Open Journal of Discrete Mathematics
Vol.06 No.04(2016), Article ID:71603,26 pages
10.4236/ojdm.2016.64026
The Z-Valued Characters for the Huge Symmetry of Hexamethylethane
Ali Moghani*, John P. Najarian
Department of Computer Science, William Paterson University, Wayne, NJ, USA
Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 5, 2016; Accepted: October 25, 2016; Published: October 28, 2016
ABSTRACT
To enumerate isomers of the fluxional molecules, some theorems for maturity and the integer-valued characters of finite groups were introduced by S. Fujita and first author. The full non-rigid group of hexamethylethane is the semi-direct product of the direct products of six copies of the cyclic group Z3 by the dihedral group of order 12 (see, Asian J. Chem. (2010) 22 (3), 1966-1972). In this paper, we continue our study on finite groups (see Int. J. Theo. Physics, Group Theory, and Nonlinear Optics (2013), 17) and all the integer-valued characters of the above molecule are successfully derived.
Keywords:
Symmetry, Dominant Class, Hexamethylethane, Character

1. Introduction
An object is called symmetrical if some movement or operation leaves the object in a position indistinguishable from its original position. The symmetry of molecules and solids is a very powerful tool for developing and understanding bonding and physical properties used to predict the nature of molecular orbitals. Chemists and physicists classify molecules in terms of their symmetry. It is of some value to recognize that all molecules that have the same basic “structure” share a number of common properties. The process of doing the rotation, reflection etc. is referred to as a symmetry operation if it does not change the appearance of the molecule. It is easy to see that all symmetry operations of a molecule form a group named symmetry group of molecule under consideration [1] - [20] . A molecule is said to be non-rigid if there are several local minima on the potential energy surface easily surmountable by the molecular system via a tunneling rearrangement. A non-rigid molecule typically possesses several potential valleys separated by relatively low energy barriers, and thus exhibits large amplitude tunneling dynamics among various potential minima. Because of this deformability, the non-rigid molecules exhibit some interesting properties of intramolecular dynamics, spectroscopy, dynamic NMR and so on, all of which can be interpreted by resorting to group theory. Group theory is the best formal method to describe the symmetry concept of molecular structures. Group theory has numerous applications to large amplitude vibrational spectroscopy or small organic molecules, in particular.
Character tables for reducing linear representations into irreducible ones belong to the standard repertoire of chemical group theory. Since they are widely applied to various fields of chemistry, textbooks on chemical group theory use many pages to introduce the concept and applications of character tables. On the other hand, the concept of mark tables for assigning permutation representations to coset representations, which has been developed by Burnside, has been unjustly neglected for a long time not only in chemical fields but also in mathematics. Among several works using mark tables, Kerber’s contribution, Redfield’s research can be mentioned for discussing mathematical applications. In order to develop new methods of combinatorial enumeration of isomers, some relationships between character tables containing characters for irreducible representations and mark tables containing marks for coset representations have been clarified by Shinsaku Fujita who proposed not only markaracter tables, which enable us to discuss characters and marks on a common basis, but Q-Conjugacy character tables, which are obtained for finite groups [2] - [11] [20] .
The present study investigates all the dominant subgroups and the integer-valued characters of the symmetry of Hexamethylethane (Figure 1) with the aid of GAP system [21] .
Figure 1. The structure of hexamethylethane.
2. Results and Discussion
In this section we first describe some notation which will be kept throughout.
Let G be an arbitrary finite group and h1, h2 Î G. We say h1 and h2 are Q-conjugate if there exists t Î G such that t−1
Suppose that H is a cyclic subgroup of order n of a finite group G and K is a conjugacy class of G. Then, the maturity discriminant of H denoted by m(H) is an integer delineated by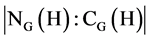 . In addition, the dominant class of K Ç H in the norma-
. In addition, the dominant class of K Ç H in the norma-
lizer 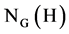 is the union of t =
is the union of t = 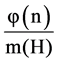 conjugacy classes of G where φ is the Euler
conjugacy classes of G where φ is the Euler
function, i.e. the maturity of G is clearly defined by examining how a dominant class corresponding to H contains conjugacy classes. The group G should be a matured group if t = 1, but if t ≥ 2, the group G is unmatured concerning subgroup H, see [4] - [9] .
Let Cu×u be a matrix of the character table for an arbitrary finite group G. Then, C is transformed into a more concise form called the Q-Conjugacy character table denoted by  containing integer-valued characters. By Theorem 4 in [5] , the dimension of a Q-conjugacy character table is equal to its corresponding markaracter table, i.e.
containing integer-valued characters. By Theorem 4 in [5] , the dimension of a Q-conjugacy character table is equal to its corresponding markaracter table, i.e.  is an m × m-matrix where m is the number of dominant classes or the number of non- conjugate cyclic subgroups denoted by SCSG, see [9] .
is an m × m-matrix where m is the number of dominant classes or the number of non- conjugate cyclic subgroups denoted by SCSG, see [9] .
Assume that N be a normal subgroup of G and H is another subgroup of G such that H Ç N = {e} and G = HN = {xy| x Î H, y Î N}, then we say that G is a semidirect product of N by H denoted by N ⋉ H. Let us make some observations about semidirect product. Since G = NH, each x Î G can be written uniquely as x = nh for some n Î N and h Î H. Fix element h in H, since N is normal in G, conjugation by h maps N to H, consequently we can define a map ϕh: N → N by ϕh(n) = hnh−1 for n Î N. It is easy to show that ϕh is an automorphism of N and also that ϕhoϕj = ϕhj for any j Î H. Therefore, we have constructed a homomorphism ϕ: H → Aut(N), where ϕ(h) = ϕh, we call ϕ conjugation homomorphism of the semidirect product G and write G = N ⋉ ϕ H. We can see that if the homomorphism ϕ: H → Aut(N) defined above is trivial, then semidirect product reduces to the direct product of N × H. It is a well-known fact that the homomorphism ϕ completely determines the semi-direct product.
The full non-rigid group of hexamethylethane is the semi-direct product of the direct products of six copies of the cyclic group Z3 by D6 (the dihedral group of order 12), i.e. (Z3 × Z3 × Z3 × Z3 × Z3 × Z3) ⋉ D6 of order 8748, see for further details in [18] .
The first author shows that the semi-direct product of the matured groups again is a matured group, but the semi-direct product is unmatured if at least one of the groups is unmatured, see [19] .
Now consider the full non-rigid group of hexamethylethane i.e. HEX = (Z3 × Z3 × Z3 × Z3 × Z3 × Z3) ⋉ D6 then, HEX is an unmatured group [19] , see Figure 1.
We will use for an arbitrary conjugacy class of G of elements of order n the notation
Table 1. The corresponding non-redundant set of cyclic subgroups of HEX.
nX, where X = a, b, c, ∙∙∙
Referring to the reported GAP program [21] - [28] , we are able to introduce all the dominant classes (i.e. Ki) as follow:
K1 = 1a, K2 = 3a È 3b, K3 = 3c È 3e, K4 = 3d, K5 = 3f È 3l, K6 = 3g È 3n, K7 = 3h È 3m, K8 = 3i, K9 = 3j È 3k, K10 = 3o È 3ad, K11 = 3p È 3af, K12 = 3q È 3ae, K13 = 3r È 3ai, K14 = 3s È 3ah, K15 = 3t È 3ag, K16 = 3u, K17 = 3v È 3aa, K18 = 3w È 3x, K19 = 3y È 3ac, K20 = 3z, K21 = 3ab, K22 = 3aj È 3bq, K23 = 3ak È 3ay, K24 = 3al È 3bs, K25 = 3am È 3bj, K26 = 3an È 3br, K27 = 3ao È 3bc, K28 = 3ap È 3bx, K29 = 3aq È 3bz, K30 = 3ar È 3by, K31 = 3at È 3bw, K32 = 3au È 3bv, K33 = 3av, K34 = 3aw È 3bu, K35 = 3ax È 3bt, K36 = 3az È 3bi, K37 = 3ba È 3bb, K38 = 3bd È 3bp, K39 = 3be È 3bo, K40 = 3bf, K41 = 3bg È 3bh, K42 = 3bk, K43 = 3bl È 3bm, K44 = 3n È 3as, K45 = 3ca È 3cm, K46 = 3cb È 3cl, K47 = 3cc È 3ch, K48 = 3cd È 3cj, K49 = 3ce, K50 = 3cf È 3ck, K51 = 3cg, K52 = 3ci, K53 = 3cn, K54 = 9a È 9b, K55 = 9c È 9e, K56 = 9d, K57 = 6a, K58 = 18a È 18b, K59 = 2a, K60 = 6b È 6c, K61 = 6d È 6f, K62 = 6e, K63 = 6g È 6j, K64 = 6h È 6i, K65 = 2b, K66 = 6k È 6l, K67 = 6m È 6p, K68 = 6n È 6r, K69 = 6o È 6q, K70 = 6s
Assume Cbe the character table of HEX = <(1, 2, 3, 4, 5, 6), (2, 6)(3, 5), (7, 8, 9), (10, 11, 12), (13, 14, 15), (16, 17, 18), (19, 20, 21), (22, 23, 24)> i.e. group HEX is generated by generators (1, 2, 3, 4, 5, 6), (2, 6)(3, 5), (7, 8, 9), (10, 11, 12), (13, 14, 15), (16, 17, 18),
Table 2. The integer-valued characters of the f-NRG of hexamethylethane.
(19, 20, 21) and (22, 23, 24), which is a 174 × 174 matrix, contains 174 irreducible characters, see [18] . Now by considering the above outputs, we calculate all the integer-valued characters by the row-reductions (respectively, column-reductions) of HEX as a 99 × 99-matrix which are stored as in Table 2, see [27] [28] for more details.
Besides, the Q-conjugacy character table of the symmetry of a given molecule would also be valuable in other applications such as in the context of chemical applications of graph theory and aromatic compounds, see [1] - [6] [23] - [28] .
Acknowledgements
The authors are indebted to the referees for useful discussion and suggestions.
Cite this paper
Moghani, A. and Najarian, J.P. (2016) The Z-Valued Characters for the Huge Symmetry of Hexamethylethane. Open Journal of Discrete Mathematics, 6, 314-339. http://dx.doi.org/10.4236/ojdm.2016.64026
References
- 1. Hargittai, I. and Hargitta, H. (1986) Symmetry through the Eyes of a Chemist. VCH, Weinheim.
- 2. Fujita, S. (1998) Maturity of Finite Groups. An Application to Combinatorial Enumeration of Isomers. Bulletin of the Chemical Society of Japan, 71, 2071-2080.
http://dx.doi.org/10.1246/bcsj.71.2071 - 3. Fujita, S. (1998) Inherent Automorphism and Q-Conjugacy Character Tables of Finite Groups. An Application to Combinatorial Enumeration of Isomers. Bulletin of the Chemical Society of Japan, 71, 2309-2321.
http://dx.doi.org/10.1246/bcsj.71.2309 - 4. Fujita, S. (1998) Markaracter Tables and Q-Conjugacy Character Tables for Cyclic Groups. An Application to Combinatorial Enumeration. Bulletin of the Chemical Society of Japan, 71, 1587-1596.
http://dx.doi.org/10.1246/bcsj.71.1587 - 5. Fujita, S. (2005) Orbits in a Molecule. A Novel Way of Stereochemistry through the Concepts of Coset Representations and Sphericities. MATCH Communications in Mathematical and in Computer Chemistry, 54, 251-300.
- 6. Fujita, S. (2006) Orbits among Molecules. A Novel Way of Stereochemistry through the Concepts of Coset Representations and Sphericities. MATCH Communications in Mathematical and in Computer Chemistry, 55, 5-38.
- 7. Fujita, S. (2006) Combinatorial Enumeration of Stereoisomers by Linking Orbits in Molecules with Orbits among Molecules. A Novel Way of Stereochemistry through the Concepts of Coset Representations and Sphericities. MATCH Communications in Mathematical and in Computer Chemistry, 55, 237-270.
- 8. Fujita, S. (2007) Mandalas and Fujita’s Proligand Method. A Novel Way of Stereochemistry through the Concepts of Coset Representations and Sphericities. MATCH Communications in Mathematical and in Computer Chemistry, 57, 5-48.
- 9. Fujita, S. (2007) Diagrammatical Approach to Molecular Symmetry and Enumeration of Stereoisomers. University of Kragujevac, Kragujevac.
- 10. Fujita, S. (1995) Dominant Representations and a Markaracter Table for a Group of Finite Order. Theoretica Chimica Acta, 91, 291-314.
http://dx.doi.org/10.1007/bf01133077 - 11. Fujita, S. (1995) Subduction of Dominant Representations for Combinatorial Enumeration. Theoretica Chimica Acta, 91, 315-332.
http://dx.doi.org/10.1007/bf01133078 - 12. Kerber, A. (2002) Enumeration under Finite Group Action, Basic Tools, Results and Methods. MATCH Communications in Mathematical and in Computer Chemistry, 46,
- 13. Fujita, S. (2007) Combinatorial Enumeration of RS-Stereoisomers Itemized by Chirality, RS-Stereogenicity, and Sclerality. MATCH Communications in Mathematical and in Computer Chemistry, 58, 611-634.
- 14. Fujita, S. (2008) Numbers of Asymmetric and Pseudoasymmetric Centers in Enumeration of Achiral and Chiral Alkanes of Given Carbon Contents. MATCH Communications in Mathematical and in Computer Chemistry, 59, 509-554.
- 15. Fujita, S. (2009) Stereoisograms for Specifying Chirality and RS-Stereogenicity. A Versatile Tool for Avoiding the Apparent Inconsistency between Geometrical Features and RS-No-menclature in Stereochemistry. MATCH Communications in Mathematical and in Computer Chemistry, 61, 11-38.
- 16. Fujita, S. (2009) Substitution Criteria Based on Stereoisograms to Determine Prochirality and Pro-RS-Stereogenicity. MATCH Communications in Mathematical and in Computer Chemistry, 61, 39-70.
- 17. Fujita, S. (2009) Itemized Enumeration of Quadruplets of RS-Stereoisomers under the Action of RS-Stereoisomeric Groups. MATCH Communications in Mathematical and in Computer Chemistry, 61, 71-115.
- 18. Darafsheh, M.R. and Moghani, A. (2010) Full Non-Rigid Group Theory for Hexamethylethane. Asian Journal of Chemistry, 22, 1966-1972.
- 19. Moghani, A. (2009) A New Simple Method for Maturity of Finite Groups and Application to Fullerenes and Fluxional Molecules. Bulletin of the Chemical Society of Japan, 82, 1103-1106.
http://dx.doi.org/10.1246/bcsj.82.1103 - 20. Balasubramanian, K. (2004) Group Theory, Nuclear Spin Statistics and Tunneling Splittings of 1,3,5-Triamino-2,4,6-Trinitrobenzene. Chemical Physics Letters, 398, 15-21.
http://dx.doi.org/10.1016/j.cplett.2004.09.032 - 21. GAP (1995) Groups, Algorithms and Programming. Lehrstuhl de für Mathematik, RWTH, Aachen.
- 22. Moghani, A. (2010) Study of Symmetries on Some Chemical Nanostructure. Journal of Nano Research, 11, 7-11.
http://dx.doi.org/10.4028/www.scientific.net/JNanoR.11.7 - 23. Darafsheh, M.R. and Moghani, A. (2008) Markaracter Table and Q-Conjugacy Character Table for the Non-Rigid Group 1,3,5-Trimethylbenzene. Bulletin of the Chemical Society of Japan, 81, 979-982.
http://dx.doi.org/10.1246/bcsj.81.979 - 24. Littlewood, D.E. (2006) The Theory of Group Characters and Matrix Representations of Groups. AMS Chelsea Publishing, Rhode Island.
http://dx.doi.org/10.1090/chel/357 - 25. Safarisabet, S.A., Moghani, A. and Ghaforiadl, N. (2013) Study on the Q-Conjugacy Characters of Some Finite Groups. International Journal of Theoretical Physics, Group Theory, and Nonlinear Optics, 17, 57-62.
- 26. Moghani, A., Ghafooriadl, N. and Asadzadeh, S. (2011) Computational Algorithms for Topological Cycle Indices of Tetr-Butyl Alcohol by Computer Science. Defect and Diffusion Forum, 6, 39-44.
- 27. Moghani, A. (2016) Computing of Z-Valued Characters for the Projective Special Linear Group L2 (2m) and the Conway Group Co3. Journal of Mathematics Research, 8, 61-67.
http://dx.doi.org/10.5539/jmr.v8n3p61 - 28. Moghani, A. (2016) Study on the Q-Conjugacy Relations for the Janko Groups. Applications and Applied Mathematics, in Press.



