Advances in Biological Chemistry
Vol. 2 No. 2 (2012) , Article ID: 18976 , 8 pages DOI:10.4236/abc.2012.22018
Purification and characterization of Aspergillus parasiticus cytosine deaminase for possible deployment in suicide gene therapy
![]()
1Department of Biochemistry, University of Maiduguri, Maiduguri, Nigeria
2Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria
Email: *zannahassan@yahoo.co.uk
Received 21 January 2012; revised 2 March 2012; accepted 17 March 2012
Keywords: Cytosine Deaminase; Cytosine; 5-fluorocytosine; Aspergillus parasiticus; Activation energy
ABSTRACT
Cytosine Deaminase (CD) from Aspergillus parasiticus was purified and characterized. Time course for maximal CD production (50 µmol/min/mg) was at 72 hrs. The enzyme was purified 387.73 folds with an overall yield of 13%. The CD had pH optimum of 7.2, a temperature optimum of 40˚C - 45˚C, activation energy (Ea) of 8.4 KJ/mol and a t1/2 of 1.10 hr. A. parasiticus CD stoichiometrically deaminated Cytosine and 5-fluorocytosine (5-FC) with the KM values of 0.19 mM and 0.30 mM respectively. Studies on the effect of pH on KM and Vmax gave pKa1 of 5.8 and pKa2 of 6.3 with enthalpy of ionization of 43.01 KJ/mole suggesting histidine in the active site. The enzyme was strongly inhibited by Ca2+, Co2+, Zn2+ and Hg2+ and completely inhibited by Cu2+ and Fe2+ at 50 mM. Therefore, A. parasiticus CD can be compared with CDs from other sources that are used in suicide gene therapy.
1. INTRODUCTION
Cytosine deaminase is of the Amidohydrolase protein superfamily and catalyzes the deamination of cytosine to uracil and ammonia in the pyrimidine salvage pathway. The pyrimidine salvage pathway is active in bacteria and fungi and absent in mammals including humans. The same enzyme also catalyzes the conversion of 5-fluorocytosine to 5-fluorouracil; this activity allows the formation of a cytotoxic chemotherapeutic agent from a noncytotoxic precursor. The enzyme is of wide spread interest both for antimicrobial drug design and for gene therapy applications against tumours [1,2].
Cytosine deaminase has been isolated and characterized from various sources by several researchers. Katsuragi et al., [3], purified the enzyme from E. coli 1200 fold to homogeneity with two bands of 35 and 46 KDa respectively. The enzyme had a pH and temperature optimum of 9˚C and 50˚C respectively and inhibited by Cu2+, Co2+, Fe2+ and Hg2+.
Porter and Austin, [4] showed that a catalytically essential divalent metal ion is contained in Escherichia coli CD after efficiently removing Fe2+ from the enzyme with O-phenanthroline to yield an apoenzyme with less than 5% of the catalytic activity of native enzyme. They reported that apoenzyme reconstituted with Fe2+, Mn2+, Co2+ or Zn2+ had Kcat values of 1,858,850 and 32 S−1 and similar Km values for cytosine (0.22 - 0.39 mM) but Fe2+ CD was inhibited by Cu2+, Zn2+ and EDTA.
E.coli CD is a hexameric protein of 300 KDa molecular mass with a single catalytic iron coordinated by three histidine residues. KM and Kcat of the wild CD for cytosine and 5-fluorocytosine are 0.2 mM, 3.3 mM and, 165 s−1 and 75.5 s−1 respectively while that of the mutagenized CD are 2.2 mM, 2.8 mM and 104 s−1 and 137 s−1 respectively [5].
Cytosine deaminase from Salmonella typhimurium was purified 419-fold to apparent homogeneity by the criteria of SDS polyacrylamide gel electrophoresis with a molecular weight of 230 KDa containing four identical subunits with each subunit having a molecular weight of 54 KDa [6]. They also reported a pH and temperature optimum of 7.3˚C and 45˚C respectively. The enzyme also had a Km and Vmax values for cytosine of 0.74 mM and 47.16 µmole/min respectively.
The extracellular CD from Chromobacterium violaceum YK391 was purified 264.7-fold with an overall yied of 14.3%. The molecular weight of the purified enzyme was estimated to be about 154 KDa containing two identical subunits of approximate molecular weight of 78 KDa each. The enzyme had a pH optimum of 7.5 and a temperature optimum of 40˚C - 45˚C.
Apart from cytosine, the enzyme deaminated 5FC, Cytidine and 5-methylcytosine and apparent Km values for them were 1.55 mM, 5.52 mM, 10.4 mM and 67.2 mM respectively. The enzyme activity was strongly inhibited by heavy metal ions such as Fe2+, Pb2+, Cd2+, Zn2+, Hg2+ and Cu2+ at 1 mM [7].
Ipata and Cercignani, [8] reported a molecular weight of 34 KDa and pH optimum of 6.5 for CD from Baker’s yeast. The enzyme preparation was stable for at least 48 hr when kept at 4˚C in the pH range of 5 - 9. Both cytosine and 5-methylcytosine were good substrates, with identical Km values of 2.5 mM.
Katsuragi et al., [9] extracted CD from Baker’s yeast and purified it 6800-fold to homogeneity. The molecular weight was 41 KDa by gel permeation with pH and temperature optimum of 7.5˚C and 30˚C respectively. The enzyme deaminated cytosine and 5FC with apparent Km values of 3.1 mM and 1.2 mM respectively. Yeast CD was strongly inhibited by Ag2+, Hg+ and Hg2+, and weakly by Fe2+, Fe3+ and Pb2+. The enzyme was unstable to heat with a half-life of about 0.5 hr at 37˚C.
Cytosine deaminase from Aspergillus fumigatus was the first CD to be found in a mould and was purified 150-fold to homogeneity with an overall yield of 0.75% [10]. The enzyme was a monomer of 32 KDa with pH and temperature optimum of 7˚C and 35˚C respectively.
Beside cytosine, the enzyme also deaminated 5-methylcytosine and 5FC with apparent Km values of 2, 36 and 6.5 mM respectively. Heavy metal ions such as Fe2+, Cu2+, Hg2+ and Pb2+ inhibited the enzyme activity at 0.1 mM.
Cytosine deaminase is employed in the so-called suicide gene therapy to treat tumours. Currently, the CD used in this therapy are either from bacterial or yeast sources. The enzyme from either of these sources has limitations because yeast CD has a higher affinity for 5-FC than E. coli CD but less thermostable. Conversely, E. coli CD has lower affinity for 5-FC but is more thermostable than yeast CD (11). Therefore there is need to look for CDs with high physiological efficiency in terms of kinetics and other properties than either bacterial or yeast CD that are currently in use for the treatment of tumours. The aim of this study is to isolate, purify and characterize CD from Aspergillus parasiticus and the objective is to see if the purified enzyme will be suitable for suicide gene therapy.
2. MATERIALS AND METHODS
2.1. Chemicals
All the chemicals used in this study were of analytical grade and purchased from various sources.
2.2. Methods
2.2.1. Inoculation
The Aspergillus parasiticus used in this study was isolated and identified by a mycologist using internet resources.
Spores of Aspergillus parasiticus were harvested from 5 day old potato-dextrose agar (PDA) slants by washing with sterile 0.2% tween 80. The spores were used to inoculate the mineral salt medium containing: distilled water 1.0 L, glucose 10 g, KH2PO4 5.0 g, MgSO4·7H2O 1.0 g, NaCl 0.5 g, NaNO3 2 g, 0.5 g peptone and pH adjusted to 5.5 with 0.1 M HCl/NaOH as described by Haq et al., [11]; Zanna et al., [12].
2.2.2. Cytosine Deaminase Extraction
One hundred mililitre (10.0 ml) liquid culture was centrifuged at 3000 ´ g for 5 minutes and the supernatant was used as crude enzyme.
2.2.3. Enzyme Assay
Cytosine deaminase activity was assayed as described by Ipata and Cercignani [1978]. Enzyme activity was measured by direct spectrophotometric assay from the fall in absorbance at 286 nm following conversion of 4-amino to 4-keto compounds.
2.2.4. Cytosine Deaminase Assay Using 5-Fluorocytosine as Substrate
This was done as described by Nishiyama et al., [13]; Mahan et al. [5].
Enzyme activity was measured spectrophotometrically at 255 and 290 nm following conversion of 5-FC to 5-FU.
2.2.5. Purification of Cytosine Deaminase
1) Ammonium Sulphate Fractionation
Crude CD was precipitated between 20% - 90% of ammonium sulphate saturation. Each fraction was centrifuged at 10,000 ´ g for 20 min after standing over night at 4˚C. Cytosine deaminase activity and total protein were assayed in the pellet as well as the supernatant for each fraction. Ninety percent (90%) fraction gave the highest specific activity.
2) Protamine Sulphate Treatment
The ammonium sulphate fraction (90%) was treated with 2% protamine sulphate (adjusted to pH 7) and allowed to stand for 10 minutes at 37˚C and then centrifuged for another 15 minutes at 10,000 ´ g. The precipitate was discarded and the supernatant used for gel purification.
3) Purification of Cytosine Deaminase Using Sephadex G-75
Sephadex G-75 column (2 ´ 60 cm) was pre-equilibrated with 0.05 M Tris-HCl buffer, pH 7.2. The column was then loaded with 1.5 ml of cytosine deaminase solution obtained after protamine sulphate treatment and eluted with the same equilibration buffer. Five milliliter (5 ml) fractions were collected at a flow rate of 1 ml/min. Each fraction was assayed for CD activity and protein concentration.
4) Cytosine Deaminase Purification on DE-52 Ion Exchange Chromatography
The cytosine deaminase active fractions from the gel purification were pooled together, concentrated by dialysis and 5 ml was applied to a DEAE-Cellulose, DE-52 column (2 ´ 30 cm). The column was eluted with a linear gradient of NaCl (0.025 - 0.50 M) prepared in 0.05 M Tris-HCl buffer pH 7.2. The Flow rate was maintained at 0.2 ml/min.
2.2.6. Characterization of Cytosine Deaminase
1) Thermostability Studies
a) Optimum Temperature of Cytosine Deaminase
This was determined by incubating a mixture of enzyme and substrate at varying temperature (30˚C - 80˚C) for 10 mins and activity assayed.
b) Enzyme Operational Stability
The operational stability of the enzyme was determined by incubating the enzyme solution in a waterbath at 37˚C and aliquots were taken every hour, and transfered into ready reaction mixture containing the substrate and buffer for assay. The residual activity remaining which is directly related to stability was determined at a constant pH, temperature and ionic strength using the same buffer in all determinations. Half-life of the enzyme was then calculated using the formula:
t1/2 = 0.693/KD where KD is the decay constant and is given by
KD = 2.303/t·log (Eº/E); where (Eº/E) is the fraction of enzyme activity remaining after incubation for time t.
c) Activation Energy of Cytosine Deaminse
Activation energy (Ea) was determined by preincubating the enzyme and substrate at various temperature for 10 mins before assaying for activity. log initial velocity was plotted against reciprocal of the temperature in Kelvin (Arrhenius plot) and the slope was used to determine Ea.
d) Effect of pH on Cytosine Deaminase Activity
pH optimum of CD was determined by assaying enzyme activity at varying pHs ranging from pH 4 - 9. The following buffers (0.1 M) were employed in the assay of the enzyme activity;
Acetate buffer for pH range of 4 - 5, phosphate buffer for pH range of 6 - 7 and Tris-HCl buffer for pH range of 8 - 9. Then a plot of activity against pH was used to determine pH optimum.
2) Kinetic Studies
a) Initial Velocity Studies
This was done by incubating the enzyme with 0.5, 1.0, 2.0, 2.5, 3.0, 3.5 and 4.0 mM of substrate to obtain their corresponding vo. Then the data obtained was used for double reciprocal plot from which KM and Vmax were deduced.
b) Effect of pH on Kinetic Parameters
This was done by carrying out initial velocity studies at varying pHs of 4, 6, 7, 8 and 9. Michaelis constant (Km) and Maximum velocity (Vmax) were determined at each pH and a plot of log of Vmax/Km against pHs (DixonWebb plot) was done to determine ionisable groups in the active site of CD.
3) 5-Fluorocytosine Specificity for CD
Cytosine deaminase was assayed using varying concentrations of 5-FC and data obtained was used for the determination of Km and Vmax.
4) Effect of Ions on CD Activity
Activity of CD was assayed in the presence of the following cations;
Ca2+, Zn2+, Hg2+, Fe2+, Co2+ and Cu2+. Fifty milimolar (50 mM) Concentrations of each cation was used. This is to examine the modulatory effect of the cations.
5) Statistical Analysis
Results are mean ± standard deviation for triplicate determination.
3. RESULTS
3.1. Purification Profile of A. parasiticus CD
Purification steps employed using different procedures were summarized in table 1. Ammonium sulphate precipitation gave a specific activity of 3.61 µmol/min/mg with fold purification of 1.50 and 38% yield. Protamine sulphate treatment lowered the specific activity by 0.31 µmol/min/mg as well as the fold purification and yield by 0.15 and 13% respectively. Purification on sephadex G-75 column gave CD with specific activity of 258.50 µmol/min/mg with fold purification of 106.0 and 13.8% yield. Ion exchange on DEAE cellulose DE52 purified CD in 12.4% yield with purification fold of 384.12. The purified enzyme had a specific activity of 937.25 µmol/min/mg based on protein concentration determined by absorbance at 280 (A280) nm.
3.2. Effect of pH on CD
The effect of different pH on CD activity is as described in Figure 1. The CD activity was assayed at pH 4.6, 7, 8 and 9. Highest CD activity was at pH 7.2 though pH 4 and 7 also gave an appreciable activity.
3.3. Effect of Temperature on Partially Purified CD Activity
The effect of temperature on partially purified CD activ-
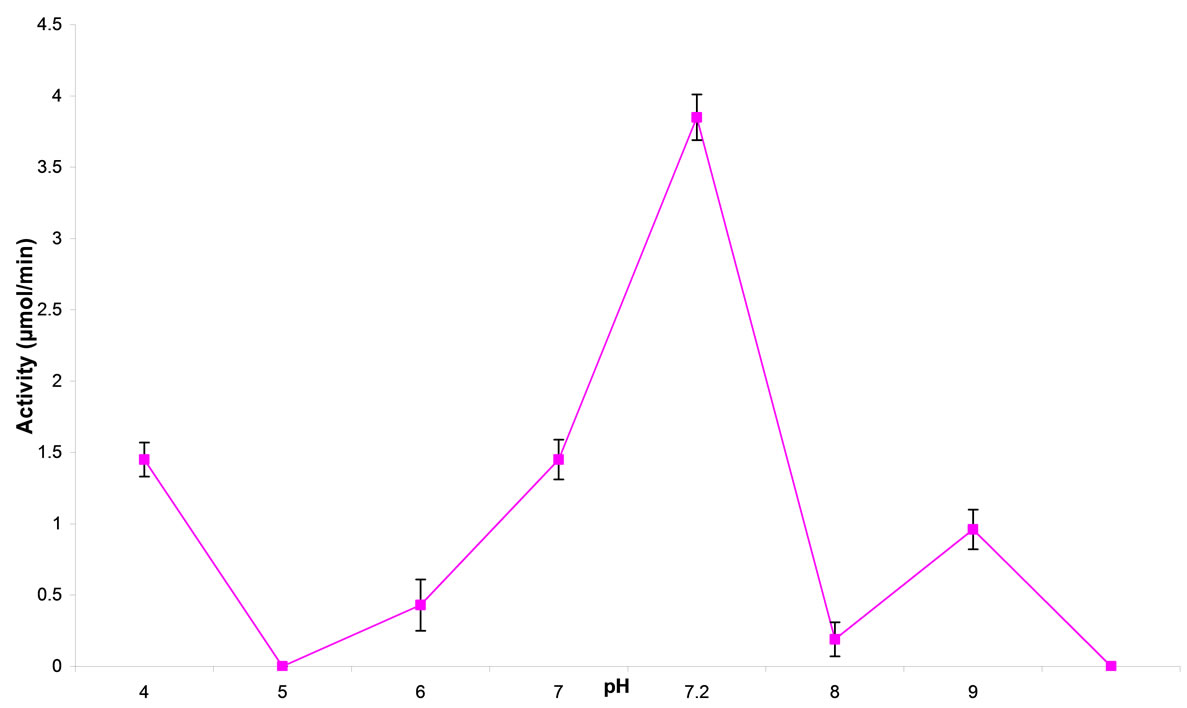
Figure 1. effect of pH on cytosine deaminase activity.
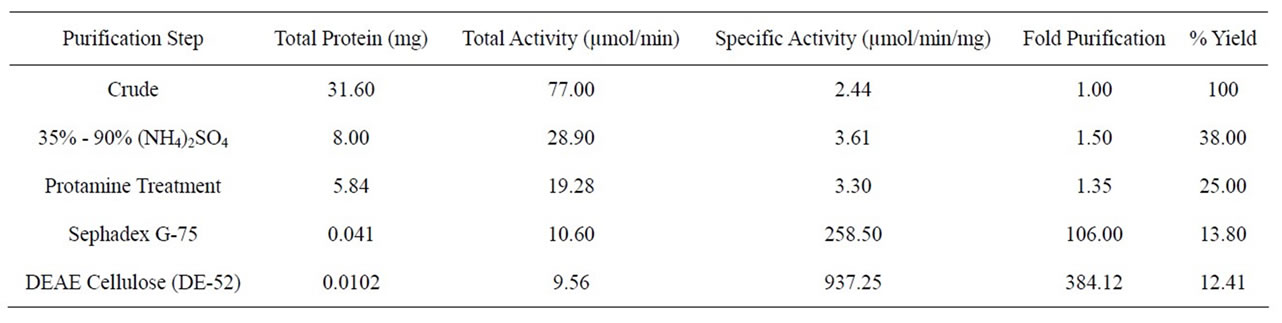
Table 1. Purification profile of A. parasiticus cytosine deaminase.
ity is as presented in Figure 2. Highest CD activity was recorded between the temperatures of 40˚C - 45˚C. Enzymic activity dropped at 50˚C but remained stable through to 80˚C. An activation energy of 8.4 KJ/mole was calculated from the slope of the plot of logvo vs reciprocal of absolute temperature (K) (Figure 3).
3.4. Operational Stability of CD
The operational stability of CD at 37˚C is as presented in Figure 4. Percentage residual activity after 1 hr was 67% and at 2 hrs it was around 33%. The t1/2 for the enzyme was 1.1 hr.
3.5. Michaelis Constants (KM)
The result of double reciprocal plots for the determination of KM for Cytosine and 5-FC gave a KM of 0.19 and 0.30 mM respectively.
3.6. Effect of Cations on Cytosine Deaminase Activity
The effect of cations on CD activity is as shown in Figure 5. The enzyme activity was strongly inhibited by Ca2+, Co2+, Zn2+ and Hg2+, and completely by Cu2+ and Fe2+ at 50mM concentration.
3.7. Determination of Ionisable Groups in the Active Site
Dixon-Webb plot (Figure 6) for the determination of ionisable groups in the active site of the enzyme gave pKa1 and pKa2 of 5.8 and 6.3 respectively which correspond to the pKa of histidine. The enthalpy of ionization (∆H) was also calculated to be 43.01 KJ/mole using the
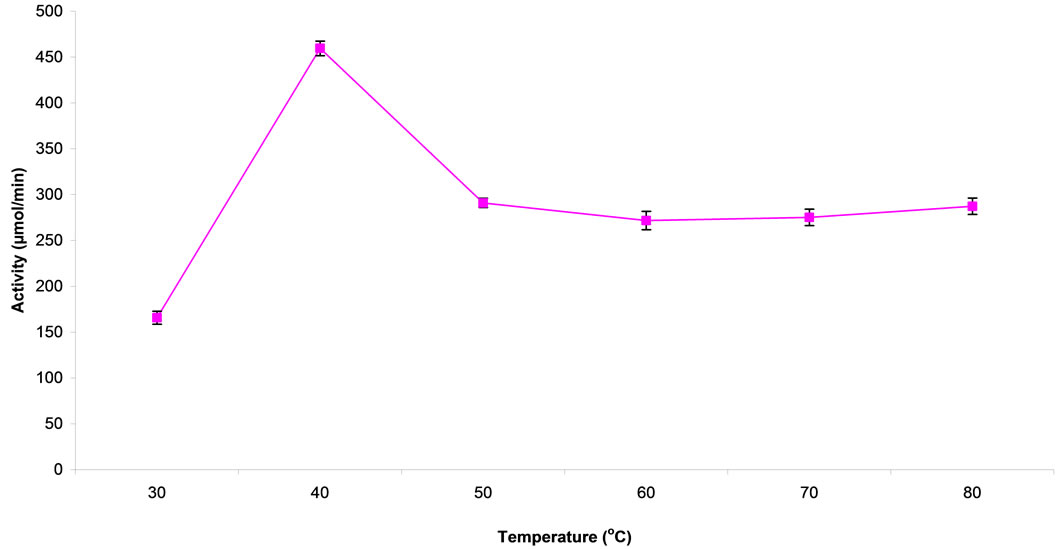
Figure 2. effect of temperature on cytosine deaminase activity.

Figure 3. Arrhenius plot for cytosine deaminase.
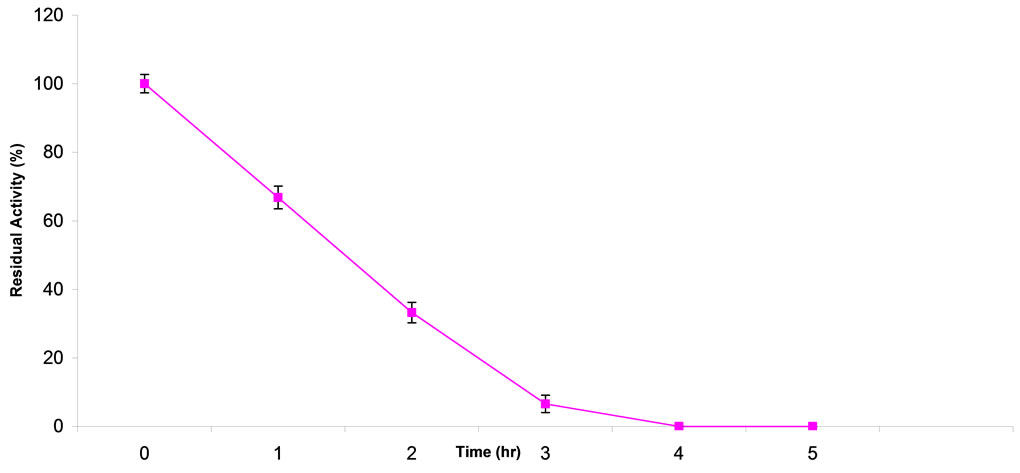
Figure 4. Operational stability of A. parasiticus cytosine deaminase at 37˚C. Residual activity of CD was 67% after 1 hr of incubation and it dropped to zero.

Figure 5. Effect of cations on cytosine deaminase activity enzyme activity was completely inhibited by Cu2+ and Fe2+, and strongly by Ca2+, Co2+, Zn2+ and Hg2+.
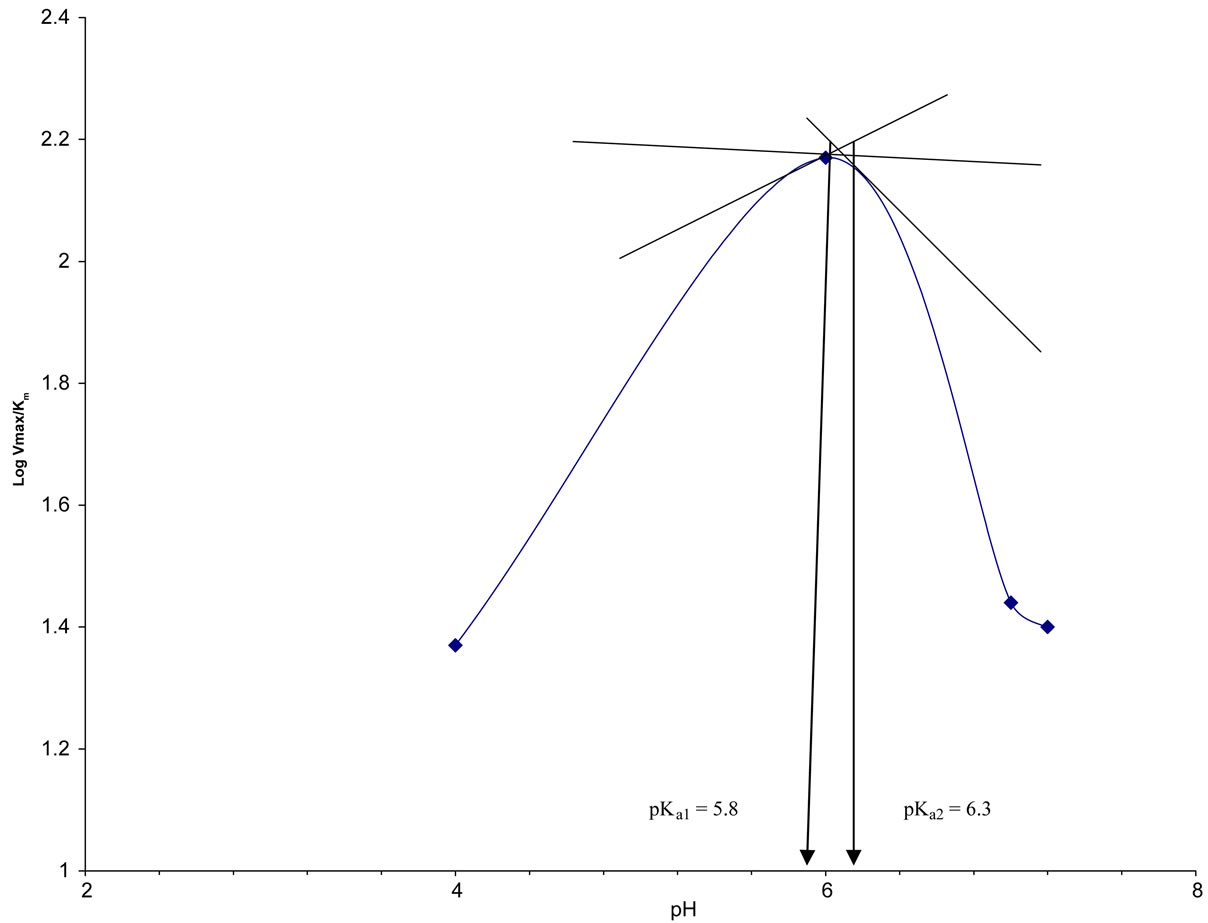
Figure 6. Plot of LogVmax/Km against pH (Dixon Plot) for A. parasiticus CD.
formula ∆G = ∆H – T∆S.
4. DISCUSSION
A. parasiticus CD was purified 384.12 fold with an overall yield of 13% and a specific activity of 937.25 µmol/min/mg.
The pH optimum of 7.2 exhibited by the native CD is characteristic of physiological pH. Thus the enzyme may operate optimally at physiological pH and hence can be deployed for cancer treatment which is promising for clinical use. This result is similar to earlier report by West et al., [6] where they reported a pH optimum of 7.3 - 7.5 for CD from Salmonella typhimurium. Tae-shick et al., [10] reported a pH optimum of 7 for A. fumigatus cytosine deaminase.
The A. parasiticus CD was maximally active at temperature of 40˚C - 45˚C with an activation energy of 8.4 KJ/mole thus capable of thriving under physiological conditions. Large polymeric enzymes are likely to be less heat-stable than the lower molecular weight single polypeptide enzyme proteins with some disulphide bonds. A temperature optimum of 50˚C and 40˚C respectively were reported for E. coli and yeast CD [3,9].
Lineweaver-Burk plots of initial velocity data at pH 7.2 gave Km values of 0.19 mM and 0.30 mM for cytosine and 5FC respectively. The enzyme from Baker’s yeast has KM of 3.1 mM for cytosine and 1.2 mM for 5FC respectively (9), while Mahan et al., [5] reported KM of 2.2 and 2.8 mM respectively for cytosine and 5FC using CD from E. coli after mutagenesis.
Aspergillus parasiticus cytosine deaminase lost 47% of its activity in the presence of Ca2+, 58% in the presence of Hg2+ and 40% in the presence of Co2+ and Zn2+ respectively. The inhibition of CD by divalent metal ions such as Cu2+, Fe2+ and Hg2+ suggest that the enzyme is an SH-enzyme [9]. Metal ions can be involved in enzyme catalysis in a variety of ways. They may accept or donate electrons to activate electrophiles or nucleophiles, or they may themselves act as electrophiles. They may bring together enzyme and substrate by means of coordinate bonds or may hold reacting groups in the required three-dimensional orientation. Metal ions may simply stabilize a catalytically active conformation of the enzyme.
The CD’s percentage residual activity was 67% after 1hr of incubation at 37˚C. The t1/2 of the enzyme was 1.1 hr. However, Katsuragi et al., [14] reported a t1/2 of 30 min at 37ºC for yeast CD.
The study on ionisable groups in the active site of A. parasiticus CD revealed the presence of groups with enthalpy of ionization of 43.01 KJ/mole. This result is suggestive of histidine in or around the active site of the enzyme. Ireton et al., [1] reported His61, His63, His214 and His246 in the active site of E. coli CD, while Ko et al., [15] reported His62, Cys91 and Cys94 in the active site of yeast CD.
5. CONCLUSION
The free CD from A. parasiticus was stable and had lower KM value for 5-FC. Therefore, cytosine deaminase from A. parasiticus is a suitable candidate for comparison with yeast and E. coli CD; and for subsequent deployment in cancer therapy either as capsule implant at the site of the tumour or in suicide gene therapy. However, the operational stability of the enzyme be improved either by immobilization or by addition of stabilizers.
![]()
![]()
REFERENCES
- Ireton, G.C., McDermott, G., Black, M.E. and Stoddard, B.L. (2002) The structure of Escherichia coli cytosine deaminase. Journal of Molecular Biology, 315, 687-697. doi:10.1006/jmbi.2001.5277
- Hamaji, Y., Fujimori, M., Sasaki, T., Matsuhashi, H., Matsui-seki, K., Shimatani-Shibata, Y., Kano, Y., Amano, J. and Taniguchi, S. (2007) Strong enhancement of recombinant cytosine deaminase activity in Bifidobacterium longum for tumor-targeting enzyme/prodrug therapy. Bioscience Biotechnology Biochemistry, 71, 874-883. doi:10.1271/bbb.60502
- Katsuragi, T., Sakai, T., Matsumoto, K. and Tonomura, K. (1986) Cytosine deaminase from Escherichia coli production, purification, and some characteristics. Agricultural Biological Chemistry, 50, 1721-1730. doi:10.1271/bbb1961.50.1721
- Porter, D.J. and Austin, E.A. (1993) Cytosine deaminase: The roles of divalent metal ions in catalysis. Journal of Molecular Biology, 268, 24005-24011.
- Mahan, S.D., Ireton, C.G., Knoeber, C., Stoddard, L.B. and Black, E.M. (2004) Random mutagenesis and selection of Escherichia coli cytosine deaminase for cancer gene therapy. PEDS, 17, 625-633.
- West, T.P., Shanley, M.S. and O’Donovan, G.A. (1982) Purification and some properties of cytosine deaminase from Salmonella typhimurium. Biochimica et Biophysica Acta, 719, 251-258
- Yu, T.S. and Kim, T.H. (1999) Purification and properties of extracellular cytosine deaminase from Chromobacterium violaceum YK391. Journal of Microbiology and Biotechnology, 9, 173-178.
- Ipata, P.L. and Cercignani, G. (1978) Cytosine and cytidine deaminase from yeast. Methods in Enzymology, 51, 394-400. doi:10.1016/S0076-6879(78)51053-7
- Katsuragi, T., Sonoda, T., Matsumoto, K., Sakai, T. and Tonomura, K. (1989) Purification and some properties of cytosine deaminase from Baker’s yeast. Agricultural Biological Chemistry, 53, 1313-1319. doi:10.1271/bbb1961.53.1313
- Tae-shick, Y., Jae-kuen, K., Tohoru, K., Takuo, S. and Kenzo, T. (1991) Purification and properties of cytosine deaminase from Aspergillus fumigatus. Journal of Fermentation and Bioengineering, 72, 266-269.
- Haq, I., Ali, S. and Quadeer, M.A. (2005) Influence of dissolved oxygen concentration on intracellular pH for regula-tion of Aspergillus niger growth rate during citric acid fermentation in stirred tank bioreactor. International Journal of Biological Sciences, 1, 34-41.
- Zanna, H., Nok, A.J., Ibrahim, S. and Inuwa, H.M. (2011) Screening some aspergillus fungi for cytosine deami-nase activity. International Journal of Biotechnology & Biochemistry, 7, 397-403.
- Nishiyama, T., Kawamura, Y., Kawamoto, K., Matsumura, H., Yamamoto, N., Ito, T., Ohyama A., Katsuragi, T. and Sakai, T. (1985) Antineoplastic effects in rats of 5-fluorocytosine in combination with cytosine deaminase capsules. Cancer Research, 45, 1753-1761.
- Katsuragi, T., Shibata, M., Sakai, T. and Tonomura, K. (1989) Stabiliation of cytosine deaminase from Baker’s yeast by immobilization. Agricultural Biological Chemistry, 53, 1515-1523. doi:10.1271/bbb1961.53.1515
- Ko, T., Lin, J., Hu, C., Hsu, Y., Wang, A.H. and Liaw, S. (2003) Crystal struture of yeast cytosine deaminase: Insights into enzyme mechanisms and evolution. The Journal of Biological Chemistry, 278, 19111-19117. doi:10.1074/jbc.M300874200
NOTES
*Corresponding author.

