes which can be useful in case of infections.
The pathological changes observed in the livers of infected mice, such as hydropic degeneration and neutrophilia have also been observed in patients with bacterial sepsis [4]. Our histopathologic data suggest that this animal model reproduces many of the pathological findings of S. aureus infections in humans and is, therefore, a useful model for further study of host-pathogen interactions in S. aureus-induced sepsis [19].
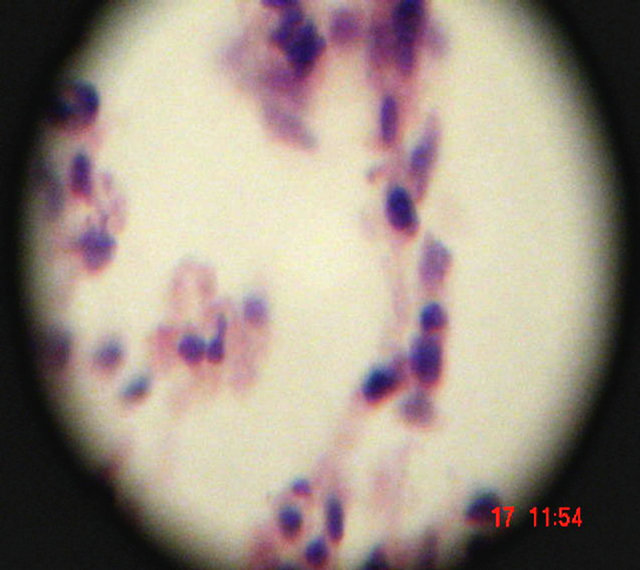
Neutrophils invading lung tissue
Figure 6. Histopathologic changes in S. aureus-infected lung of mice. Hematoxylin and eosin-stained section of infected tissue are shown.
5. Conclusion
The murine model of S. aureus sepsis showed a significant effect of deferoxamine on activation of neutrophil with addative killing action of gentamicin for bacteria.
REFERENCES
- V. R. Gordeuk, P. E. Thuma, G. M. Brittenham, et al., “Iron Chelation as a Chemotherapeutic Strategy for Falciparum Malaria,” The American Journal of Tropical Medicine and Hygiene, Vol. 4, No. 4, 1993, pp. 193-197.
- R. C. Hider and Z. Liu, “The Treatment of Malaria with Iron Chelators,” Journal of Pharmacy and Pharmacology, Vol. 49, No. 1, 1997, pp. 59-64. doi:10.1111/j.2042-7158.1997.tb06162.x
- R. S. Bhimani, Y. Vendrov and P. Furmanski, “Influence of Lactoferrin Feeding and Injection against Systemic Staphylococcal Infections in Mice,” Journal of Applied Microbiology, Vol. 86, Vol. 1, 1999, pp. 135-144.
- S. Baveye, E. Elass, J. Mazurier, et al., “A Multifunctional Glycoprotein Involved in the Modulation of the Inflammatory Process,” Clinical Chemistry and Laboratory Medicine, Vol. 37, No. 3, 1999, pp. 281-286. doi:10.1515/CCLM.1999.049
- S. H. Hartzen, N. Frimodt-Moller and V. F. Thomsen, “The Antibacterial Activity of a Siderophore. 2. The Influence of Deferoxamine Alone and Combined with Ascorbic Acid on the Activity of Antibiotics against Staphylococcus aureus,” Acta Pathologica, Microbiologica et Immunologica Scandinavica, Vol. 99, No. 10, 1991, pp. 879- 886. doi:10.1111/j.1699-0463.1991.tb01274.x
- B. V. Asbeck, J. J. Marx, A. Struyvenberg, et al., “Deferoxamine Enhances Phagocytic Function of Human Polymorphonuclear Leukocytes,” Blood, Vol. 63, No. 3, 1984, pp. 714-720.
- A. Emine, G. Suveyda, C. Ozlem, et al., “Effect of Granulocyte Colony-Stimulating Factor in Experimental Methicillin Resistant Staphylococcus aureus Sepsis,” BMC Infectious Diseases, Vol. 4, No. 2, 2004, p. 43.
- C. G. Gemmell and C. W. Ford, “Virulence Factor Expression by Gram-Positive Cocci Exposed to Subinhibitory Concentrations of Linezolid,” Journal of Antimicrobial Chemotherapy, Vol. 50, No. 2, 2002, pp. 665-672. doi:10.1093/jac/dkf192
- V. Shery, T. Yue and A. James, “Contrasting Sensitivities of Escherichia coli Aconitases A and B to Oxidation and Iron Depletion,” Journal of Bacteriology, Vol. 185, No. 1, 2003, pp. 221-230. doi:10.1128/JB.185.1.221-230.2003
- G. A. Somerville, M. S. Chaussee, C. I. Morgan, et al., “Staphylococcus aureus Aconitase Inactivation Unexpectedly Inhibits Post-Exponential-Phase Growth and Enhances Stationary-Phase Survival,” Infection and Immunity, Vol. 70, No. 11, 2002, pp. 6373-6382. doi:10.1128/IAI.70.11.6373-6382.2002
- G. A. Somerville, S. B. Beres, L. R. Fitzgerald, et al., “In Vitro Serial Passage of Staphylococcus aureus: Changes in Physiology, Virulence Factor Production, and Agr Nucleotide Sequence,” Journal of Bacteriology, Vol. 185, No. 5, 2002, pp. 1430-1437. doi:10.1128/JB.184.5.1430-1437.2002
- G. Kahlmeter, “Non-Betalactam Antibiotics—Clinical MICValues and Species-Related MIC-Breakpoints. European Committee on Antimicrobial Susceptibility Testing (EUCAST),” 2005. http://www.srga.org/MICTAB/Mictab2.htm
- C. G. Gemmell, P. K. Peterson, D. Schmeling, et al., “Potentiation of Opsonization and Phagocytosis of Streptococcus pyogenes Following Growth in the Presence of Clindamycin,” Journal of Clinical Investigation, Vol. 67, No. 3, 1981, pp. 1249-1256. doi:10.1172/JCI110152
- A. M. Shibl and I. A. Al-Sowaygh, “Differential Inhibition of Bacterial Growth and Hemolysin Production by Lincosamide Antibiotics,” Journal of Bacteriology, Vol. 137, No. 5, 1979, pp. 1022-1023.
- S. E. Unkles and C. G. Gemmell, “Effect of Clindamycin, Erythromycin, Lincomycin, and Tetracycline on Growth and Extracellular Lipase Production by Propionibacteria in Vitro,” Antimicrobial Agents and Chemotherapy, Vol. 21, No. 1, 1982, pp. 39-43. doi:10.1128/AAC.21.1.39
- A. M. Shibl and I. A. Al-Sowaygh, “Antibiotic Inhibition of Protease Production by Pseudomonas aeruginosa,” Journal of Medical Microbiology, Vol. 13, No. 2, 1980, pp. 345-348. doi:10.1099/00222615-13-2-345
- A. M. Shibl and C. G. Gemmell, “ Effect of Four Antibiotics on Haemolysin Production and Adherence to Human Uroepithelial Cells by Escherichia coli,” Journal of Medical Microbiology, Vol. 16, No. 3, 1983, pp. 341-349. doi:10.1099/00222615-16-3-341
- S. Herbert, P. Barry and R. P. Novick, “Subinhibitory Clindamycin Differentially Inhibits Transcription of Exoprotein Genes in Staphylococcus aureus,” Infection and Immunity, Vol. 69, No. 5, 2001, pp. 2996-3003. doi:10.1128/IAI.69.5.2996-3003.2001
- M. M. Al-Jebouri and N. A. Jafar, “Neutroplilia-Induced Deferoxamine in Mice Infected with Staphylococcus aureus,” International Symposium, Abstracts, The Neutrophil in Immunity, Quebec, 2012, p. 130.

