Open Journal of Animal Sciences
Vol. 2 No. 4 (2012) , Article ID: 23988 , 16 pages DOI:10.4236/ojas.2012.24037
Immuno-contraceptive potential of sperm specific LDHC4 and SPAM-1 (PH-20) sub units in dog
![]()
1Department of Veterinary Gynaecology and Obstetrics, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India; *Corresponding Author: ranjna.cheema@gmail.com
2Department of Zoology, Punjab Agricultural University, Ludhiana, India
3Division of Biochemistry, National Dairy Research Institute, Karnal, India
Received 21 August 2012; revised 25 September 2012; accepted 30 September 2012
Keywords: Immunocontraception; Dog; Sperm; LDHC4; PH-20
ABSTRACT
PH-20 (Spam-1) antigen appears to be a bifunctional sperm plasma membrane protein with hyaluronidase activity and its role is related to cumulus penetration as well as zona binding. (LDH-C4) is a key enzyme, distributed specifically in testis and is highly immunogenic, function of which relates to energy metabolism and capacitation of the sperm. Therefore, in this study, we observed the effect of purified native PH-20 and LDH-C4 antigens on sperm function and morphology in dog. Native PH-20 (46, 32 kDa) and LDH-C4 (36, 30, 28) sub units, purified from dog sperm SDS-extracts were used to test as immunocontraceptive candidates in stray dog. Dogs 1, 2, 3, 4, 5/6 and 7/8 were immunized with 46, 32, 36, 30/28, 46/32, 36/30/28 kDa sub units respectively by i/m route and semen was analysed at weekly intervals. High titre of 3200 - 6400 was achieved in all dogs after three boosters. Immune response in treated dogs was confirmed by increase in Ig level in blood serum and reaction of partially purified serum Ig of immunized dogs with respective antigens in double immune diffusion and immunoblots. Immunization of dogs either with individual PH-20/LDHC sub units or pooled PH-20/LDHC4 sub units showed a significant effect on sperm count, percent motility, viability, HOST and sperm morphology. All the sperm parameters declined to a significant level (p < 0.05) between 45 - 115 DPI and remained lower than that of the recommended values for fertile dog semen till 175 DPI. The incubation of spermatozoa in HOS solution in the presence of anti-PH-20 and anti-LDHC Ig significantly reduced the percent HOS positive spermatozoa. A significant decline (p ≤ 0.05) in acrylamide penetration assay was also observed in the presence of anti 46 and 30/28 kDa Ig. Since percent motility, sperm count, HOST and percent normal spermatozoa in immunized dogs were significantly less than that of recommended fertile dog semen, it can be concluded that PH-20 (46/32 kDa) and LDHC4 (36/30/28 kDa) sub units had an immunocontraceptive potential in dogs.
1. INTRODUCTION
Immunocontraception is a process of using a reproductive protein to produce a humoral immune response that leads to the infertility in animals for a defined time period. Sperm-specific proteins are able to induce immune responses in males because they are synthesized and incorporated into spermatozoa only at puberty, long after the neonatal period, during which the immune system sorts “self” from “non-self” [1]. Interference with fertility could occur at several points: during sperm production in the testis, during sperm maturation in the epididymis or during interaction with the egg in the female reproductive tract. A few of the sperm specific antigens have been isolated and characterized by biochemical and immunological techniques and genes encoding for some of these antigens have been characterized, cloned and sequenced. Notable among these are FA-1 [2], PH-20 [3,4], SP-10 [5], FA-2 [6], SP-17 [7], NZ-1 [8] and LDH-C4 [4,9,10]. Active immunization of female animals with some of these antigens (FA-1, PH-20 and SP-17) has been shown to reduce fertility in vivo [11,12]. Sperm surface PH-20 antigen has been cloned and studied in a number of species including the guinea pig [13], rat [14], mouse [13], horse [15], macaque and human [16]. PH-20/2B1 antigen appears to be a bi-functional sperm plasma membrane protein with hyaluronidase activity and sperm-zona pellucida binding. Antibodies generated against PH-20 and 2B1 significantly reduce sperm zona pellucida binding in vitro assay [17,18]. The immunization trials in both male and female guinea pigs were reported to lead to infertility in all immunized animals [3]. Similarly, another testis specific LDHC4 has been well characterized. The sperm specific isozyme LDHC4 was purified from mouse testis and was shown to suppress the fertility of female mice, rabbits, and baboon [19]. O’Hern et al. [20] used LDHC4 sequence (amino acids 9 - 20) for fertility testing in baboons and detected reversible immunocontraceptive effect with no side effects for one year. For the last two decades, LDHC4 has been reported to be an excellent antigen for use in a contraceptive vaccine. Therefore, LDHC4 and PH-20 sub units were purified from dog’s sperm and used for immunization of dogs to observe their immune-contraceptive effect.
2. MATERIAL AND METHODS
2.1. Maintenance of Animals
Before starting the experiment, approval of local ethical as well as national ethical committee (CPCSEA) was obtained. Ten healthy adult mongrel dogs weighing about 16 - 20 Kg and of 2 - 3 years were maintained for semen collection and immunization. Starting 4 weeks prior to experimentation, animals were housed in concrete floored kennels with access to outside runs and fed commercial dog feed (nutripet). The water was available at libitum.
2.2. Collection and Processing of Semen
The semen (entire second sperm rich fraction and part of 3rd fraction) was collected by manual manipulation in clean graduated tube attached to a glass funnel. Ten ejaculates were collected from each dog with a minimum interval of 4 days before immunization. Semen was immediately centrifuged to separate out spermatozoa and seminal plasma.
2.3. Extraction of Sperm Membrane Proteins and Purification of LDHC4 and PH-20 Sub Units
SME were prepared by suspending pooled spermatozoa of all dogs in 62.5 mM Tris-HCl, pH 6.8 containing 2% SDS, 1 mM PMSF, 25 mM benzidine, 10 mM aprotinin, 10 mM pepstatin and 5 mM soyabean trypsin inhibitor, sonicated (3 bursts of 20 sec each) and centrifuged at 15,000 rpm for 30 minutes (SDS-SME) [21]. For purification of proteins of interest, SDS-PAGE of SDS-SME was run. After SDS-PAGE, one strip cut from each side of the gel was stained with 0.5% CBB and destained with 7% acetic acid and 10% methanol, rest was kept on glass plates at 4˚C. Then stained side strips were lined up along the unstained gel and used as a guide to cut out LDHC4 (36, 30, 28 kDa) and PH-20 (46, 32 kDa) sub units with a sharp knife. Washed the bands three times (5 minutes each) with 250 mM Tris buffer, pH 7.4 followed by three washings of 5 minutes each with distilled water. Then chopped the gel and added 1.0 ml of extraction buffer i.e. 20 mM Tris-buffer of pH 7.4 and 0.1% SDS, sonicated for 3 minutes (6 × 30 sec) in an ice bath with a 3 mm probe sonicator (Misonix ultrasonic liquid processor CML-4, Coleparmer, USA). Gel containing sample in extraction buffer was centrifuged through mini syringe G-25 sephadex column at 10,000 g for 10 minutes. Purified protein in extraction buffer was obtained free of gel matrix. Samples were concentrated by filtering through ultrafiltration units (Millipore). 20 µg protein of each band was re-run by SDS-PAGE under reducing conditions.
2.4. Immunization of Dogs
Each dog was immunized intramuscularly. Three injections were given at an interval of 15 days. Blood was collected at an interval of 15 or 7 days. Immunoglobulins, partially purified at 33% (NH4)2SO4 were used for further analysis. Detail of immunization is given in the following table.
2.5. Detection and Monitoring of Immune Response
Immune response was detected by DID, Ig level in blood serum, ELISA and Immunoblotting.
2.5.1. DID [22]
Immunodiffusion was performed on 0.5% agarose slides. 3.0 µl of antigen and antibody were loaded in two separate wells and allowed to diffuse for 48 hr at room temperature in petri plates lined with moist filter paper. The slides were kept in saline so as to remove unbound proteins, rinsed with distilled water, properly dried and stained with Coomassie Blue Brilliant R-250.
2.5.2. ELISA [23]
ELISA plates were coated with 5 µg protein per well by incubating at 37˚C for three hrs. After washing thrice with PBS, antigen coating was blocked by incubating with 300 µl of 2% BSA per well for overnight at 4˚C. Again washed thrice with PBS pH 7.4 and added serial dilutions of partially purified immuno-globulins into the wells and incubated at 37˚C for three hours. Washed again with PBS and incubated with 100 µl/well of anti dog IgG for three hours at 37˚C. Washed the plate twice with PBS and incubated with 100 µl of o-phenyldiamine + 0.06% H2O2 as a substrate for 20 min at room temperature. Stopped the reaction with 5 N H2SO4 and measured the absorbance at 492 nm using ELISA reader.
2.5.3. SDS-PAGE and Immunoblotting [24,25]
Primary antibodies raised in dogs against LDHC4/ PH-20 sub units were reacted with respective antigens. Proteins separated by SDS-PAGE under reducing conditions were transferred to nitrocellulose/PVDF membrane using wet electrophoresis transfer apparatus at 100 V for 2.30 hr. Transfer quality was checked by 0.2% ponceau dye and proteins were blocked in 3% BSA as blocking solution for overnight at 4˚C. After washing the membrane with PBS + 0.05% Tween-20, it was incubated in 1:200 diluted primary antibodies for 2.5 hr. Again washed thrice with PBS + 0.05% Tween-20 and incubated with 1:10000 anti dog IgG as secondary antibody for 45 min. Washed thrice with PBS + Tween-20 and incubated with substrate (0.05% Diaminobenzidine + 0.015% 4-Chloro Napthol + 0.06% Hydrogen Peroxide) for 10 min. Gel images were captured on Syngene gel doc using GeneSnap image acquisition software and analyzed by using GeneTools gel analysis software (Syngene).
2.6. Effect of Immunization on Semen Quality
Semen was analysed for percent motility, sperm concentration, percent viability (Live Dead), morphology (abnormalities) and sperm membrane integrity (HOS-test) at week intervals. Dog # 1, 2 and 5, 6 immunized with PH-20 sub units were observed till 270 and 115 DPI respectively. Whereas, dogs # 3, 4 and 7, 8, immunized with LDHC4 sub units were observed/M till 100 and 115 DPI respectively.
2.7. Histopathology of Testis
Testis of normal and immunized dogs with LDHC4 sub units were fixed in aqueous bouines fixative, dehydrated with increasing concentrations of ethanol (70%, 90%, 100%), cleared in benzene and embedded in paraffin wax (58˚C - 60˚C). The paraffin blocks of tissues were cut into 7 µ thick sections, stained with Haematoxylin and eosin and observed at 400×.
2.8. Effect of Anti PH-20 and Anti LDHC4 Sub Units on HOST
Spermatozoa were incubated in 60 mM HOS solution supplemented with partially purified anti 46 kDa, 36 kDa, 32 kDa and 30/28 kDa Ig for 30 min. A control was also run with pre immunized partially purified Ig of each dog.
2.9. Effect of Anti PH-20 and Anti LDHC4 Sub Units on Acrylamide Penetration Assay
1.5% acrylamide gel was prepared, dialyzed against Tris pH 8.8. Partially purified Ig of pre-immunized and immunized dogs were mixed with acrylamide in the ratio of 1:100 and filled into the glass capillaries (marked in cm), which were sealed from one side and pre heated at 37˚C. Approximately 100 μl of semen was placed at the bottom of an eppendorf tube and a capillary tube was placed with its open end in the semen. After 60 min incubation at 37˚C, the capillary tube was viewed under microscope at 400×. The length of the tube was then scanned to establish the distance furthest from the semen reservoir attained by spermatozoa. The maximum distance of migration of spermatozoa after 1 h of incubation was defined as the migration distance. Number of migrated spermatozoa was counted in the uppermost one cm.
2.10. Statistical Analysis
The Analysis of Factorial Experiment in CRD (computer software programme) or one way variance analysis was used to evaluate the significance levels between the parameters studied. The critical difference (CD) of two factors—A (within the dogs), B (time of immunization) and AB (interaction between two factors) were used to find the level of significance. A p value of 0.05 was selected as a criterion for statistically significant differences.
3. RESULTS
3.1. Purification of PH-20 and LDHC Sub Units
SDS-PAGE and Immunoblotting of Purified PH-20 and LDHC4 Sub Units
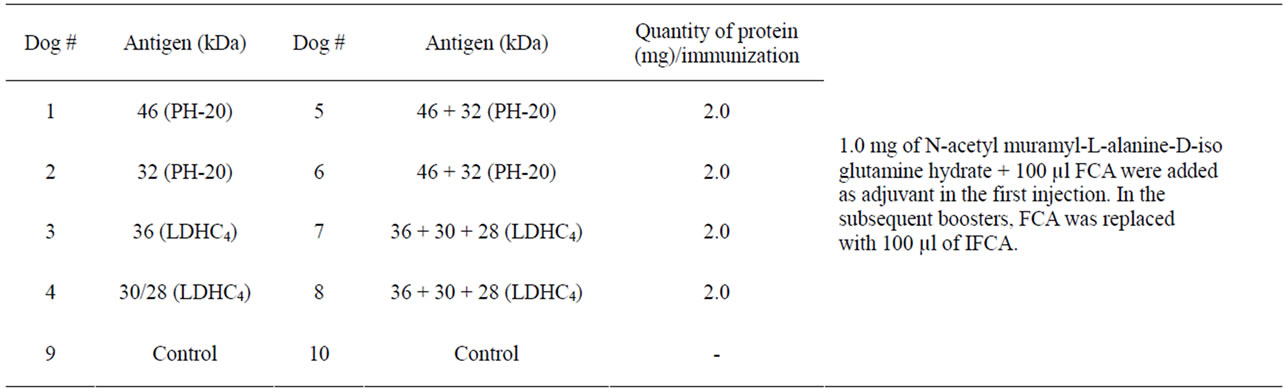
A Single band of 46, 36, 32, 30 and 28 kDa purified proteins with little alteration in their molecular weights on acrylamide gels under reducing conditions confirmed their purification (Figure 1). Purification of PH-20 (46/32 kDa) and LDHC4 (32/30/28 kDa) sub units was further confirmed by the detection of respective bands on immunoblots upon reaction with anti spam-1 anti LDHC respectively (Figure 2).
3.2. Immune Response to PH-20 and LDHC Sub Units
3.2.1. Immunodiffusion
A single sharp precipitation line in immunodiffusion

Figure 1. SDS-PAGE of purified PH-20 (46/32 kDa and LDHC (36/30/28 kDa) antigens).
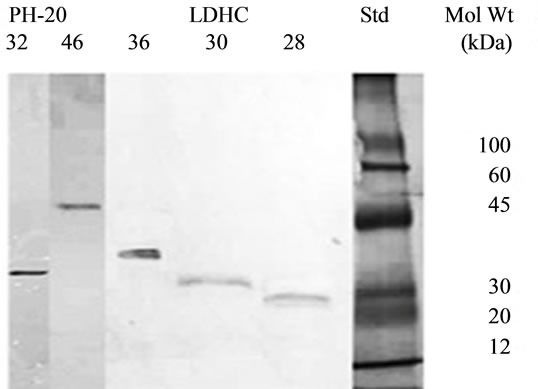
Figure 2. Immunpblotting of purified PH-20 (46/32 kDa and LDHC (36/30/28 kDa) with anti-Spam-1 and anti LDHC).
reaction between partially purified Ig of immunized dogs 1, 2, 3, 4, 5, 6, 7 and 8 against LDHC4/PH-20 sub units and respective antigens on 60 day confirmed the production of antibodies (Figures 3 and 4). Such precipitation line was not noticed in partially purified Ig of D-0 and 45 DPI serums of immunized and control dogs respectively.
3.2.2. Ig Level in Blood Serum
There was progressive increase in Ig level from day of immunization (D-0) to 75 days of post immunization (DPI) in the blood serum of immunized dogs (1 - 8). An increase of 50.37%; 52.27%; 51.66%, 48.58%, 51.49%, 64.87%, 57.21% and 54.30% in Ig level from D-0 to 75 DPI was observed in the blood serum of dogs 1, 2, 3, 4, 5, 6, 7 and 8 respectively (Figures 5 and 6). Thereafter, Ig level showed an inconsistent trend, but was still at a higher level till 105 DPI than that of D-0 and control dogs.
3.2.3. Ag-Ab Titre by ELISA
A titre of 1600 was obtained in the blood serum of 8 dogs immunized with either PH-20 or LDHC4 sub units on 45 DPI, which increased to 6400 between 75 - 90 DPI. Titre remained at a level of 3200 in dogs 1 - 8 till 115 DPI.
3.2.4. Immunoblotting of Partially Purified Ig
Reaction of purified PH-20 (46, 32 kDa) and LDHC4 (36, 30, 28 kDa) sub units with partially purified Ig of 45 DPI serum of immunized dogs (1 - 8) on immunoblots
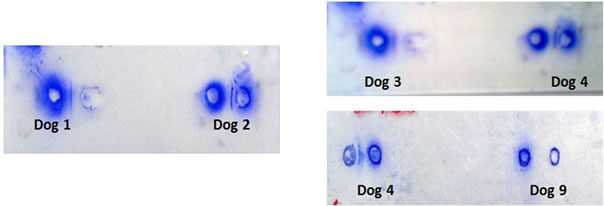
Figure 3. Double immunodiffusion reaction between anti PH- 20 and LDHC and their respective antigens. Dog 1 (PH-20, 46 kDa); Dog 2 (PH-20, 32 kDa); Dog 3 (LDHC, 36 kDa); Dog 4 (LDHC, 30/28 kDa) and Dog 9 (Control).
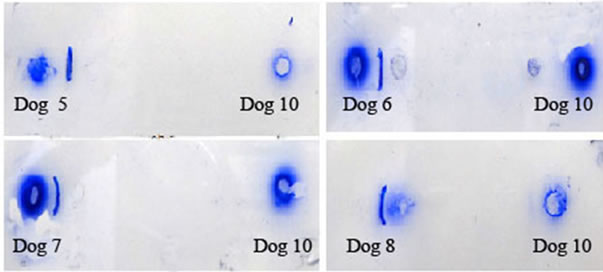
Figure 4. Double immunodiffusion reaction between anti PH- 20 and LDHC Ig and their respective antigens. Dog 5, 6 (PH-20, 46/32 kDa); Dog 7, 8 (LDHC, 36/30/28 kDa) and Dog 10 (Control).

Figure 5. Immunoglobulin level in blood serum of dogs immunized with PH-20 and LDHC sub units. An increase in Ig level with increase in time of immunization was observed in the blood serum of immunized dogs.
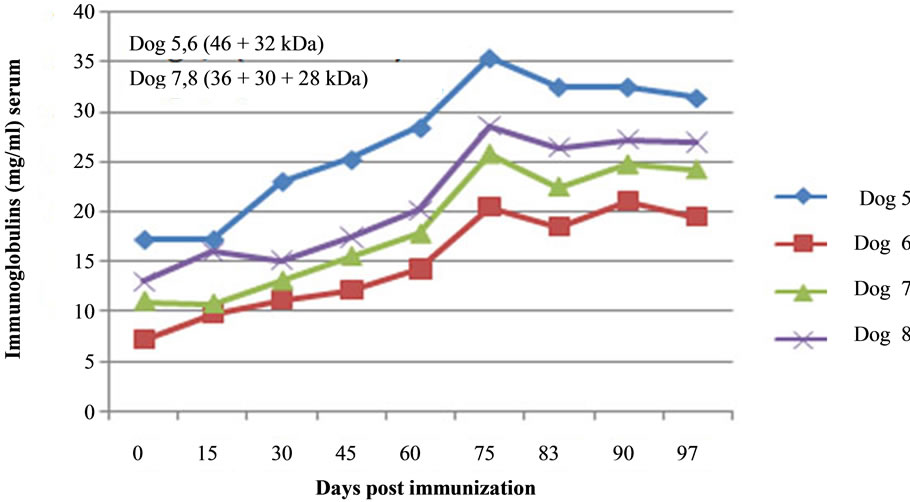
Figure 6. Immunoglobulin level in blood serum of dogs 5, 6, 7 and 8 immunized with PH-20 and LDHC sub units. An increase in Ig level with increase in time of immunization was observed in the blood serum of immunized dogs.
further confirmed the production of antibodies against the respective sub units (Figure 7). No reaction was found between partially purified Ig and purified PH-20 and LDHC4 sub units on D-0 and 45 DPI in dogs 1 - 8 and 9, 10 respectively.
3.2.5. Effect of Immunization with PH-20 Sub Units (46 and 32 kDa) on Sperm Parameters
Effect of immunization of dogs 1, 2 and 5, 6 with individual and pooled PH 20 sub units on volume of semen, percent motility, viability, HOST, total abnormalities and sperm count is shown in Figures 8-10.
3.2.5.1. Dogs 1 and 2
There was no significant (p < 0.05) change in volume of semen of dogs 1 and 2 immunized respectively with 46 kDa and 32 kDa sub units from D-0 to 115 DPI (Table 1). However significant difference was observed in volume of these two dogs in comparison to that of control. A significant (p < 0.05) gradual decline in percent motility, percent viability, percent HOS and sperm count (106/ml) and significant increase in percent abnormalities was observed from day 0 to 115 DPI in the dogs, immunized with 46 and 32 kDa subunits. Similarly, a significant (p < 0.05) difference in all these parameters was

Figure 7. Immunoblotting of partially purified Ig of dogs 1 - 8 immunized against Ph-20 and LDHC sub units with respective antigens. 1 - 46 kDa, 2 - 32 kDa, 3 - 36 kDa, 4 - 30/28 kDa, 5, 6 - 46/32 kDa, 7, 8 - 36/30/28 kDa.
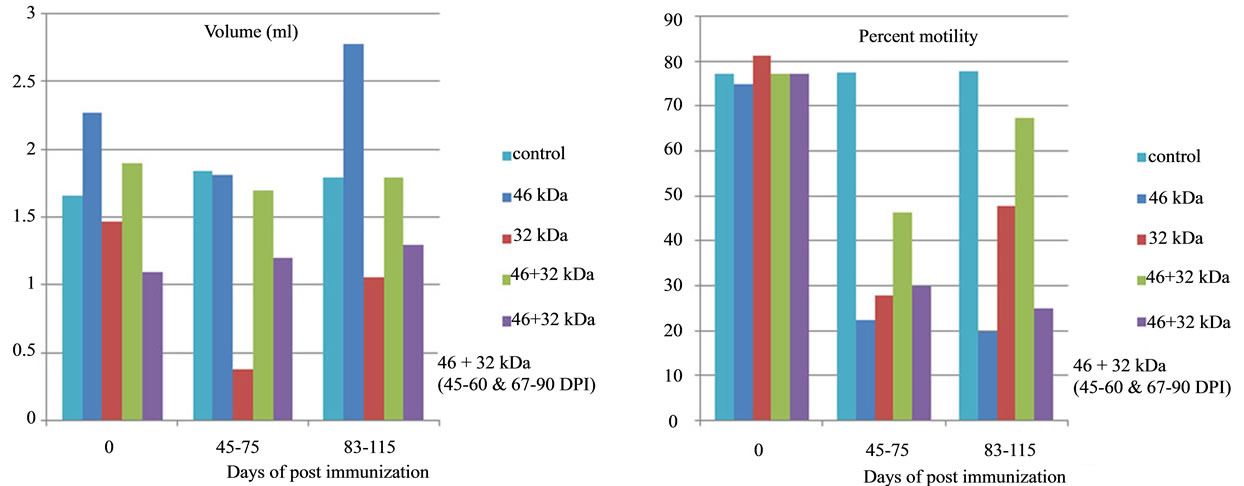
Figure 8. Effect of immunization with PH-20 sub units on semen volume and percent motility. Immunization of dogs with either 46, 32 kDa or 46 + 32 kDa sub units showed significant effect on volume of semen and percent motility as compared to control dog and zero day of the immunized dogs.
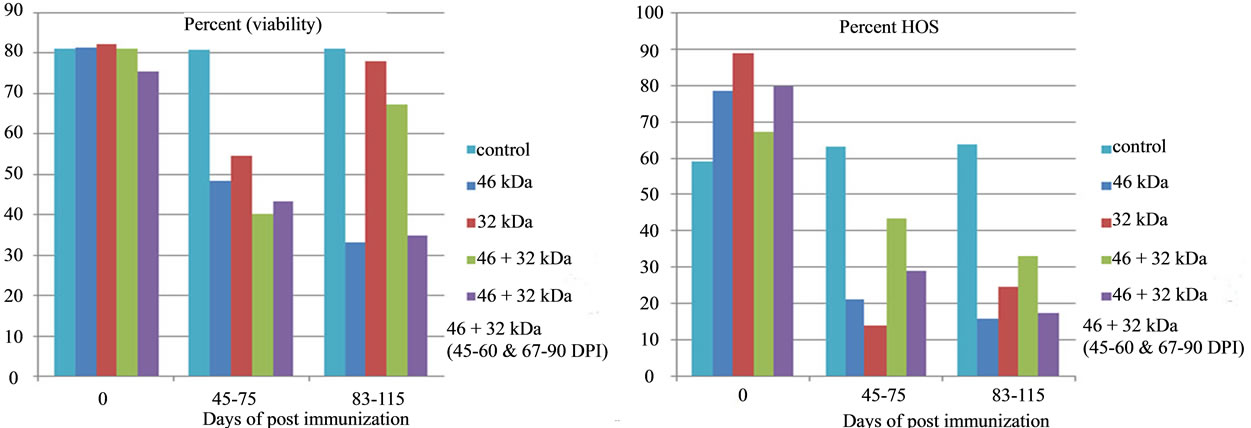
Figure 9. Effect of immunization with PH-20 sub units on percent viability and HOS. Immunization of dogs with either 46, 32 kDa or 46 + 32 kDa sub units showed significant effect on viability and HOS as compared to control god and zero day of the immunized dogs.
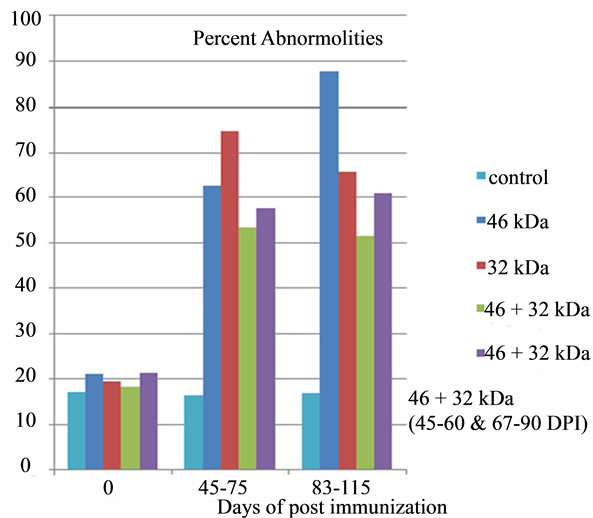
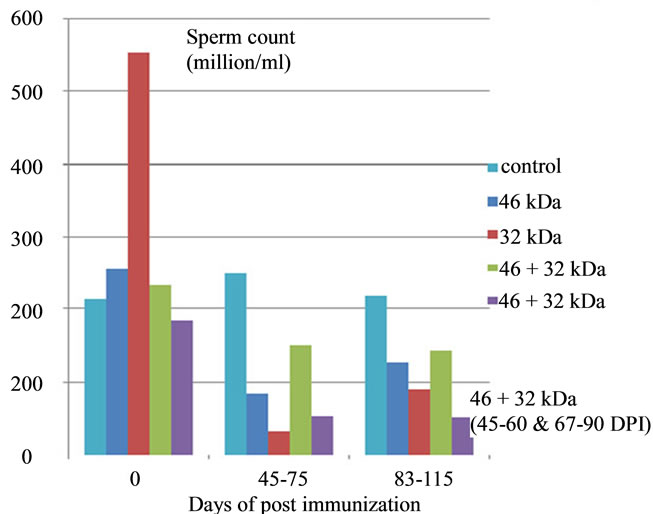
Figure 10. Effect of immunization with PH-20 sub units on sperm abnormalities and count. Immunization of dogs with either 46, 32 kDa or 46 + 32 kDa sub units showed significant effect on morphology and sperm count as compared to control dog and zero day of the immunized dogs.
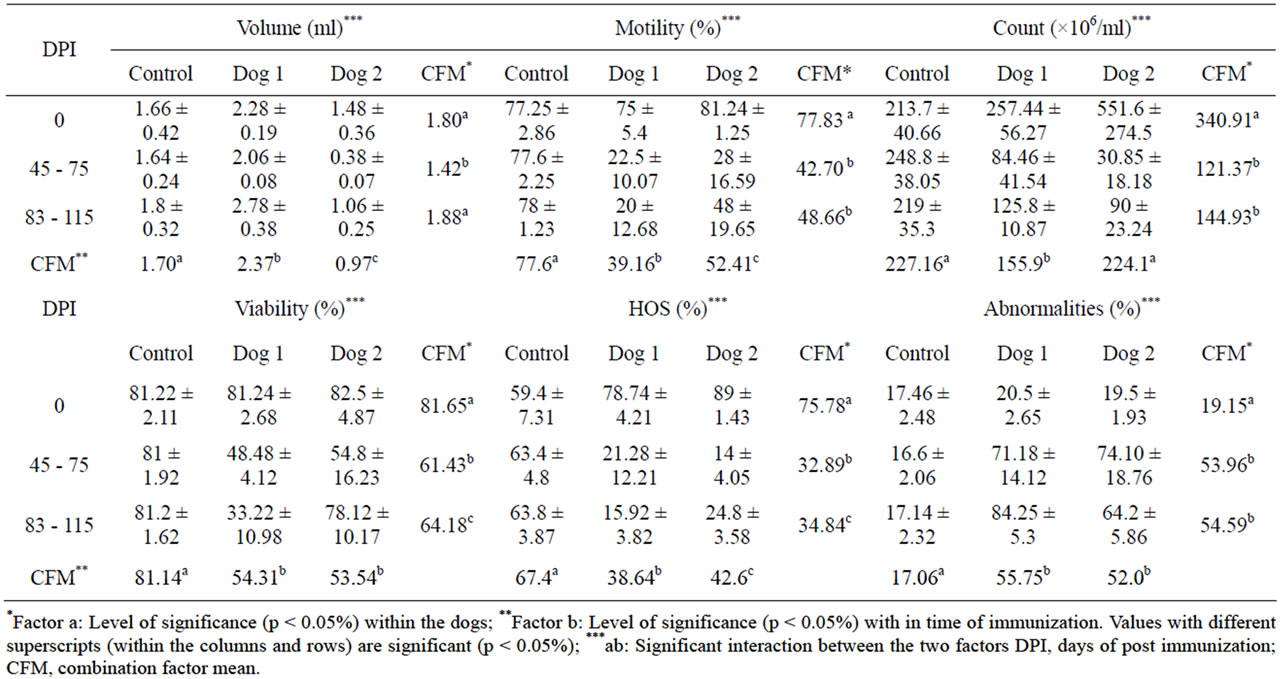
Table 1. Effect of immunization with purified PH-20 sub units on sperm parameters in dog 1 (46 kDa) and 2 (32 kDa).
also observed in the dogs immunized with PH-20 subunits in comparison to that of control. Level of significance was observed within the dogs and time of immunization in all parameters (Table 1). Sperm parameters of dog 1 and 2 immunized with 46 and 32 kDa sub units were still lower than that of pre-immunized parameters even on 175 DPI (Table 2).
3.2.5.2. Dogs 5 and 6
A non-significant effect on volume of semen was observed in dogs 5 and 6 immunized with PH-20 (46 + 32 kDa) subunits w. r. t time of immunization in comparison to that of control dogs 9, 10. There was significant (p < 0.05) reduction in percent motility, percent viability, percent HOS and sperm count (106/ml) in dogs 3 and 4 from day 0 to 115 DPI in comparison to that of control dogs 9 and 10. A significant (p < 0.05) increase in percent abnormalities was noticed in both the dogs w. r. t time of immunization in comparison to day 0 and that of control dog. Level of significance was observed within the dogs and time of immunization in all parameters except volume (Table 3).
3.2.5.3. Effect of PH-20 Sub Units on Sperm Morphology
Different type of abnormalities in the semen of immunized dogs with PH-20 and LDHC4 sub units are shown in Figures 11(a) and (b). The number of spermatozoa with morphological defects of CT, BT, AH, DH, CD, MPT were within the permissible range in the un-immunized dogs 1, 2, 5 and 6 and showed an increase in the immunized dogs between 45 - 75 and 83 - 115 DPI. Whereas, morphological defects like DAG, TSH and multiple were observed only in the immunized dogs between 45 - 75 and 83 - 115 DPI. The highest number of spermatozoa with morphological defects like CT, BT, AH, DH, CDMPT, DAG, TSH and MA were 25.5 ± 6.2 (dog 1), 22.8 ± 7.6 (dog 2), 9.9 ± 2.9 (dog 5), 39.76 ± 6.26 (dog 1), 14.5 ± 1.1 (dog 6), 14.1 ± 0.7 (dog 6), 2.6 ± 0.9 (dog 6), 1.7 ± 1.2 (dog 2), 5.3 ± 1.8 (dog 2), respectively (Table 4).
3.3. Effect of Immunization with LDHC4 Sub Units (36 and 30/28 kDa) on Sperm Parameters and Morphology of Dogs 3 and 4
A percent decline of 83.3, 69.93, 58.13, 38.84, 93.64; 74.4, 69, 37.24, 60.69, 95.27 was observed between 30 - 45 DPI in dog 3 (36 kDa) and 4 (30/28 kDa) respectively. On the other hand, there was an increase of 78% and 75.64% in the sperm abnormalities between 30 to 45 DPI
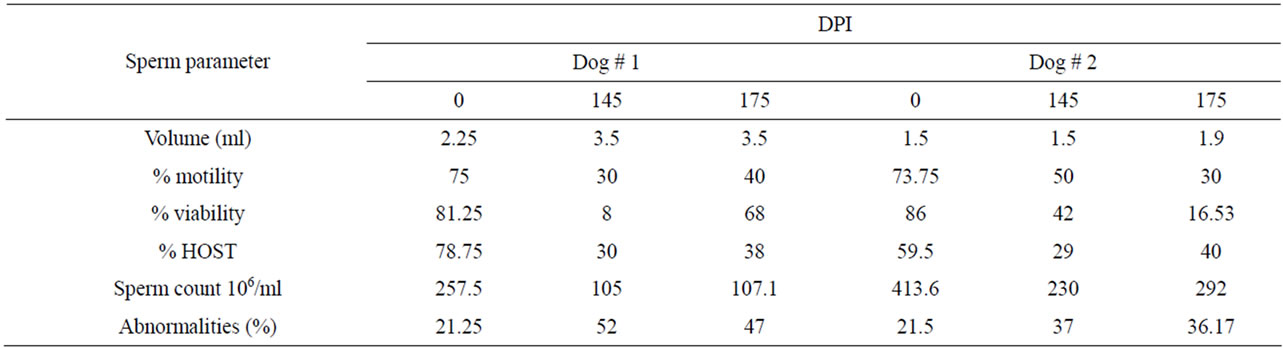
Table 2. Effect of immunization with PH-20 sub units on sperm parameters of dogs 1 and 2 after 115 DPI.
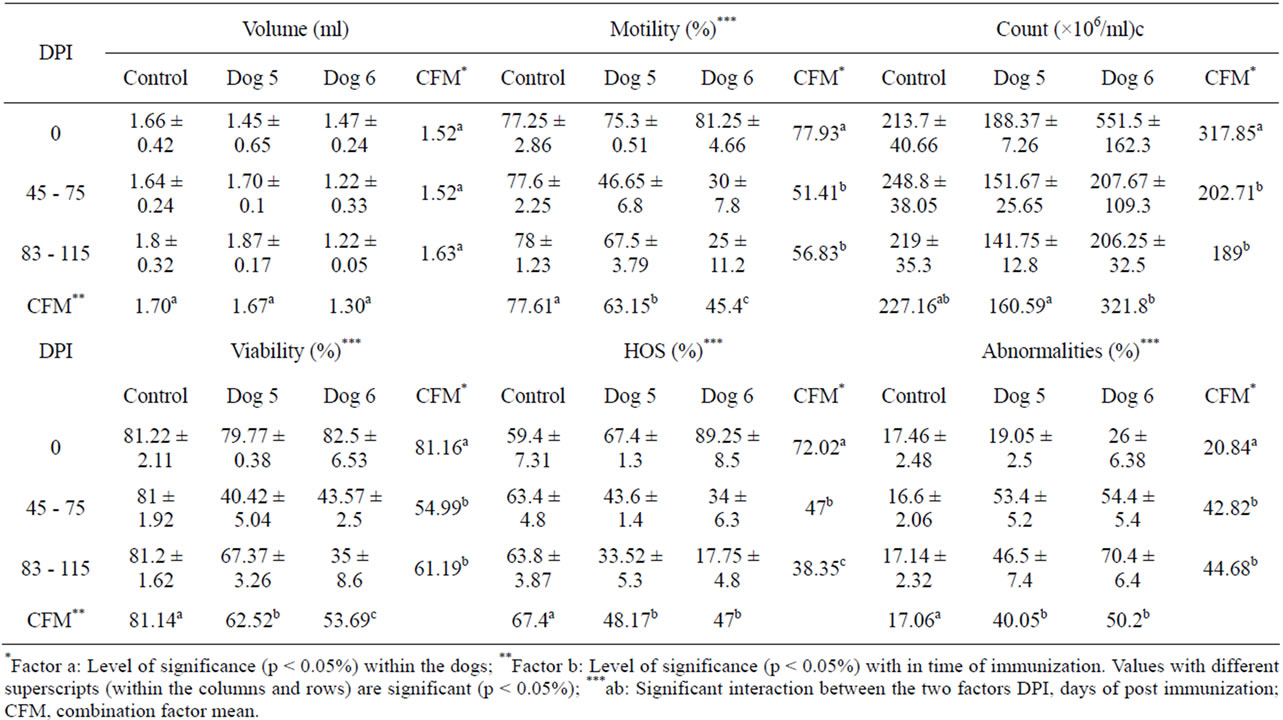
Table 3. Effect of immunization with purified PH-20 (46 + 32 kDa) sub units on sperm parameters in dogs 5 and 6.
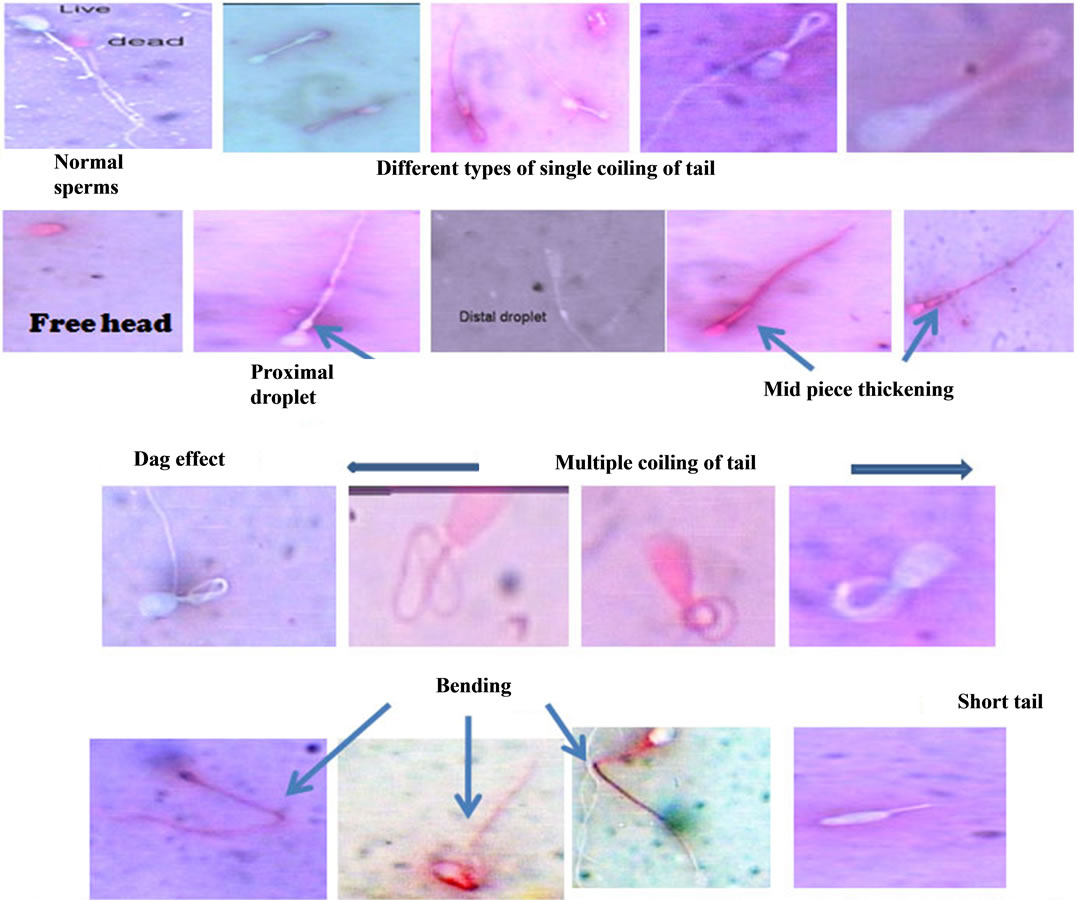 (a)
(a) (b)
(b)
Figure 11. (a) Showing various sperm morphological changes in dogs 1 - 8, immunized with PH-20/LDHC; (b) Showing various sperm abnormalities in dogs immunized with PH-20 and LDHC sub units.
in dog 3 (36 kDa) and 4 (30/28 kDa) respectively. There was an increase in morphological defects like CT, BT, DH, AH, MPT between 30 - 45 DPI. Whereas, defects like DAG, CD and MA were observed only between 30 - 45 DPI in dog 3 and 4 (Table 5). Both the dogs stopped donating semen from 45 DPI onwards, Therefore, one of the gonads was removed surgically on 70 DPI and observed for the presence of spermatozoa in caput, corpus and cauda epididymis, There were no sperms in the three regions of epididymis in dog 3. A few sperms were seen in the epididymal fluid of dog 4, which were not countable with haemocytometer. About 99.98%, 61.28% and 82.9% sperm morphological defects were observed in eosin-nigrosine stained smears of epididymal fluid of caput, corpus and cauda respectively in dog 4 (Table 5). Second gonad was removed on 100 DPI to see the reversal effect of immunization, but still no sperms were found in the three regions of the epididymis of dog 3 and only a few sperms with 64.55% morphological defects were observed in eosine-nigrosine stained smears of caput epididymis of dog 4.
3.3.1. Effect of Immunization with Pooled LDHC4 Sub Units (36 + 30 + 28 kDa) on Sperm Parameters and Morphology of Dogs 7 and 8
Volume of semen, percent motility, viability, HOS, abnormalities and sperm count/ml of dog 7 and 8 were within the normal range required for the fertility of a dog (Table 6). Both the dogs stopped donating semen from 45 DPI onwards. Therefore, removed one gonad on 90 DPI and observed for the presence of spermatozoa in epididymis. Spermatozoa were missing in caput, corpus epididymis of dog 7. But there were only 10 million sperms with 20% motility, 42.1% viability, 18.91% HOS and 36.6% morphological defects in cauda epididymal fluid. Total number of spermatozoa in caput, corpus and cauda epididymal fluid of dog 8 were 75 × 106, 85 × 106 and 225 × 106 respectively (Table 6). There were 40% motile, 34.78% viable, 49.2% HOS positive and 36.18% abnormal spermatozoa in the cauda epididymis. Second gonad of both dogs was removed on 115 DPI and still found no sperms in the three regions of epididymis of dog 7. The number of spermatozoa further declined to 35 × 106, 18 × 106 and 180 × 106 in caput, corpus and cauda epididymis of dog 8 on 115 DPI. The number of motile, viable and HOS positive spermatozoa in cauda epididymis of dog 8 also declined to 20%, 20% and 2.5% respectively. Whereas, number of spermatozoa with morphological defects further increased to 70% on 115 DPI (Table 6).
3.3.2. Histopathological Changes in Testis
Pachytene stage of spermatogonia and intact germinal epithelium was observed in the sections of testis of dogs immunized with LDHC4 like that of control dog (Figure 12). Whereas, dispersal of cells and empty lumen as compared to control was clear in the sections. Therefore, it indicated that spermatogenesis is inhibited/arrested in the dogs, immunized with LDHC4.
3.4. Effect of Anti PH-20 (46, 32) and LDHC4 (36/30/28) Sub Units on HOST and Acrylamide Penetration Assay
The incubation of spermatozoa in HOS solution in the presence of anti-PH-20 and anti-LDHC Ig significantly reduced the percent HOS positive spermatozoa. Percent HOS positive spermatozoa declined from 53.23% ± 6.01% to 32.95% ± 4.07%, 31.6% ± 4.06% and 34% ± 2.76% and 38.11% ± 2.76% respectively in the presence
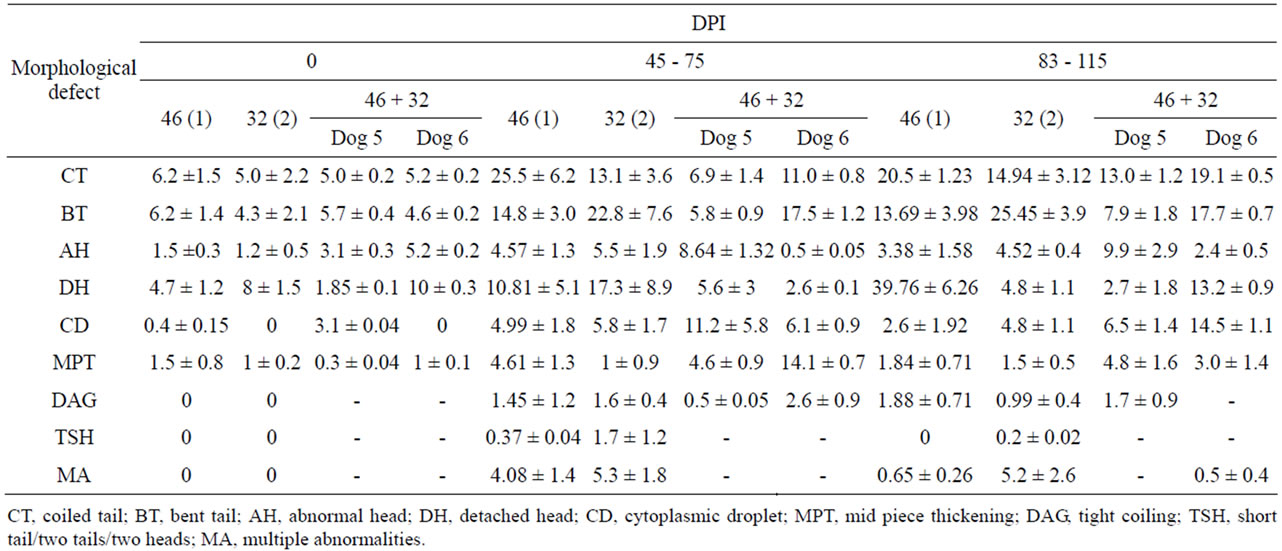
Table 4. Effect of immunization with PH-20 subunits on sperm morphology of dog 1 (46 kDa), 2 (32 kDa) and 5, 6 (46 + 32 kDa).
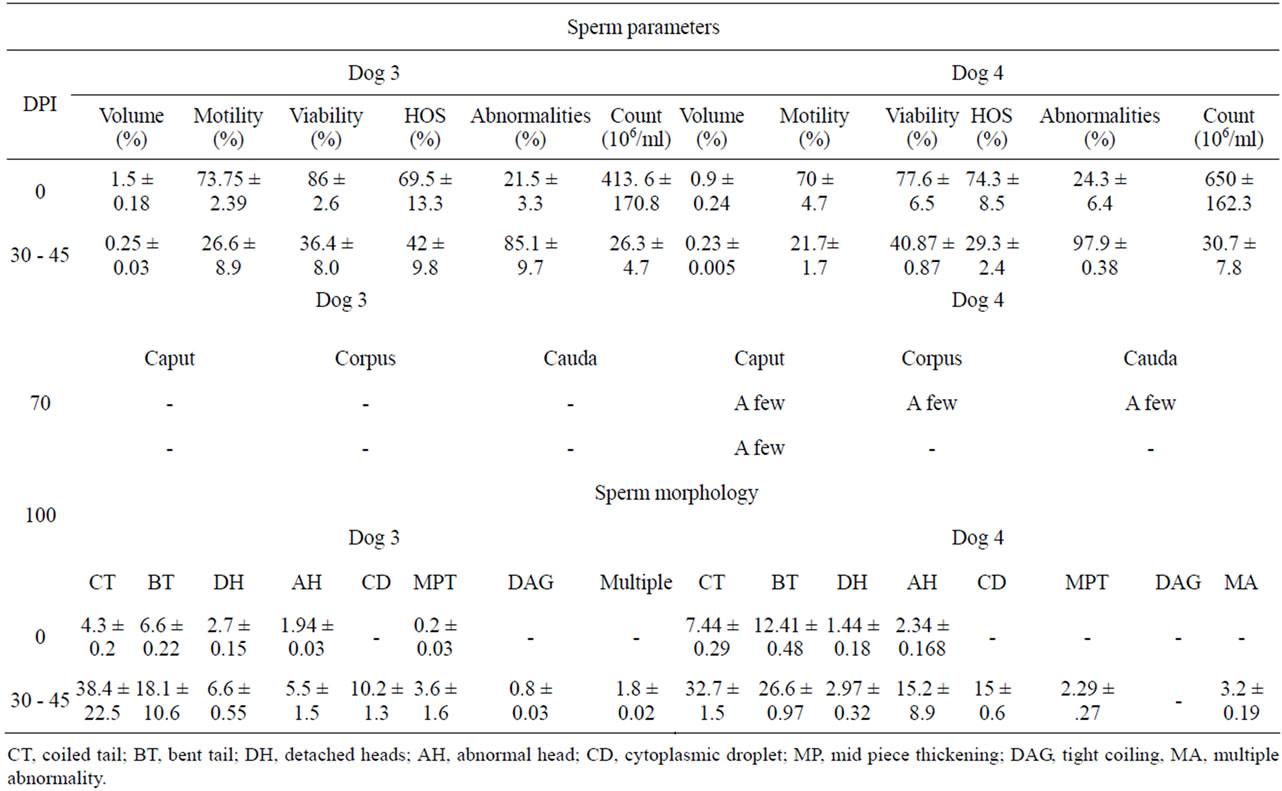
Table 5. Effect of immunization with purified LDHC sub units on sperm parameters and morphology of dogs 3 (36 kDa) and 4 (30/28).
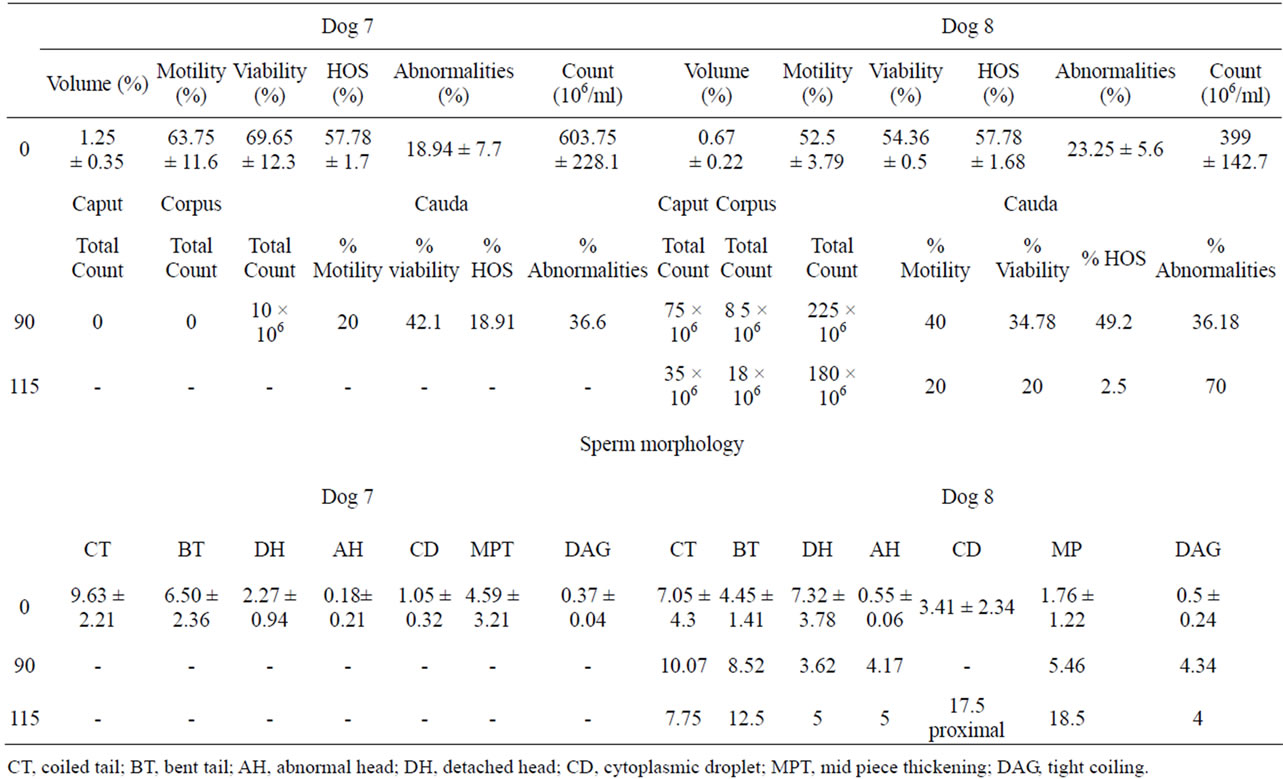
Table 6. Effect of immunization with purified LDHC (36/30/28 kDa) sub units on sperm parameters of dogs 7 and 8.

Figure 12. Histopathology of control dog (1) and immunized #7 (A, B) and #8 (C, D, E) with LDHC. FL, Lumen filled with sperms; EL, Empty lumen; L, Lumen with few sperms; GE, Germinal epithelium; DC, Dospersed cells; CC, Compact cells.
of anti 46, 36, 32 and 30/28 Ig respectively (Figure 13). Migration of spermatozoa through acrylamide per 30 min declined to 2.16 ± 0.03, 2.20 ± 0.08, 2.13 ± 0.10 and 0.6 ± 0.49 in the presence of anti 46, 36, 32, 30/28 Ig respectively. Similarly number of spermatozoa penetrated in 30 min in upper one cm declined to 1.0 ± 0, 1.33 ± 0.33, 1.0 ± 0, 0.83 ± 0.33 in the presence of anti 46, 36, 32, 30/28 Ig respectively. Although decline in both sperm penetration distance and number of spermatozoa was observed with anti 46, 36, 32 and 30/28 Ig, but it was significant (p ≤ 0.05) only with anti-46 and anti-30/28 Ig.
4. DISCUSSION
4.1. Immune Response to PH-20 and LDHC Sub Units
A single precipitation line in DID reaction between partially purified Ig of immunized dogs against PH-20 and LDHC4 sub units and respective antigens indicated the production of antibodies. Comparison of Ig level in the blood serum of pre-immunized and immunized dogs also revealed the production of antibodies against purified PH-20 and LDHC4 sub units. Significantly higher values of antibodies produced in the serum of dogs immunized with PH-20 and LDHC4 sub units showed their efficacy as immunocontraceptive antigens as it has been also reported that higher serum antibody levels achieved after immunization with epididymal specific protein (DE) resulted into a higher rate of reduction in the fertility of male rats [26,27]. An antigen-antibody titre of 1600 was observed after second booster, which remained at an elevated level of 3200 - 6400 between 60 to 115 DPI. It also indicated higher level of antibodies against the respective antigens in the blood serum of dogs 1 - 8 during this period. In the similar studies in Macaca radiata, Rana et al. [28] also observed an elevated antibody titre after the first booster which was significantly higher from week 8 - 12 against human sperm associated antigen 9. Immunization of bulls against spermatozoa resulted in transient rise in the level of IgM antibodies and persistent elevated levels of IgG1 and IgG2 in the serum [29]. The prevalence of IgG anti-sperm antibodies in serum has been reported in humans and is consistent with a systemic immune response [30]. Therefore, elevated level of immunoglobulins, antigen-antibody titre in blood serum of immunized dogs and detection of 46; 32; 36; 30/28; 46/32; 36/30/28 kDa antigens in the immunoblots, developed between the reaction of partially purified Ig and purified PH-20, LDHC4 sub units indicated the production of antibodies against purified PH-20 and LDHC4 sub units. Naz and Zhu [31] also opined that an increase in the level of ASA in blood serum of immunized mice has a direct and significant correlation with the reduction in fertility.
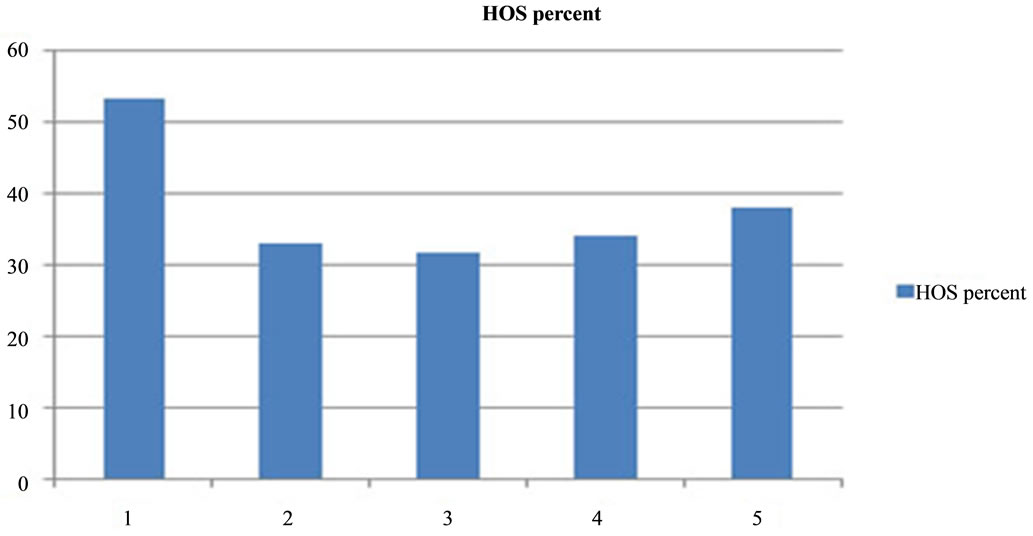
Figure 13. Effect of anti PH-20 and LDHC sub units on HOST of non-immunized dogs. 1-control, 2-anti 46 kDa, 3-anti 32 kDa, 4-anti 36 kDa, 5-anti 30/28 kDa. Significant effects of anti PH-20 and LDHC sub units antibodies was observed on HOST.
4.2. Effect of Antibodies against PH-20 and LDHC4 Sub Units on Sperm Parmeters and Morphology
A decline of about 0% - 74.32%, 38.04% - 74%, 33.57% - 49.33%, 35.31% - 84.27% in volume of semen, sperm motility, viability, HOS was observed in dogs 1, 2 and 5, 6 immunized with 46, 32 and 46 + 32 kDa sub units after 3rd booster dose. However, percent increase in morphological defects ranged from 19.48% to 94.41% in dog 1, 2, 5 and 6. Reduction in volume of semen, percent motility, viability, HOS and sperm count/ml was 74.4% - 83.3%, 63.9% - 69%, 47.3% - 57.67%, 39.56% - 60.56% and 93.77% - 95.27% in dog 3, 4 immunized with 36 and 30/28 kDa sub units respectively after 3rd booster. Level of significance in all sperm parameters within the dogs indicated the individual variation to the immune response. Since, PH-20 is likely to play a role in both sperm maturation and storage [32], therefore, increase in morphological abnormalities and increase in HOS positive spermatozoa in the dogs immunized with PH-20 may be due to its effect on sperm maturation during epididymal transit. Moreover, increase in morphological defects of CT, BT, AH, DH, CD and appearance of that of mid piece, two tail, two heads, short tail, DAG and multiple defects in the semen of only immunized dogs further indicated that both PH-20 and LDHC4 sub units affected sperm maturation process either during spermatogenesis/spermiogenesis or epididmal transit. Earlier studies reveal the possibility of abnormal spermatozoa in reducing the fertility/failure to reach the fertilizing site/ inability to fertilize the ovum or to sustain development of early embryo. HOS-test was evaluated as an additional test to standard semen analysis for the identification of males that may be subfertile despite a normal spermiogram [33]. In male guinea pigs, immunization with gpPH20 in Freund’s adjuvant led to complete infertility due to the loss of normal sperm in the epididymis and associated autoimmune orchitis [34]. Infact, >40% of total head/mid piece abnormalities or 20% head/acrosome/retained cytoplasmic droplets or <60% sperm with normal morphology results in infertility of dog semen. Moreover, major defects of head, acrosome, mid piece and tail occur during spermatogenesis and have more pronounced effects on sperm function. Oettle [35] was also of the view that fertility was more (61%) in dogs with >60% normal sperms than that of with <60% normal sperms (13%). In our study, spermatozoa with normal morphology were <60% in the semen samples of dogs immunized either with PH-20 or LDHC4 sub units from 45 DPI onwards. Therefore, significant decline in HOS positive spermatozoa and <60% of spermatozoa with normal morphology in immunized dogs with both PH-20 and LDHC4 sub units confirmed the infertility of immunized dogs. In our earlier observations, immunization of dogs with SDS-sperm membrane extracts also resulted in a reversible immunocontraceptive effect [36].
The percent decline in sperm concentration was higher in dogs immunized with LDHC4 than that immunized with PH-20. Moreover the dogs immunized with three sub units of LDHC4 to gether did not donate semen after 3rd booster. Fadiloglu et al. [37] have reported a correlation between LDHC4 activity and sperm count in humans. Therefore, lack of ejaculates in the dogs immunized with LDHC4 sub units may be due to impaired activity of LDHC4 like antigens. Contraceptive anti-eppin antibodiies from male monkeys in the studies of O’Rand et al. [38] inhibit human sperm progressive motility in vitro and seem to disrupt the cAMP regulatory pathway of the sperm. Odet et al. [39] reported that motility of Ldhc null human sperm (mutated) was impaired and their progressive motility decreased over time suggesting that Ldhc null human sperm are unable to swim through the female tract. In another study carried out by Zhang et al. [40] on anti-eppin antibodies inhibited the sustained increase in Ca2+ and reduced the human sperm motility in vitro. Also production of higher ASA in blood circulation has been found to decrease the sperm motility [41]. Fayemi et al. [42] have also concluded that sperm antibodies have negative association with sperm motility in mice immunized with sperm proteins. It has been reported that antibodies against LDH-C4 resulted into impaired motility, decreased forward progressive motility and immotile or dead spermatozoa in humans [43].
There is a direct role of immunization with sperm membrane proteins in fertility regulation, as hematoepididymal barrier is much more permeable than the blood-testes barrier especially for the molecules of size of IgG [44] and results in the entry of immunoglobulins into the epididymis [45]. Then these ASA get attached to the sperms, resulting in the reduction of fertility [27,46]. The extent to which ASA are present in the reproductive tract secretions would be expected to influence the degree of fertility impairment [47]. Therefore, alteration in sperm parameters of immunized dogs observed during the present study was due to immobilization of spermatozoa with anti PH-20 and LDHC4 sub units antibodies that crossed the testis/epididymis and affected the process of spermatogenesis and sperm maturation. Recommended values for fertile dog semen are >70% motility, 300 × 106/ml, >50% HOST, >80% normal spermatozoa. Primary (detached/ abnormal heads, mid piece etc.) and secondary (bent tail, DAG, double coiled tail etc.) abnormalities should not exceed 10% and 30% respectively [47]. In the normal reproductive male, sperms are stored in the extragonadal compartments of the epididymis and the ductus deferens. Total sperm counts in a sexually rested male encompass sperm reserves plus daily sperm produced by the testes. Extragonadal sperm reserves (109) based on 7 days sexual rest are 0.07 × 109, 1.10 × 109 and 2.06 × 109 in caput, corpus and cauda epididymis of dogs weighing about 10 - 34 Kg [48]. Therefore absence of sperms in epididymii of dog # 5, 7; a few sperms in dog 6 and only 75 × 106, 85 × 106 and 225 × 106 spermatozoa in caput, corpus, cauda epididymis respectively of dog # 8 further indicates effect of LDHC4 on spermatogenesis. Inhibtion/arresting of spermatogenesis were also clear in the histological sections of testis of immunized dogs with LDHC4. Since percent motility, viability, HOST, sperm count and percent normal spermatozoa in immunized dogs were less than that of recommended fertile dog semen, it can be concluded that PH-20 (46/32 kDa) and LDHC4 (36/30/28 kDa) sub units has an immunocontraceptive potential in dogs.
5. ACKNOWLEDGEMENTS
The authors are thankful to Department of Biotechnology, Ministry of Science and Technology, New Delhi, for providing financial support to carry out this work.
REFERENCES
- Tung, K.S.K. (1998) Autoimmune disease of the testis and the ovary. In: Rose N.R. and Mackay, I.R., eds., The Autoimmune Disease, Academic Press, New York, 687-704.
- Naz, R.K., Alexander, N.J., Isahakia, M. and Hamilton, M.D. (1984) Monoclonal antibody to a human sperm membrane glycoprotein that inhibits fertilization. Science, 225, 342-344. doi:10.1126/science.6539947
- Primakoff, P., Lathrop, W., Woolman, L., Cowan, A. and Myles, D. (1988) Fully effective contraception in male and female guinea pigs immunized with the sperm protein PH-20. Nature, 335, 543-546. doi:10.1038/335543a0
- Ranjna, C.S., Vashishat, N. and Bhakri, G. (2012) Identification and characterization of PH-20 and LDHC like antigens in dog spermatozoa for the development of immunocontraceptives. Theriogenology, under revision.
- Herr, J.C., Wright, R.M., John, E., Foster, J., Kays, T. and Flickinger, C.J. (1990) Identification of human acrosomal antigen SP-10 in primates and pigs. Biology of Reproduction, 42, 377-382. doi:10.1095/biolreprod42.2.377
- Naz, R.K., Morte, C., Garcia-Framis, V., Kaplan, P. and Martinez, P. (1993) Characterization of a sperm-specific monoclonal antibody and isolation of a 95-kilodalton fertilization antigen-2 from human sperm. Biology of Reproduction, 49, 1236-1244. doi:10.1095/biolreprod49.6.1236
- Lea, I.A., Richardson, R.T., Widgren, E.E. and O’Rand, M.G. (1996) Cloning and sequencing of cDNAs encoding the human sperm protein, SP-17. Biochemistry and Biophysics Acta, 1307, 263-266. doi:10.1016/0167-4781(96)00077-2
- Naz, R.K. and Zhu, X. (1997) Molecular cloning and sequencing of CDNA encoding for a novel testis-specific antigen. Molecular Reprodction and Development, 48, 449-457. doi:10.1002/(SICI)1098-2795(199712)48:4<449::AID-MRD5>3.3.CO;2-#
- Goldberg, E. and Herr, J.C. (1999) LDH-C4 as a contraceptive vaccine. In: Gupta, S.K., ed., Reprodutive Immunology, Narosa Publishing House, New York, 309-315. doi:10.1007/978-94-011-4197-0_33
- Sabeur, K., Foristall, K. and Ball, B.A. (2002) Characterization of PH-20 in canine spermatozoa and testis. Theriogenology, 57, 977-987. doi:10.1016/S0093-691X(01)00697-5
- Primakoff, P., Woolman-Gamer, L., Tung, K.S.K. and Myles, D.G. (1997) Reversible contraceptive effect of PH-20 immunization in male guinea pigs. Biology of Reproduction, 56, 1142-1146. doi:10.1095/biolreprod56.5.1142
- Naz, R.K., Gupta, S.K., Gupta, J.C., Vyas, H.K. and Talwar, G.P. (2005) Recent advances in contraceptive vaccine development. Human Reproduction, 20, 3271-3283. doi:10.1093/humrep/dei256
- Lathrop, W.F., Carmichael, E.P., Myles, D.G. and Primakoff, P. (1990) cDNA cloning reveals the molecular structure of a sperm surface protein, PH-20, involved in sperm-egg adhesion and the wide distribution of its gene among mammals. Journal of Cell Biology, 111, 2939-2949. doi:10.1083/jcb.111.6.2939
- Hou, S.T., Jones, R. and Hall, L. (1996) Molecular cloning and characterization of rat sperm surface antigen 2B1, a glycoprotein implicated in sperm-zona binding. Molecular Reprodution and Development, 45, 193-203. doi:10.1002/(SICI)1098-2795(199610)45:2<193::AID-MRD12>3.3.CO;2-F
- Meyers, S.A. and Rosenberger, A.E. (1999) A plasma membrane-associated hyaluronidase is localized to the posterior acrosomal region of stallion sperm and is associated with spermatozoal function. Biology of Reprodution, 61, 444-451. doi:10.1095/biolreprod61.2.444
- Lin, Y., Kimmel, L.H., Myles, D.G. and Primakoff, P. (1993) Molecular cloning of the human and monkey sperm surface protein PH-20. Proceedings of National Academy of Sciences USA, 90, 10071-10075. doi:10.1073/pnas.90.21.10071
- Primakoff, P., Hyatt, H. and Myles, D.G. (1985) A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucid. Journal of Cell Biology, 101, 2239-2244. doi:10.1083/jcb.101.6.2239
- Shalgi, R., Matityahu, A., Gaunt, S.J. and Jones, R. (1990) Antigens on rat spermatozoa with a potential role in fertilization. Molecular Reproduction and Development, 25, 286-296. doi:10.1002/mrd.1080250311
- Goldberg, E. and Shelton, J.A. (1986) Immunologic properties of LDH-C4 for contraceptive vaccine development. In: Zatuchni, G.I., Goldsmith, A., Sciarra, J.J. and Spieler, J., eds., Male Contraception Advances and Future Prospects, Harper and Row, Philadelphia, 435-446.
- O’Hern, P.A., Bambra, C.S., Isahakia, M. and Goldberg, E. (1995) Reversible contraception in female baboons immunized with a synthetic epitope of sperm-specific lactate dehydrogenase. Biology of Reproduction, 52, 331-339. doi:10.1095/biolreprod52.2.331
- Cheema Ranjna, S., Vashishat, N., Bhakri, G. and Gandotra, V.K. (2011) SDS-page of sperm membrane-, epididymal tissueand fluid proteins of indian mongrel: Identification and characterization of sperm specific proteins. International Journal of Innovations in Biological and Chemical Sciences, 2, 22-34.
- Ouchterlony, O. (1949) Antigen-antibody reactions in gels. Acta Pathology and Microbiology Scandavia, 26, 507-515.
- Crowther, J.R. (1995) Methods in molecular biology. Humana Press, New York.
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685.
- Towbin, H., Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of National Academy of Sciences, 76, 4350-4354.
- Suri, A. (2005) Contraceptive vaccines targeting sperm. Experiments in Biology Therapy, 5, 381-392. doi:10.1517/14712598.5.3.381
- Chen, Z., He, W., Liang, Z., Yan, P., He, H., Tang, Y., Zhang, J., Shen, Z., Ni, B., Wu, Y. and Li, J. (2009) Protein prime-peptide boost as a new strategy induced an eppin dominant B-cell epitope specific immune response and suppressed fertility. Vaccine, 27, 733-740. doi:10.1016/j.vaccine.2008.11.025
- Rana, R., Jagadish, N., Garg, M., Mishra, D., Dahiya, N., Chaurasiya, D. and Suri, A. (2006) Immunogenicity study of recombinant human sperm-associated antigen 9 in bonnet macaque (Macaca radiata). Human Reproduction, 21, 2894-2900. doi:10.1093/humrep/del068
- Kim, C.A., Parrish, J.J., Momont, H.W. and Lunn, D.P. (1999) Effects of experimentally generated bull antisperm antibodies on in vitro fertilization. Biology of Reproduction, 60, 1285-1291. doi:10.1095/biolreprod60.6.1285
- De Almeida, M., Zouari, R., Jouannet, P. and Feneux, D. (1991) In-vitro effects of anti-sperm antibodies on human sperm movement. Human Reproduction, 6, 405-410.
- Naz, R.K. and Zhu, X. (1998) Recombinant fertilisation antigen-1 causes a contraceptive effect in actively immunized mice. Biology of Reproduction, 59, 1095-1100. doi:10.1095/biolreprod59.5.1095
- Martin-Deleon, P.A. (2006) Epididymal SPAMI and its impact on sperm function. Molecular Cell Endocrinology, 250, 114-121.
- KumiDiaka, J. (1993) Subjecting canine semen to the hypo-osmotic test. Theriogenology, 39, 1279-1289. doi:10.1016/0093-691X(93)90230-3
- Tung, K.S., Primakoff, P., Woolman-Gamer, L. and Myles, D.G. (1997) Mechanism of infertility in male guinea pigs immunized with sperm PH-20. Biology of Reproduction, 56, 1133-1141. doi:10.1095/biolreprod56.5.1133
- Oettle, E.E. (1993) Sperm morphology and fertility in the dog. Journal of Reproduction and Fertility, 47, 257-260.
- Cheema Ranjna, S., Vashishat, N., Bhakri, G. and Gandotra, V.K. (2012) Characterization of antigenic proteins in dog spermatozoa and effect of immunization with sperm membrane proteins on semen quality. Theriogenology Insight, 2, 13-31.
- Fadiloglu, M., Ulman, C., Onvural, B. and Onvural, A. (1998) The seminal fluid isoenzyme LDH-C4 in infertile men,” Tropical Journal of Medicine Science, 28, 609-613.
- O’Rand, M.G., Widgren, E.E., Beyler, S. and Richardson, R.T. (2009) Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: Effect on cyclic adenosine monophosphate. Biology of Reproduction, 80, 279-285. doi:10.1095/biolreprod.108.072942
- Odet, F., Duan, C., Willis, W.D., Goulding, E.H., Kung, A., Eddy, E.M. and Goldberg, E. (2008) Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility,” Biology of Reproduction, 79, 26-34. doi:10.1095/biolreprod.108.068353
- Zhang, J., Ding, X., Bian, Z., Xia, Y., Lu, C., Wang, S., Song, L. and Wang, X. (2010) The effect of anti-eppin antibodies on ionophore A23187-induced calcium influx and acrosome reaction of human Spermatozoa. Human Reproduction, 25, 29-36. doi:10.1093/humrep/dep356
- Yan, J.H. (1988) Use of cauda epididymis extract for immunocontraception in male rats. Fertility Sterility, 49, 174.
- Fayemi, O., Joo, H.S. and Hunter, G. (2006) Effect of sperm immunization of male rabbits on sperm quality, conception rate and litter size. Pakistan Veterinary Journal, 26, 36-40.
- Velasco, J.A.N., Zapata, I.T., Hernandes, P.M., Albacete, M.P., Oltra, J.T. and Paricioo, J.J.P. (1993) Lactic dehydrogenase-C4 activity in seminal plasma and male infertility. Fertility and Sterility, 60, 331-335.
- Hinton, B.T. and Howards, S.S. (1981) Permeability characteristics of the epithelium in the rat caput epididymis. Journal of Reproduction and Fertility, 63, 95-99. doi:10.1530/jrf.0.0630095
- Cuasnicu, P.S., Echeverria, F.G., Piazza, A.D., Cameo, M.S. and Blaquier, J.A. (1984) Antibodies against epididymal glycoproteins block fertilizing ability in rat. Reproduction, 72, 467-471. doi:10.1530/jrf.0.0720467
- Meinertz, H., Linnet, L., Wolf, H. and Hjort, T. (1991) Antisperm antibodies on epididymal spermatozoa. American Journal of Reproduction and Immunology, 25, 158-162.
- Bronson, R.A., Cooper, G.W. and Rosenfeld, D.L. (1984) Sperm antibodies: Their role in infertility. Fertility Sterility, 42, 171-184.
- Pamela, A.D. (2001) Canine reproduction. Reproduction and the male dog, part 4. www.labbies.com/canine_reproduction
ABBREVIATIONS
Spam: Sperm adhesion molecule; LDHC4: Lactatae dehydrogenase; SDS: Sodium dodecyl sulphate; HOST: Hypo-osmotic swelling test; DPI: Days of post immunization; Ig: Immunoglobulin; SP: Sperm protein; FA: Fertility antigen; SME: Sperm membrane extract; CBB: Commissie brilliant blue, PAGE: Polyacrylamide gel electrophoresis; ELISA: Enzyme linked immuoobsorbant assay; DID: Double immune diffusion; PBS: Phosphate buffered saline; BSA: Bovine seum albumin; CT: Coiled tail; BT: Bent; AH: Abnormal head; DH: Detached head; CD: Cytoplasmic droplet; MPT: Mid piece thickening; DAG: Tight coiling; TSH: Short tail/two tails/two heads; MA: Multiple abnormalities; ASA: Anti sperm antibody.

