New Journal of Glass and Ceramics
Vol.3 No.1(2013), Article ID:27350,5 pages DOI:10.4236/njgc.2013.31009
Structural Changes by Thermal Treatment up to Glass Obtention of P2O5-Na2O-CaO-SiO2 Compounds with Bioglass Composition Types
![]()
CETMIC Centro de Tecnología de Recursos Minerales y Cerámica, Buenos Aires, Argentina.
Email: volzcris@netverk.com.ar
Received November 13th, 2012; revised December 13th, 2012; accepted December 23rd, 2012
Keywords: Glass Ceramics; Structure; Thermal Treatment
ABSTRACT
P2O5-Na2O-CaO-SiO2 compounds are the base of certain glass types. Glasses are solids obtained by fast cooling of melted mix of certain compounds. Different compositions give origin to many products with a variety of applications such as: bottles, coatings, windows, tools for chemical industry, laboratory equipment, optics, as bioceramics, etc. The aim of this work was to analyze structural changes of different composition in the P2O5-Na2O-CaO-SiO2 systems thermally treated up to 1250˚C, that is to say, before glass formation, by X ray diffraction. Intermediate and final developed phases up to 1100˚C thermal treatment in samples were generated as a function of Na2O/CaO (1 and 1.62) and P2O5/Na2O ratios (0, 0.2 and 0.245). Highand low-combeites, calcium and sodium-calcium silicate were found at the highest studied temperature.
1. Introduction
Certain glass compositions can bonds to bone and they are classified as bioactive glasses [1]. The main characteristic is that on the surface a biologically active hydroxyapatite is developed [2] which interacts with bone tissues. Generally, the compositions include P2O5, Na2O, CaO and SiO2 components. Nevertheless, the specific proportion of the components is highly important in order to have an adequate application as bioglass or as bioctive glass-ceramics. The first bioglass was obtained by Hench in 1977 and it is known as 45S5 Bioglass, where 45 is the percentage in wt of SiO2 and 5 is the molar ratio of Ca to P. Such bioglass had higher bioactivity than metals and dense ceramics [3,4]. The proportions of Na2O, CaO, SiO2 for obtaining bioglass with biocompatibility/bioactive behaviour (bone-bonding) is circumscriptive/restrictive to a central zone of the corresponding ternary diagram, including or not 6% P2O5 [1,5]. The devitrification [6] which can be done afterwards for obtaining bioactive glass-ceramics is also very important because it increases the mechanical resistance of the solid [7,8]. There are many studies about devitrification of glass, nevertheless it will be interesting to analyze the development of phases with temperature before bioglass formation. The aim of this work was to evaluate structural changes of the bioglass different compound mixes by thermal treatment previous to glass formation.
2. Materials and Methods
Powders of technical grade silicon oxide (SiO2), and reactive grade calcium carbonate (CaCO3), sodium carbonate (Na2CO3), and ammonium monobasic phosphate (NH4H2PO4) were used. The percentages of Ca, Na, Si and P expressed as oxides are shown in Table 1 and correspond to three prepared compositions for bioglass type. The composition of the sample named B6 was the same as the well known commercial bioglass B4505 [1]. The sample, B6N, was prepared taking into account the same amount of P2O5 and SiO2, with respect to B6, but changing the amount of Na2O y CaO, in higher and smaller of 6%, respectively. Finally, in the sample B0NC, the amount of CaO and SiO2 were the same as B6, the Na2O was 6% higher and P2O5 was not added. The mixtures
Table 1. Samples compositions*.

*wt%.
were treated at different temperatures in air atmosphere by using Pt crucibles.
The mixtures were heated at 500˚C, 800˚C, 900˚C, 1000˚C, 1100˚C and 1250˚C during one hour and then quenched. The heated samples were grinded M:100 (<74 μm) before to test.
The crystalline phases were identified by X-ray diffraction by using a Philips 3020 Goniometer with PW 3710 Controller, CuKα radiation (λ = 1.5405 Å) at 40 kV and 20 mA, and Ni filter and powder diffraction files were used for the identification of crystalline phases [9].The relative quantities of crystalline phases were obtained by measuring the highest intensity diffraction peaks of each compound and comparing each other.
3. Results and Discussion
X-ray diffractograms of three samples (B6, B6N, B0NC) after thermal treatment (500˚C, 800˚C, 900˚C, 1000˚C, 1100˚C) are shown in Figures 1-3. Modifications on the diffracttograms with an increase of temperature can be appreciated. The treatment up to 1250˚C caused development of visible amorphous phase (Figure 4).
Sample B6 treated up to 500˚C (Figure 1) showed the original carbonated and quartz reactives [10], whereas the initial ammonium dibasic phosphate component was not observed as a crystal phase and sodium phosphate (Na3PO4) was formed. Besides, the appearance of a double carbonate of sodium and calcium (NyereriteNa2Ca(CO3)2) took place due to the partial disappearance of the carbonates [11]. After 800˚C treatment, the sodium phosphate amount slightly increased, with an almost total reduction of initial sodium and calcium carbonates [12] and a reduction of quartz too, whose con-

Figure 1. Diffractograms of B6 treated at different temperatures. q: SiO2, cc: CaCO3; nc: Na2CO3; np: Na3PO4; ny: Na2Ca(CO3)2;c5ps: Ca5(PO4)2(SiO4); n15cs: Na15Ca3.84Si12O36; c: combeite.

Figure 2. Diffractograms of B6N treated at different temperatures. q: SiO2, cc: CaCO3; nc: Na2CO3; np: Na3PO4; ny: Na2Ca(CO3)2; n15cs: Na15Ca3.84Si12O36; n4cs: Na4Ca4Si6O18; co: CaO; c: combeite.

Figure 3. Diffractograms of B0NC treated at different temperatures. q: SiO2, cc: CaCO3; nc: Na2CO3; ny: Na2Ca(CO3)2; n2s: Na2SiO3; n2cs: Na2CaSiO4; n15cs: Na15Ca3.84Si12O36; n4cs: Na4Ca4Si6O18; co: CaO; c2s: Ca2SiO4.
tributed for sodium and calcium silicate
(Na15Ca3.84Si12O36) and calcium silicate-phosphate
(Ca5(PO4)2(SiO4)) appearance. Up to 900˚C, quartz suffered almost a total reduction, and sodium phosphate disappeared, both probably responsible for sodium and silicon contribution to the new phase named low combeite (Na4.2Ca2.8(Si6O18)). The Na15Ca3.84Si12O36 apparition was also more notable and calcium silicatephosphate phase was still present. The last three mentioned compounds were observed at 1000˚C. The disappearance of calcium silicate-phosphate, Ca5(PO4)2(SiO4), and Na15Ca3.84Si12O36 was produced by a subsequent increase in temperature up to 1100˚C, and then two phases were

Figure 4. Diffractograms of B6, B6N and B0NC treated at 1250˚C.
only shown, both belonging to the family of combeites (as it will be later discussed).
According to B6 and B6N composition, the main difference was Na2O/CaO ratio (Table 1). This way, B6N has a higher Na2O amount, and a corresponding CaO decrease of equivalent content in the starting mix. B6N treatment up to 500˚C (Figure 2) produced a similar behavior to the one corresponding to B6 at the same temperature. Up to 800˚C, the sodium phosphate presence, Na3PO4, and Na15Ca3.84Si12O36 were like B6 under the same heat treatment, while the quartz amount was lower, and carbonates were not seen.
Another important difference between B6 and B6N was due to the apparition of CaO and sodium-calcium silicate (Na4Ca4Si6O18). This developed phase has the same elements proportions than that found by Clupper and Hench [13] (Na2Ca2Si3O9) which, according to mentioned authors, presented bioactive behavior.
After 900˚C treatment, quartz, calcium oxide and Na4Ca4Si6O18 disappeared, while sodium phosphate was reduced and Na15Ca3.84Si12O36 was still present. Low combeite, Na4.2Ca2.8Si6O18, was the only new phase which presents a similar molecular formula to the phase which was gone, Na4Ca4Si6O18. Up to 1000˚C, sodium phosphate disappeared, the relative low combeite amount increased and high combeite appeared at the expense of Na15Ca3.84Si12O36 similar amount disapperance, as both molecular compositions are similar. After 1100˚C treatment, the still existing phases were the same as such presented in B6.
A detailed analysis showed that B6-1100 and B6N- 1100 samples had different amounts of two types of combeite: high and low. Differences between both phases in studied samples were determined by comparison of the peaks placed on 23.8˚, 33.8˚ and 34.1˚ (2 theta) whose relative intensities are unlike between both samples. This way, the corresponding composition of B6 sample was about 60% of low combeite and 40% of high combeite, and 30% and 70%, respectively for B6N sample.
B0NC thermal treatment up to 500˚C had a similar behavior about phase transformations as previous samples (B6, B6N), (Figure 3), except for sodium phosphate formation, which was not present because of the phosphorous absence in original composition.
At 800˚C, raw material presence was not detected, and new phases were originated: CaO, Na4Ca4Si6O18, Na15Ca3.84Si12O36, Na2SiO3 and Na2CaSiO4. The last two phases were absent in samples B6 and B6N treated up to 500˚C. Treatment at 900˚C leaded to almost CaO extinction and sodium-calcium double silicate reduction, with sodium silicate, Na4Ca4Si6O18 and Na15Ca3.84Si12O36 increase and calcium silicate (Ca2SiO4) appearance (probably induced by calcium supply because of CaO disappearance). After rise in temperature up to 1000˚C, still existing phases were the same, except CaO, which was gone. At 1100˚C, Na4Ca4Si6O18, Na15Ca3.84Si12O36 and Ca2SiO4 were the majority phases. The most outstanding thing of this composition treated at the temperature previously mentioned, was that highand low-combeites were not formed. However, Na4Ca4Si6O18 and Na15Ca3.84Si12O36 phases, which we could call respectively lowand high-combeite forerunners, were present. This gives us an idea that formation processes of combeites are delayed due to phosphorous absence in the mix.
Figures 5-7 show the presence of phases between 800 and 1100˚C heat treatment in B6, B6N and B0NC, respectively, in arbitrary units. Comparative analyses of the figures allowed to highlight the following:
• High-combeite formation was observed at lower temperatures (1000˚C for B6N and between 1000 and 1100˚C for B6) when phosphorous/sodium ratio was higher (0.245 for B6 and 0.200 for B6N). This might suggest that, for a zero relation, as B0NC case, the probability of high combeite appearance would increase at temperatures higher than 1100˚C, without passing liquid temperature of the mix.
• Highand low-combeites would be formed by similar formulation sodium and calcium silicate, which could be called forerunners.
• Higher sodium content in the initial composition with phosphorous (B6 and B6N) originated a higher relative amount of Na15Ca3.84Si12O36 or high-combeite at high temperature (eg. 1000˚C - 1100˚C).
• As it was mentioned before, both samples (B6 and B6N) showed combeite phases.
• In general, disappearance or reduction of Na4Ca4Si6O18 or Na15Ca3.84Si12O36 causes the lowor high-combeite appearance, respectively.
• Na4Ca4Si6O18 phase was developed, which has the same minimal molecular formula than that found by Clupper and Hench, and could have bioactive behaviour.
• Phosphorous absence on B0NC originated, after high temperature treatments, two phases that were not present in the other three samples (B6 and B6N): Sodium silicate Na2SiO3, calcium silicate Ca2SiO4 and sodium-calcium silicate Na2CaSiO4. However, it seemed clear that any type of combeite could be formed.
4. Conclusions
Developed phases along the applied thermal treatments on both B6 and B6N samples were different, probably due to different Na2O/CaO ratio contents with 1 and 1.62 values for B6 and B6N samples, respectively, still holding the same values of initial phosphorous and silicon amount. The highest Na2O/CaO ratio value, 1.62, originnated intermediate CaO and Na4Ca4Si6O18, and also high-combeite at lower temperature and with higher final content respect to the sample with ratio equal to 1 (B6 sample). For the sample ratio value 1, phosphorous takes part of Ca5(PO4)2(SiO4) compound. This phase bioactiveity was shown as be good [14].
The Na4Ca4Si6O18 and Na15Ca3.84Si12O36 phases can be considered as forerunner phases for low- (Na4.2Ca2.8(Si6O18) and highcombeite (Na15.78Ca3(Si6O12)), respectively.
Na4Ca4Si6O18 could have bioactivity because its similarity to the phase Na2Ca2Si3O9 found by Clupper and Hench, which showed amorphous calcium phosphate formation on its surface when it was contacted with simulated body fluid.
The other relevant result was correlated to the P2O5/ Na2O ratio value of the samples. The combeite phases were formed at lower temperatures while such ratio increases. This way, combeite in B6 was found at a lower temperature than B6N, whose P2O5/Na2O ratio values were 0.245 and 0.200, respectively, and for zero ratio as shown B0NC sample, the combeite was not formed up to least 1100˚C. However, on B0NC, sodium and calcium silicates, which were previously called combeite forerunners, were present. This indicated that if it is possible to increase P2O5/Na2O ratio, the processes of combeites formation can be accelerated. Besides, it is possible to obtain these included phases at higher temperatures with phosphorous absence, always without passing liquid temperature.
Another thing is that this study can help to elucidate developed crystalline phases by devitrification of glass formulation of biomaterials, by using the found out ten-

Figure 5. Relative phases evolutions vs. temperature of B6 composition.

Figure 6. Relative phases evolutions vs. temperature of B6N composition.
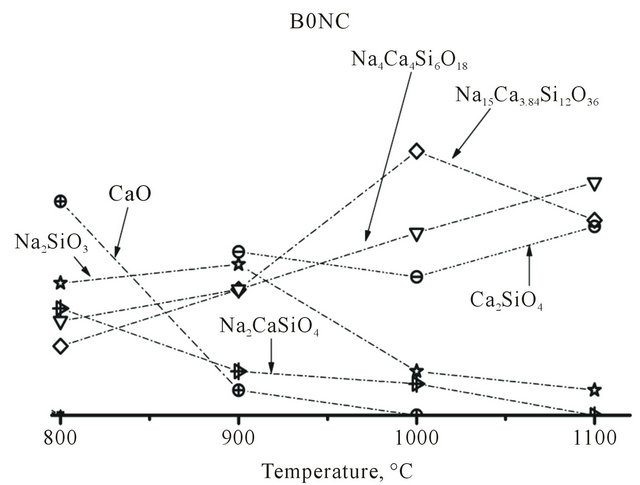
Figure 7. Relative phases evolutions vs. temperature of B0NC composition.
dencies.
Finally, future works will be done for evaluate the bioactive behavior highand low-combeite, the last which has the more similar structure as Na4Ca4Si6O18.
REFERENCES
- L. L. Hench, “Bioceramics: From Concept to Clinic,” Journal of American Ceramic Society, Vol. 74, No. 7, 1991, pp. 1487-1510. doi:10.1111/j.1151-2916.1991.tb07132.x
- O. H. Anderson and K. H. Karlsson, “Calcium Phosphate Formation at the Surface of Bioactive in vivo,” Journal of Non-Crystalline Solids, Vol. 119, No. 3, 1990, pp. 290- 296. doi:10.1016/0022-3093(90)90301-2
- L. L. Hench and H. A. Paschall, “Direct Chemical Bond of Bioactive Glass-Ceramic Materials to Bone and Muscle,” Journal of Biomedical Materials Research, Vol. 7, No. 3, 1973, pp. 25-42. doi:10.1002/jbm.820070304
- G. Piotrowski, L. L. Hench, W. C. Allen and G. L. Miller, “Mechanical Studies of Bone Bioglass Interfacial Bond,” Journal of Biomedical Materials Research, Vol. 9, No. 4, 1975, pp. 47-61. doi:10.1002/jbm.820090408
- J. Wilson, F. J. Schoen, G. H. Pigott and L. L. Hench, “Toxicology and Biocompatibility of Bioglasses,” Journal of Biomedical Material Research, Vol. 15, No. 6, 1981, pp. 805-817. doi:10.1002/jbm.820150605
- S. Agathopoulos, D. U. Tulyaganov, J. M. G. Ventura, S. Kannan, A. Saranti, M. A. Karakassides and J. M. F. Ferreira, “Structural Analysis and Devitrification of Glasses Based on the CaO-MgO-SiO2 System with B2O3, Na2O, CaF2 and P2O5 Additives,” Journal of Non-Crystalline Solids, Vol. 352, No. 4, 2006, pp. 322-328. doi:10.1016/j.jnoncrysol.2005.12.003
- D. Williams, “An Introduction to Medical and Dental Materials. Concise Encyclopedia of Medical & Dental Materials,” 2nd Edtion, The MIT Press, Cambridge, 1990.
- P. N. De Aza, A. H. De Aza, P. Pena and S. De Aza, “Bioactive Glasses and Glass-Ceramics,” Boletin de la Sociedad Española de Cerámica y Vidrio, Vol. 46, No. 2, 2007, pp. 45-55. doi:10.3989/cyv.2007.v46.i2.249
- Joint Committee on Powder Diffraction Standards, JCPDS.
- G. W. Brindley and G. Brown, “Crystal Structures of Minerals and Their X-Ray Identification. Mineralogical Society,” Mineralogical Society and the Royal Society, Melbourne, 1980.
- R. C. Roth, T. Negas and L. Hook, “Phase Diagrams for Ceramists,” American Ceramic Society Inc., Westerville, 1975.
- R. C. Mackenzie, “Differential Thermal Analysis,” Academic Press, New York, 1970.
- D. C. Clupper and L. L. Hench, “Crystallization Kinetics of Tape Cast Bioactive Glass 45S5,” Journal Non-Crystalline Solid, Vol. 318, No. 1-2, 2003, pp. 43-48. doi:10.1016/S0022-3093(02)01857-4
- W. Lu, W. Duan; Y. Guo and C. Ning, “Mechanical Properties and in Vitro Bioactivity of Ca5(PO4)2SiO4 Bioceramic” Journal of Biomaterials Applications, Vol. 26, No. 6, 2012, pp. 637-650. doi:10.1177/0885328210383599

