Open Journal of Clinical Diagnostics
Vol.3 No.2(2013), Article ID:33600,7 pages DOI:10.4236/ojcd.2013.32013
Comparative prevalence of MRSA in two Nepalese tertiary care hospitals
![]()
Department of Microbiology, Tri-Chandra Campus, Tribhuvan University, Kathmandu, Nepal
Email: b_shrestha_07@hotmail.com
Copyright © 2013 Bidya Shrestha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 25 April 2013; revised 26 May 2013; accepted 2 June 2013
Keywords: MRSA; Nepal; Hospital; Antibiotic; Resistance
ABSTRACT
This comparative study has been focused on the prevalence of MRSA types and their antibiotic resistance in two tertiary care hospitals of Nepal. During November 2007 to June 2009, clinical samples from patients with nosocomial infection from two Nepali hospitals, Kathmandu Based Hospital (KBH) and Lalitpur Based Hospital (LBH) were cultured and antibiotic susceptibility tests done following standard methodology in Microbiology laboratory, Tribhuvan University Teaching Hospital, Kathmandu, Nepal. Occurrence of MRSA (52.9% of 304 Staphylococcus aureus isolates) in KBH and that of MSSA (62% of 100 S. aureus isolates) in LBH were significant. No association of age was observed with MRSA or MSSA. Among MSSA from both hospitals, the highest resistance was found against penicillin. KBH urinary isolates were resistant to norfloxacin (51.4%), while isolates from other sites were resistant to ciprofloxacin (30.6%), erythromycin (12%), gentamicin (10.3%). LBH isolates were resistant to co-trimoxazole (22.6%), erythromycin (17.2%), ciprofloxacin (13.8%), gentamicin (12.9%). Among MRSA, most of the isolates from both hospitals were resistant to a wide array of antibiotics. A majority of the MSSA and MRSA isolates were susceptible to rifampicin and chloramphenicol. Most of KBH MRSA were homogeneous MRSA, 80.5% (significant), of which, 99.2% were multiresistant oxacillin resistant S. aureus (MORSA). And among the heterogeneous MRSA isolates from KBH, 71% were MORSA. On the other hand, among LBH MRSA isolates, 52.6% were homogeneous MRSA, cent percent of which were MORSA while 47.4% were heterogeneous MRSA of which 44.5% were MORSA. Since almost all of the homogeneous MRSA and most of the heterogeneous MRSA from both hospitals were MORSA, there is a possibility that a hospital acquired S. aureus could be MORSA. Hence, every infected patient should be considered as a potential source of MORSA.
1. INTRODUCTION
Previously, methicillin sensitive Staphylococcus aureus (MRSA) was responsible for most of the staphylococcal infections. In recent years, MRSA has accounted for approximately 80% of all S. aureus infections [1], emerging as a major nosocomial pathogen worldwide [2,3]. A continued rise in the MRSA incidence has been reported by various researchers [4,5], engendering a significant risk to patients and contributing to a substantial financial burden on healthcare resources. A considerable variation in the number of clinical infections among units, hospitals, countries and among individual isolates has been reported [6].
An altered penicillin binding protein (PBP), PBP2a, which has low affinity for binding with β lactam antibiotics is responsible for methicillin resistance in S. aureus [7]. PBP2a is encoded in the mecA gene whose transcription is regulated by regulatory regions mecI and mecR1 genes located 5’ to mecA gene. The gene complex consisting of mecI, mecR1 and mecA has been referred to as the mec complex [8]. The mecA gene is carried in SCCmec cassette, which can accommodate a number of genetic elements for antibiotic resistance (leading to multiple resistant strains) and a panoply of virulence factors. These chromosome mediated genes have been widely distributed among many Staphylococcal species [9]. MRSA is produced when methicillin sensitive S. aureus (MSSA) acquires the genetic element, staphylococcal cassette chromosome, SCCmec [10].
Nosocomial MRSA strains are resistant to multiple antibiotics. Patients infected with MRSA need to be treated with second and third choice of drugs, which tend to be less effective, more toxic and more expensive [11]. Therefore, people infected with MRSA have longer hospital stays, further adding to the cost while making the hospital facility unavailable to other patients. Unfortunately, MRSA infection may even end in death due to the multiple resistance.
Methicillin resistant strains may be homogeneous or heterogeneous. Homogeneous strains are composed of a single population of highly resistant cells [12,13] and hence can grow in the presence of high concentrations of methicillin (>50 μg/ml). In heterogeneous resistant strains, only 1 in 108 cells grows in the presence of high concentration of methicillin (e.g. 50 μg/ml)—most cells appear susceptible to relatively low concentration of methicillin. Accordingly, heterogeneous strains have been considered to be composed of 2 populations of cells: relatively susceptible cells and highly resistant cells [12].
2. MATERIAL AND METHODS
Consecutive Clinical samples (non repetitive) were cultured for the isolation of S. aureus from patients admitted in two hospitals, Kathmandu based hospital (KBH) and Lalitpur based hospital (LBH). S. aureus isolates (n = 404, 304 from KBH and 100 from LBH) were identified and antibiotic susceptibility tests were performed in the Department of Microbiology, Institute of Medicine, Kathmandu, Nepal. The tests were performed during the period of Nov 2007 to June 2009.
Records including clinical history, type of infection, ward, gender, age, consumption of antibiotics, were obtained from patients whose sample S. aureus had been isolated. Their duration of stay, development of infection 48 - 72 hours after admission to the hospital, use of prosthetic devices, use of invasive procedures such as catheterization, and use of hospital instruments were also recorded. Only those cases that developed infection post 48 hours of admission were included in the study. Patients who had been admitted in the hospital previously and submitted sample for culture within one month of discharge from the hospital were also included as per definition of nosocomial infection.
2.1. Isolation and Identification
All clinical samples (urine, urinary catheter, sputum, endotracheal tube, blood, body fluids, pus, wound swab, high vaginal swab, tissue, discharges, etc.) of hospitalized patients were processed as described by the American Society for Microbiology, ASM 2004 [14]. Samples were inoculated onto recommended medium within 30 minutes of collection and incubated for 18 hours at 35˚C.
Any golden yellow or cream colored or white colony 2 - 5 mm in size, entire, smooth, convex or domed, opaquebutyrous, hemolytic or non hemolytic in Blood agar (BA) plate and lactose fermenting in MacConkey agar (MA) plate were Gram stained. Gram positive cocci isolates grown on BA were tested for various biochemical properties along with S. aureus ATCC 25923. Gram positive cocci occurring in clusters or pairs or occurring singly, non sporing; and colonies that were positive for catalase, mannitol fermentation, lactose fermentation, VP, DNase, clumping factor, staphylocoagulase, staphytect plus and negative in oxidase were identified as S. aureus. Staphylocoagulase test was taken as the diagnostic test for the identification of S. aureus.
2.2. Antibiotic Sensitivity Test
Antibiotic susceptibility of the S. aureus isolate was done as recommended by the Clinical Laboratory Standard Institute [15]; the antibiotics discs (Oxoid, UK) used were penicillin (10 units), oxacillin (1 µg), cefoxitin (30 µg), vancomycin (30 µg), teicoplanin (30 μg), erythromycin (15 µg), clindamycin (2 µg), co-trimoxazole (1.25/23.75 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), rifampicin (5 µg) and tetracycline (30 µg). For urinary isolates, nitrofurantoin (300 µg), norfloxacin (10 µg) and novobiocin (5 μg) were used in place of erythromycin, clindamycin, chloramphenicol and ciprofloxacin. Determination of the minimum inhibitory concentration by microbroth dilution method was done as recommended by the Clinical Laboratory Standard Institute [16]. Control strain S. aureus ATCC 25923 was run in parallel.
2.3. Identification of MRSA
Isolates from both KBH and LBH were tested by disc diffusion using oxacillin and cefoxitin, isolates resistant to these were identified as MRSA. Methicillin resistance of these isolates was confirmed by microbroth dilution method, the minimum inhibitory concentration test technique. Those isolates that exhibited small colonies within the zone of inhibition and had MIC <100 µg/ml were regarded as heterogeneous MRSA; and those that had confluent growth up to the disc and had MIC >100 µg/ml were regarded as homogeneous MRSA.
2.4. Grading of MRSA
MRSA were identified as multiresistant oxacillin resistant S. aureus (MORSA) and non-multiresistant oxacillin resistant S. aureus (NORSA) as described by Gosbell et al., 2001 [17]. Multi-resistant S. aureus exhibiting resistance to ≥3 non-β lactam antibiotics were classified as MORSA and those exhibiting resistance to ≤2 non-β lactam antibiotics as NORSA.
The data was analyzed using SPSS 11.5 program.
3. RESULT
Of 304 isolates from KBH, 159 were identified as MRSA (52.3%), making it more prevalent than its methicillin sensitive counterpart (47.7%). On the contrary, in LBH, the occurrence of MSSA (62%) was found to be higher compared to MRSA (38%). MRSA infection among the nosocomial infections in KBH was high compared to that in LBH. The higher occurrence of MRSA in KBH and that of MSSA in LBH was significant (χ2 = 5.16, P < 0.05).
3.1. Prevalence of the Infection among Different Age Groups
Patients were categorized into 5 age groups: <1 year, 1.1 to 14 years, 15 to 45 years, 46 to 60 years, and 61 years and above.
In KBH, higher occurrence of nosocomial infection was observed in the 15 to 45 years age group (Figure 1). MSSA was found to be prevalent in the <1 year and 1.1 to 14 years age groups, MRSA and MSSA prevalence were equal in 14 to 45 years age group and MRSA was more prevalent in the remaining age groups 46 to 60 years, and >61years.
In regression calculation, taking <1 year age group as the base value, an increase in the infection rate with the increase in age was observed. S. aureus infection increased steadily with age till the 46 - 60 years age group. The increase in infection rates in 1.1 to14 years age group (Odd’s ratio = 2.105, CI 0.207 to 21.449), in 15 to 45 years age group (Odd’s ratio = 4.0, CI 0.438 to 36.548) and in 46 to 60 years age group (Odd’s ratio = 6.476, CI 0.677 to 61.927) were steady while a slight upward trend was noted in the >61 years age group (Odd’s ratio = 6.889, CI 0.714 to 66.479). However the increase in infection with age was not statistically significant.
Similarly, in LBH, the occurrence was higher in 14 to 45 years age group. MSSA infection was prevalent in all groups except in the <1 year group (Figure 2).
However, regression calculation revealed a different picture for LBH compared to KBH. A steady decrease in
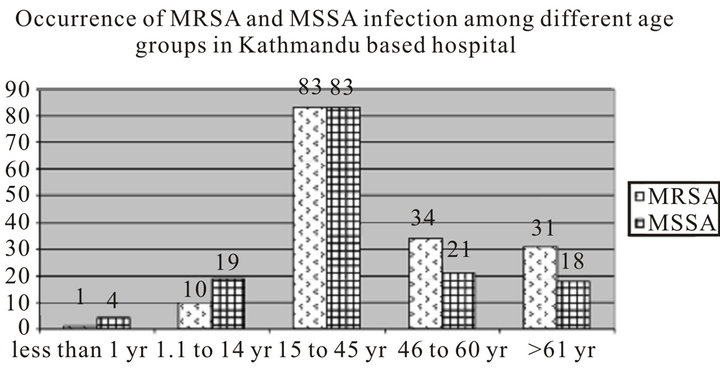
Figure 1. MRSA and MSSA infections according to age groups (Kathmandu Based Hospital).
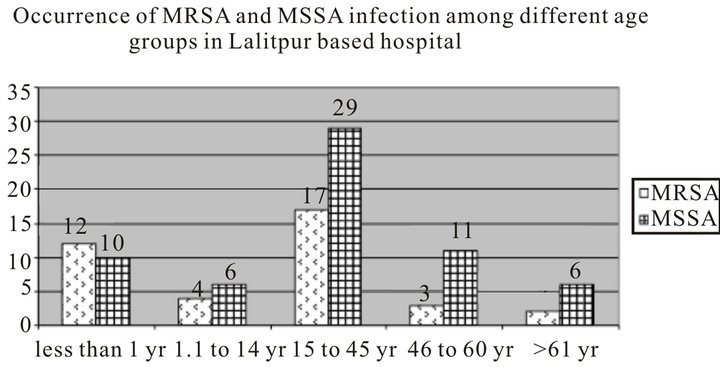
Figure 2. MRSA and MSSA infections according to age groups (LalitpurBased Hospital).
infection with increasing age was observed till the 45 to 60 years age group. Taking <1 year as base value for regression calculation, the infection rate decreased in 1.1 to 14 years age group (Odd’s ratio = 0.556, CI 0.122 to 2.536), in 15 to 45 years age group (Odd’s ratio = 0.489, CI 0.174 to 1.370) and in 46 to 60 years age group (Odd’s ratio = 0.227, CI 0.049 to 1.047), and then became almost stable in 61 and above age group (Odd’s ratio = 0.278, CI 0.046 to 1.692). However this was also not statistically significant.
Of note, in LBH, the higher occurrence of infection in the <1 year age group was conferred by umbilical infection in the neonatal unit.
3.2. Antibiotic Susceptibility Test
For antibiotic susceptibility test, the Food Drug Administration recommended antibiotics (penicillin, erythromycin, co-trimoxazole, chloramphenicol, ciprofloxacin, rifampicin, gentamicin, tetracycline, norfloxacin and nitrofurantoin were used in addition to oxacillin and cefoxitin.
Among KBH isolates, resistance was observed in a smaller number of MSSA isolates compared to MRSA isolates. Within the MSSA isolates, resistance was observed highest to penicillin (86.9%), followed by norfloxacin (51.4%) in urinary isolates and ciprofloxacin (30.6%) in other isolates. MSSA resistance to the latter 2 antibiotics, norfloxacin and ciprofloxacin, both of which are categorized as fluoroquinolones indicate a heightened MSSA resistance to fluoroquinolones. The urinary isolates were categorically susceptible to nitrofurantoin and isolates from other sites were all susceptible to rifampicin. Most of the MSSA isolates were susceptible to most of the antibiotics tested (Table 1).
Among MRSA isolates obtained from the same hospital, a large number of the isolates were resistant to most of the antibiotics tested. The highest resistance (100%) observed was to norfloxacin (by urinary isolates) and to penicillin. Resistance to other antibiotics was also notable: ciprofloxacin (98.5%), gentamicin (98.1%), erythromycin (90.9%), co-trimoxazole (83%), tetracycline (79.2%), and so on. Most of the MRSA isolated from sources other than the urinary tract were susceptible to chloramphenicol (93.9%) and rifampicin (88.1%). Similar to MSSA, resistance was high against fluoroquinolones among MRSA isolates as well. All urinary MRSA isolates were susceptible to nitrofurantoin, as were urinary MSSA isolates (Table 1).
Similar to KBH isolates, resistance was observed in a small number of LBH MSSA isolates compared to MRSA. Among MSSA, the resistance was observed to co-trimoxazole (22.6%), erythromycin (17.2%), ciprofloxacin (13.8%), gentamicin (12.9%). Most of the isolates were susceptible to chloramphenicol and tetracycline (Table 2). Unlike KBH, fluoroquinolone resistance was limited. All urinary isolates were susceptible to both norfloxacin and nitrofurantoin. Of 62 MSSA isolates, rifampicin was tested in 48 isolates and all of them were susceptible.
All MRSA isolates obtained from sources other than the urinary tract were susceptible to chloramphenicol while most were resistant to many of the tested antibiotics. Of 38 MRSA isolates, rifampicin was tested in 18 isolates and all of them were found to be susceptible.
3.3. Prevalence of Homogeneous and Heterogeneous MRSA
In KBH, MRSA demonstrated a higher occurrence (52.3%, n = 159) than MSSA; and among the MRSA isolates, homogeneous MRSA (80.5%, 128/159) was more prevalent than the heterogeneous MRSA (19.5%, 31/159). Conversely in LBH, the prevalence of MSSA (62%, n = 62) was higher than that of MRSA (38%, n = 32). Homogeneous MRSA (52.6%, 20/38) and heterogeneous MRSA (47.4%, 18/38) were almost equal in occurrence (Table 3).
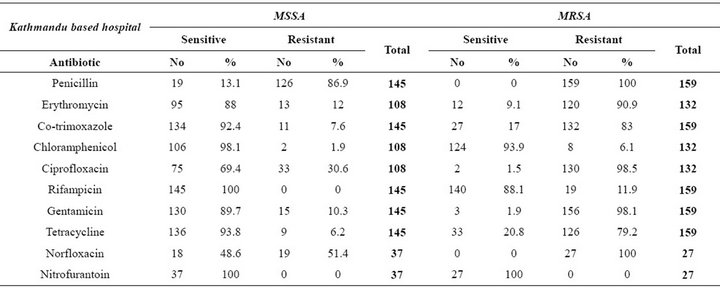
Table 1. Antibiotic sensitivity pattern among MSSA and MRSA isolated from Kathmandu based hospital.
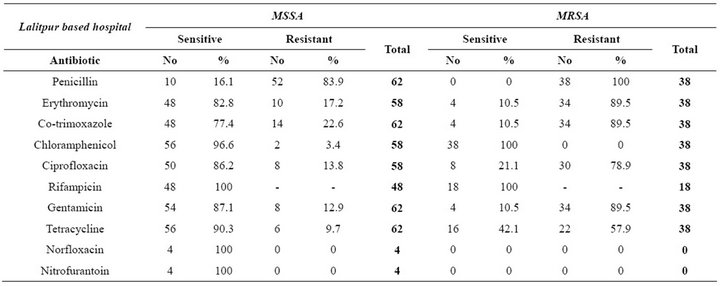
Table 2. Antibiotic sensitivity pattern among MSSA and MRSA isolated from Lalitpur based hospital.
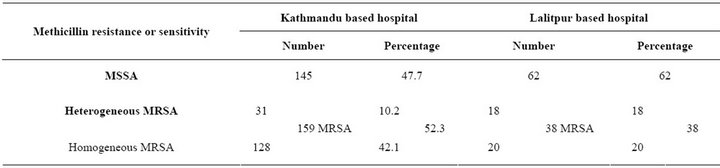
Table 3. Occurrence of MSSA and MRSA in Kathmandu based hospital and Lalitpur based hospital.
Homogeneous MRSA occurrence was significantly higher in KBH as compared to LBH isolates (χ2 = 12.75, P < 0.05).
3.4. Prevalence of MORSA and NORSA
In KBH, where homogeneous MRSA (80.5%) was found in much higher prevalence compared to heterogeneous MRSA (19.5%); 99.2% of the homogeneous MRSA and 71% of heterogeneous MRSA were found to be MORSA. Following a similar pattern 100% of the LBH homogeneous MRSA were MORSA while only 44.5% of heterogeneous MRSA were MORSA.
4. DISCUSSION AND CONCLUSION
The significantly higher rate of MRSA infection observed in KBH as compared to LBH can be justified by the fact that KBH is a big and busy hospital with a remarkable load of referral cases. The enormity of MRSA infection, specifically homogeneous MRSA in KBH compared to LBH can be justified by the fact that once MRSA becomes endemic within a hospital, such resident bacteria may account for 5% to 50% of all nosocomial S. aureus infections in that hospital [18]. Infection control program should be effectively implemented to breach the transmission of MRSA among patients and to the community.
In LBH, no relation between age and the rate of S. aureus nosocomial infection—whether MSSA or MRSA could be observed. In KBH however, a steadily escalating infection rate with increasing age was observed, albeit insignificantly. Of note, there have been studies suggesting an association of S. aureus infection with older age group [19-21]. On the contrary, in LBH, infection rates appeared to decline with increase in age, a trend that was once again statistically insignificant. This trend in LBH could be attributed to a higher infection rate in <1 year age group, reflected by the multiple umbilical pus samples obtained from this hospital, clearly depicting the requisition of stringent infection control measures in the delivery room and neonatal units.
Among the MSSA isolates, most were susceptible to many of the tested FDA recommended antibiotics. Among MSSA from both the hospitals, highest rate of resistance was observed against penicillin, as has been previously reported [22]. Resistance to norfloxacin in urine isolates and to ciprofloxacin in isolates from other sites was found to be high in KBH. Fluoroquinolone antibiotics are used frequently in Nepal and their unrestrained use could have led to the development of resistance against these antibiotics, as has been stated in a previous study [23]. High rates of resistance were observed against co-trimoxazole and erythromycin in LBH—once again, the unrestrained use of these antibiotics in Nepal could explain the development of resistance. Among MRSA isolates, resistance to most of the antibiotics apart from chloramphenicol and rifampicin was observed. Similar resistance has been reported in Nepal [24]. Higher susceptibility to rifampicin and chloramphenicol without any predilection either to MRSA or to MSSA can be justified by the fact that rifampicin is not used in infections other than tuberculosis and that chloramphenicol resistance, once incurred by unrestricted usage [25], has been lost due to discontinuation of chloramphenicol in clinical practice.
Regarding the prevalence of MRSA and MSSA, contrasting results were obtained from these hospitals.
MRSA was more prevalent in KBH compared to LBH. Further the occurrence of homogeneous MRSA was quite high in KBH, as has been reported [26]. LBH, although a tertiary care hospital, is with relatively less patient flow and referral cases; hence, a lower occurrence of MRSA can be justified. KBH, on the other hand, is a large tertiary care hospital where patients from all over the country seek treatment; higher occurrence of MRSA in such relatively large-volume hospitals has been reported [27]. Furthermore, the referral cases from other hospitals make a large part of the KBH patient population. It may be deduced that due to the constant inflow of chronic patients in this hospital, hospital-acquired infection by MRSA is more prevalent in KBH [28]. Furthermore, it is also important to note that MRSA spreads more easily than MSSA due to the selection under antibiotic pressure and/or due to an unknown intrinsic factor within MRSA [29]. Therefore, to control the emergence of highly resistant strains, antibiotic pressure favoring the selection of MRSA should be minimized [30].
It is due to the antibiotic selection pressure that MRSA infections have been confined exclusively to hospitals, long term hospital care facilities or similar healthcare settings [31]. Present finding that almost all of the homogeneous MRSA and a huge part of heterogeneous MRSA were MORSA is supported by published reports [26,32]. Patients infected with such strains should be kept in isolation and be treated with a strict regimen of second and third choice of drugs, which tends to come with both a toxicity and a financial toll. Economic burden due to MRSA infection is pretty high compared to that related to MSSA infection. The treatment cost of a patient infected with MRSA ($65,000) has been reported to be much higher compared to that of MSSA infection ($24,500) [33]. Concomitantly, these nosocomial infections contribute to 0.7% to 10.1% of the deaths and confer 0.1% to 4.4% of all deaths occurring in hospitals [34]. Moreover, once MRSA resides in a hospital environment, it is difficult to get rid of the bacteria, and the hospital environment may continually serve as a source of future nosocomial infections [18]. Therefore, stringent infection control measures should be implemented effectively in both hospitals.
![]()
![]()
REFERENCES
- Wenzel, R.P. (1994) Healthcare workers and the incidence of nosocomial infection: Can treatment of one influence the other? A brief review. Journal of Chemotherapy, 6, 33-37, 39-40.
- Simor, A., Ofner-Agostini, M. and Patin, S. (1997) The Canadian Nosocomial Infection Surveillance Program: Results of the first 18 months of surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospitals. Canada Communicable Disease Report, 23, 41-45.
- Witte, W. (1999) Antibiotic resistance in gram positive bacteria: Epidemiological aspects. Journal of Antimicrobial Chemotherapy, 44, 1-9. doi:10.1093/jac/44.suppl_1.1
- Panlilio, A.L., Culver, D.H., Gaynes, R.P., Banerjee, S., Hendersen, T.S., Tolson, J.S. and Martone, W.J. (1992) Methicillin resistant Staphylococcus aureus in U.S. hospitals, 1975-1991. Infection Control and Hospital Epidemiology, 13, 582-586. doi:10.1086/646432
- Bartlett, M., Mclntyre, L., Mosoley, R., O’Connor, j., Shaw, J. and Whitehead, C. (2002) Methicillin resistant Staphylococcus aureus rates increase 260% in hospitals participating in HIP’s international monitoring systems. Center for Disease Control and National Center for Infectious Diseases Focus, 9, 4.
- Kim, T., Oh, P.I. and Simor, A.E. (2001) The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals. Infection Control and Hospital Epidemiology, 22, 99-104. doi:10.1086/501871
- Hartman, B.S. and Tomasz, A. (1984) A low affinity penicillin binding protein associated in beta lactam resistance in Staphylococcus aureus. Journal of Bacteriology, 158, 513-516.
- Katayama, Y., Ito, T. and Hiramatsu, K. (2001) Genetic organization of the chromosome region surrounding mecA Clinical Staphylococcal strains: Role of IS431-mediated mecI deletion in expression of resistance in mecA carrying low level methicillin resistance Staphylococcus haemolyticus. Antimicrobial Agents and Chemotherapy, 45, 1955-1963. doi:10.1128/AAC.45.7.1955-1963.2001
- Hurlimenn-Dalel, R.L., Ruffel, C., Kayser, F.H. and Berger-Bachi, B. (1992) Survey of methicillin resistance associated genes mecA, mecR1-mecI and femA-femB in clinical isolates of MRSA. Antimicrobial Agents and Chemotherapy, 36, 2617-2621. doi:10.1128/AAC.36.12.2617
- Ito, T., Max, X., Tekuchi, F., Okuma, K., Yuzawa, H. and Hiramatsu, K. (2004) Novel type V SCCmec driven by a novel cassette chromosome recombinaseccrC. Antimicrobial Agents and Chemotherapy, 48, 2637-2651. doi:10.1128/AAC.48.7.2637-2651.2004
- Centers for Disease Control and Prevention (2010) About antimicrobial resistance: A brief overview. www.cdc.gov/drugresistance/about.htm
- Chambers, H.F. (1988) Methicillin resistant staphylococci. Clinical Microbiology Reviews, 1, 173-186.
- Chambers, H.F. (1997) Methicillin resistance in staphylococci: Molecular and Biochemical basis and clinical implications. Clinical Microbiology Reviews, 10, 781- 791.
- Isenberg, H.D. (2004) Clinical microbiology procedure handbook Vol. 1, American Society for Microbiology. ASM Press, Washington DC.
- Clinical Laboratory Standards Institute (2007) Performance standard for antimicrobial susceptibility testing: Seventeenth informational supplement M100-S17, Vol. 27, No. 1. Clinical Laboratory Standards Institute, Wayne.
- Clinical Laboratory Standards Institute (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-seventh edition. M7-A7, Vol. 27, No. 2. Clinical Laboratory Standard Institute, Wayne.
- Gosbell, I.B., Neville, S.A., Mercer, J.L., Fernandes, L.A. and Fernandes, C.J. (2001) Evaluation of the MRSAscreen test in detecting oxacillin resistance in community and hospital isolates of Staphylococcus aureus. Pathology, 33, 493-495. doi:10.1080/00313020120083214
- Boyce, J.M. (1989). Methicillin resistant Staphylococcus sureus detection, epidemiology and control measures. Infectious Disease Clinics of North America, 3, 901-913.
- Klevens, M.R., Morrison, M.A., Nadle, J., Petit, S., Gershman, K., Ray, S., Harrison, L.H., Lynfield, R., Dumyati, G., Townes, J.M., Craig, A.S., Zell, E.R., Forsheim, G.E., McDougal, L.K., Carey, R.B. and Fridkin, S.K. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Journal of American Medical Association, 298, 1763-1771. doi:10.1001/jama.298.15.1763
- Lepelletier, D., Ferreol, S. and Villers, D. (2004) Methicillin resistant Staphylococcus aureus nosocomial infection in ICU: Risk factors, morbidity and cost. PathologieBiologie, 52, 474-479. doi:10.1016/j.patbio.2004.06.002
- Huang, H., Flynn, M., King, J.H. and Monchaud, C. (2006) Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. Journal of Clinical Microbiology, 44, 2423-2427. doi:10.1128/JCM.00254-06
- Centers for Disease Control and Prevention (2005) Laboratory detection of: Oxacillin/methicillin-resistant Staphylococcus aureus. www.cdc.gov/mrsa/lab/lab-detection.html
- Kotilainen, P., Nikoskelainen, J. and Huovinen, P. (1990) Emergence of ciprofloxacin resistant coagulase negative staphylococcal skin flora in immunocompromised patients receiving ciprofloxacin. Journal of Infectious Diseases, 161, 41-44. doi:10.1093/infdis/161.1.41
- Shrestha, B., Pokhrel, B.M. and Mohapatra, T.M. (2009) Antibiotic susceptibility pattern of nosocomial isolates of Staphylococcus aureus in a tertiary care hospital, Nepal. Journal of Nepal Medical Association, 48, 234-238.
- Alestig, K. (2004) Tetracyclines and chloramphenicol. In: Cohen, J. and Powdwely, W.G., Eds., Infectious diseases, 2nd Edition, Harcourt Publishers Limited, Edinburgh, 1843-1847.
- Shrestha, B., Pokhrel, B.M. and Mohapatra, T.M. (2009) Phenotypic characterization of nosocomial isolates of Staphylococcus aureuswith reference to MRSA. Journal of Infection in Developing Countries, 3, 554-560. doi:10.3855/jidc.474
- Zinn, C.S., Westh, H., Rosdahl, V.T. and Sarisa Study Group (2004) An international multicenter study of antimicrobial resistance and typing of hospital Staphylococcus aureus isolates from 21 laboratories in 19 countries or states. Microbial Drug Resistance, 10, 160-168. doi:10.1089/1076629041310055
- Haley, R.W., Hightower, A.W., Khabbaz, R.F., Thornsberry, C., Martone, W.J., Allen, J.R. and Hughes, J.M. (1982) The emergence of methicillin resistant Staphylococcus aureus in United States hospitals. Possible role of house staff-patient transfer circuit. Annals of Internal Medicine, 97, 297-308. doi:10.7326/0003-4819-97-3-297
- Vriens, M.R., Fluit, A.C., Troelstra, A. and Vander, W.C. (2002) Is methicillin resistant Staphylococcus aureus more contagious than methicillin susceptible S. aureus in a surgical intensive care unit? Infection Control and Hospitalepidemiology, 23, 491-494. doi:10.1086/502094
- Hsueh, P.R., Teng, L.J., Chen, W.H., Pan, H.J., Chen, M.L., Chang, S.C., Luh, K.T. and Lin, F.Y. (2004) Increasing prevalence of methicillin resistant Staphylococcus aureus causing nosocomial infections at a university hospital in Taiwan from 1986 to 2001. Antimicrobial Agents and Chemotherapy, 48, 1361-1364. doi:10.1128/AAC.48.4.1361-1364.2004
- Thompson, R.L., Cabezudo, I. and Wenzel, R.P. (1982) Epidemiology of nosocomial infections caused by methicillin resistant Staphylococcus aureus. Annals of Internal Medicine, 97, 309-317. doi:10.7326/0003-4819-97-3-309
- Udo, E.E, Panigrahi, D. and Jamsheer, A.E. (2008) Molecular typing of methicillin resistant Staphylococcus aureus isolated from Bahrain hospital. Medical Principles and Practice, 17, 308-314. doi:10.1159/000129611
- Cosgrove, S.E. (2006) The relationship between antimicrobial resistance and patient outcome: Mortality, length of hospital stay, and health care cost. Clinical Infectious Diseases, 42, S82-S89. doi:10.1086/499406
- Centers for Disease Control and Prevention (1992) Public health focus: Surveillance, prevention and control of nosocomial infections. Morbidity Mortality Weekly Report, 41, 783-787.

