 American Journal of Analytical Chemistry, 2011, 2, 989-995 doi:10.4236/ajac.2011.28116 Published Online December 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Characterization of Chemical Constituents of Luffa operculata (Cucurbitaceae) Cléia Rocha de Sousa Feitosa1,3, Robério Costa da Silva1, Raimundo Braz-Filho2, Jane Eire Silva Alencar de Menezes4, Sônia Maria Costa Siqueira5, Francisco José Queiroz Monte1 1Programa de Pós-Graduação em Química-DQOI-CC, Universidade Federal do Ceará, Fortaleza, Brazil 2Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brazil 3Universidade Estadual do Ceará, Faculdades de Educação de Crateús, Fortaleza, Brazil 4Universidade Estadual do Ceará, Itapipoca Fortaleza, Brazil 5Universidade Estadual do Ceará, e campos do Itaperi, Fortaleza, Brazil E-mail: fmonte@dqoi.ufc.br Received August 4, 2011; revised September 15, 2011; accepted September 28, 2011 Abstract A mixture of new ceramides (1, 2, 3, 4 and 5) together with a binary mixture of ceramides with long chain alkyl (6 and 7), triterpenoid (10) and steroids (11 and 12) have been isolated from bark of the fruits and of the stems of Luffa operculata (Cucurbitaceae). The structures were elucidated by comprehensive spectro- scopic analysis including 1H and 13C NMR, DEPT (distortionless enhancement by polarization transfer), COSY (correlated spectroscopy), HMQC (heteronuclear multiple quantum coherence), HMBC (heteronu- clear multiple bond connectivity), IR (infrared), HR-ESI-MS (electrospray ionization-high resolution mass spectra) and LR-MS (low resolution electron ionization mass spectra) experiments. All the ceramides are reported for the first time in Cucurbitaceae and this is the first report of the rare triterpene 10 isolated from Luffa operculata. The ceramides 6 and 7 showed a high acetylcholine esterase inhibitory effect. Keywords: Cucurbitaceae, Ceramides, Triterpenes, Spectroscopic Data 1. Introduction As a part of our continuing chemical studies on plants of Cucurbitaceae family, we have investigated the bark of the fruits and the stems of Luffa operculata specie. L. operculata Cogn. (Cucurbitaceae), locally known as “cabacinha”, a perennial shrub widely distributed in Northeastern Brazil where an aqueous solution from its fruits has been used in popular medicine for the treat- ment of sinusitis [1]. In the previous paper [2], we re- ported the isolation and structure elucidation of triper- penes cucurbitane type from these fruits. In this paper, we report the isolation and structure elucidation of cera- mides (1-5, 6 and 7), triterpene oleanane type (10) and steroids (11 and 12) from the bark of the fruits and stems of this plant. In plants, recent studies indicate that cera- mides may be involved in signal transduction, membrane stability, host-pathogen interactions, and stress responses [3].The compound 6 and 7, as well as the steroids mix- ture (12), showed an acetylcholine esterase inhibitory effect. Inhibition of acetylcholinesterase (AchE) is used as a strategy for the treatment of Alzheimer's disease (AD), a neurodegenerative malady characterized by cog- nitive impairment and personality changes. One of the most promising approaches for treating this disease is to enhance the acetycholine level in rain using acetylcho- line esterase (AChE) plant-derived inhibitors [4]. In this work we report an evaluation of the cholinesterase inhi- bition effect of the ceramides 6 and 7 following the methodology of Elmann, adapted by Rhee [5] for the layer chromatography (TLC). 2. Materials and Method 2.1. General Procedures 1H and 13C NMR spectra were recorded on Bruker DPX 300 and DRX 500 spectrometers in CDCl3, with TMS as an internal standard. DEPT and all 2D experiments (COSY, HMQC and HMBC) with standard Bruker pulse sequence; IR spectra were carried out on Perkin-Elmer 2000 series FT-IR; electrospray ionization mass spectra 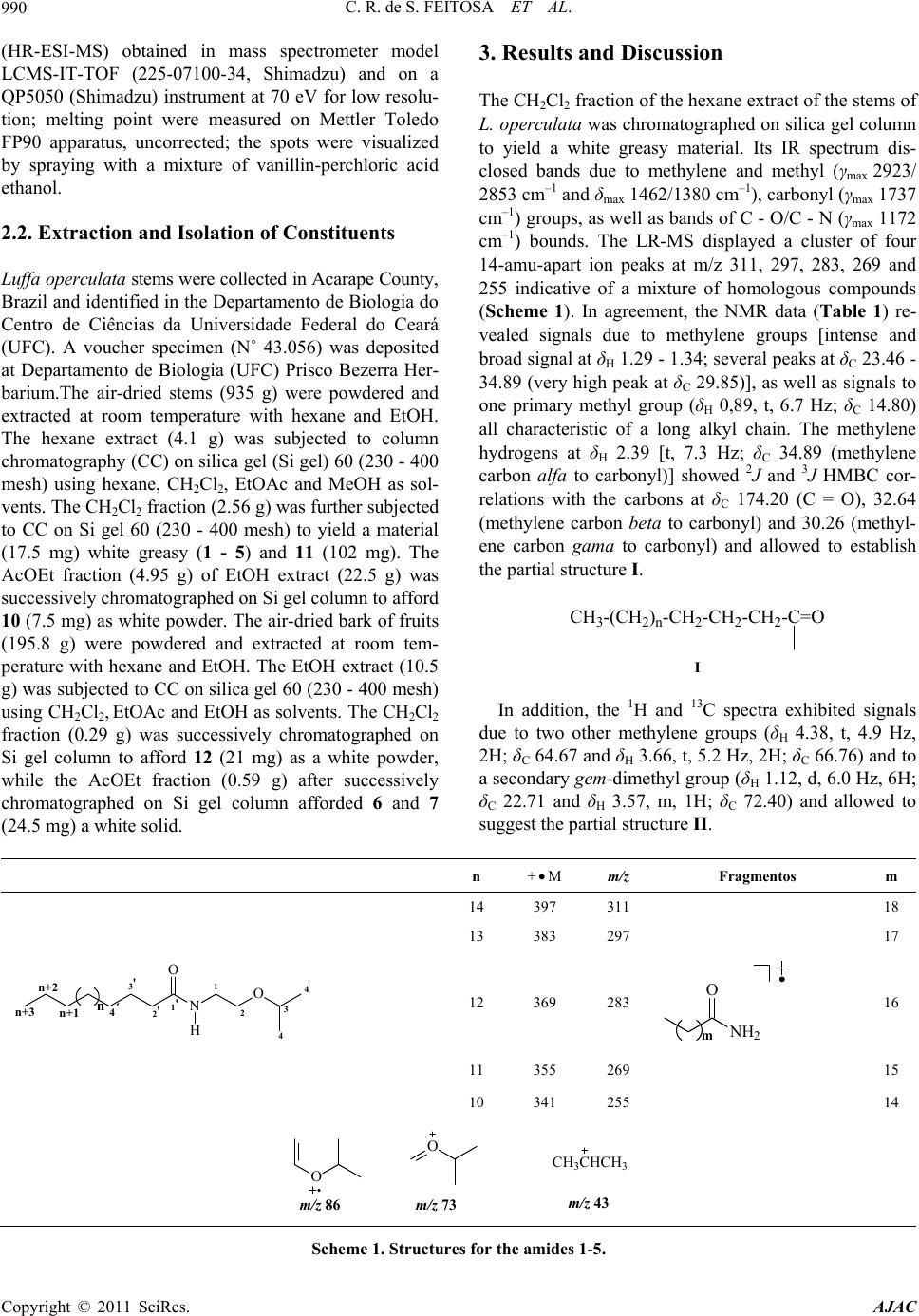 C. R. de S. FEITOSA ET AL. 990 (HR-ESI-MS) obtained in mass spectrometer model LCMS-IT-TOF (225-07100-34, Shimadzu) and on a QP5050 (Shimadzu) instrument at 70 eV for low resolu- tion; melting point were measured on Mettler Toledo FP90 apparatus, uncorrected; the spots were visualized by spraying with a mixture of vanillin-perchloric acid ethanol. 2.2. Extraction and Isolation of Constituents Luffa operculata stems were collected in Acarape County, Brazil and identified in the Departamento de Biologia do Centro de Ciências da Universidade Federal do Ceará (UFC). A voucher specimen (N˚ 43.056) was deposited at Departamento de Biologia (UFC) Prisco Bezerra Her- barium.The air-dried stems (935 g) were powdered and extracted at room temperature with hexane and EtOH. The hexane extract (4.1 g) was subjected to column chromatography (CC) on silica gel (Si gel) 60 (230 - 400 mesh) using hexane, CH2Cl2, EtOAc and MeOH as sol- vents. The CH2Cl2 fraction (2.56 g) was further subjected to CC on Si gel 60 (230 - 400 mesh) to yield a material (17.5 mg) white greasy (1 - 5) and 11 (102 mg). The AcOEt fraction (4.95 g) of EtOH extract (22.5 g) was successively chromatographed on Si gel column to afford 10 (7.5 mg) as white powder. The air-dried bark of fruits (195.8 g) were powdered and extracted at room tem- perature with hexane and EtOH. The EtOH extract (10.5 g) was subjected to CC on silica gel 60 (230 - 400 mesh) using CH2Cl2, EtOAc and EtOH as solvents. The CH2Cl2 fraction (0.29 g) was successively chromatographed on Si gel column to afford 12 (21 mg) as a white powder, while the AcOEt fraction (0.59 g) after successively chromatographed on Si gel column afforded 6 and 7 (24.5 mg) a white solid. 3. Results and Discussion The CH2Cl2 fraction of the hexane extract of the stems of L. operculata was chromatographed on silica gel column to yield a white greasy material. Its IR spectrum dis- closed bands due to methylene and methyl (γmax 2923/ 2853 cm–1 and δmax 1462/1380 cm–1), carbonyl (γmax 1737 cm–1) groups, as well as bands of C - O/C - N (γmax 1172 cm–1) bounds. The LR-MS displayed a cluster of four 14-amu-apart ion peaks at m/z 311, 297, 283, 269 and 255 indicative of a mixture of homologous compounds (Scheme 1). In agreement, the NMR data (Table 1) re- vealed signals due to methylene groups [intense and broad signal at δH 1.29 - 1.34; several peaks at δC 23.46 - 34.89 (very high peak at δC 29.85)], as well as signals to one primary methyl group (δH 0,89, t, 6.7 Hz; δC 14.80) all characteristic of a long alkyl chain. The methylene hydrogens at δH 2.39 [t, 7.3 Hz; δC 34.89 (methylene carbon alfa to carbonyl)] showed 2J and 3J HMBC cor- relations with the carbons at δC 174.20 (C = O), 32.64 (methylene carbon beta to carbonyl) and 30.26 (methyl- ene carbon gama to carbonyl) and allowed to establish the partial structure I. CH3-(CH2)n-CH2-CH2-CH2-C=O I In addition, the 1H and 13C spectra exhibited signals due to two other methylene groups (δH 4.38, t, 4.9 Hz, 2H; δC 64.67 and δH 3.66, t, 5.2 Hz, 2H; δC 66.76) and to a secondary gem-dimethyl group (δH 1.12, d, 6.0 Hz, 6H; δC 22.71 and δH 3.57, m, 1H; δC 72.40) and allowed to suggest the partial structure II. n +M m/z Fragmentos m 14 397 311 18 13 383 297 17 n+3 3' n+1 n+2 4 4 1 2 1' 2'NO O H 3 4´ n 12 369 283 NH2 O m 16 11 355 269 15 10 341 255 14 CH3CHCH3 m/z 43 O m/z 86m/z 73 O Scheme 1. Structures for the amides 1-5. Copyright © 2011 SciRes. AJAC  C. R. de S. FEITOSA ET AL.991 Table 1. 13C (125 MHz) and 1H (500 MHz) data of compounds 1 - 5 in pyridine-d5, δ in ppm, J in Hz and multiplicities, in parenthesis. No. 1 - 5 C δC δH 2,3 JCH 1’ 174.20 - H-1; H-2’; H-3’ CH 3 72.40 3.57 (m) H-4; H-2 CH2 1 64.67 4.38 (t, 4.9) H-2 2 66.76 3.66 (t, 5.2) H-1 2’ 34.89 2.39 (t, 7.3) H-3´ 3’ 25.81 1.67 (m) H-2´ 4’ 30.26 1.29 - 1.34 (m) 5’-n 29.85 - 30.51 1.29 - 1.34 (m) - n + 1 32.64 1.29 - 1.34 (m) 3H-n + 3 n + 2 23.46 1.29 - 1.34 (m) - CH3 4 22.71 1.12 (d, 6.0) - n + 3 14.80 0.89 (t, 6.7) H-2; H-4 N-CH2-CH2-O-CH (C H 3)2 II In the 1H - 1H COSY spectrum, the mutual correla- tions between the signals at δH 4.38 and 3.66, as well as between the signals at δH 1.12 and 3.57, supported the fragment II. The linkage of theses partial structures (I and II) to each other was based on additional long-range connectivities observed between the hydrogens at δH 4.38 (-NCH2-) and the carbon atom in δC 174.20 (C = O) in the 1H-13C HMBC spectrum and resulted in the gen- eral structure III, corresponding to amides mixture. Oth- ers correlations in the HMBC spectrum were assigned in the Table 1. n+3 3' n+1 n+2 4 4 1 2 1' 2'NO O H 3 4´ n III Finally, the fragments in the mass spectrum due to the peaks at m/z 311, 297, 283, 269 and 255 obtained by McLafferty rearranjement from molecular ion peaks at m/z 397, 383, 369, 355 and 341 (observed at 395, 381, 367, 353 and 339, respectively), respectively, allowed the possible structures for the amides 1 - 5 (Scheme 1), unknown ceramides up to date. Others important peaks as m/z 86 (100%), 73 and 43 all are in agreement with the proposed structures (Scheme 1). 1 n = 14M+• 397N-(2-isopropoxy-ethyl)eicosamide 2 n = 13M+• 383N-(2-isopropoxy-ethyl)nonadecanamide 3 n = 12M+• 369N-(2-isopropoxy-ethyl)octadacanamide 4 n = 11M+• 355 N-(2-isopropoxy-ethyl)heptadacanamide 5 n = 10M+• 341N-(2-isopropoxy-ethyl)hexadecanamide The AcOEt fraction of the EtOH extract from barc fruit of L. operculata was chromatographed on silica gel column to afford a white solid whose high-resolution high-resolution ESI mass spectrometry in the negative mode displayed two 14-amu-apart quasimolecular ion peaks [M-H]- at m/z 736.5277 and 722.3396, indicative of a binary mixture of homologous compounds. The IR spectrum of this solid disclosed bands at 3336/3218, 2918/2849 and 1621 cm–1 suggestive of OH and/or NH, CH3/CH2 and C = O groups, respectively, as well as bands at 1070/1025 cm–1 of C-O/C-N bound; further Copyright © 2011 SciRes. AJAC 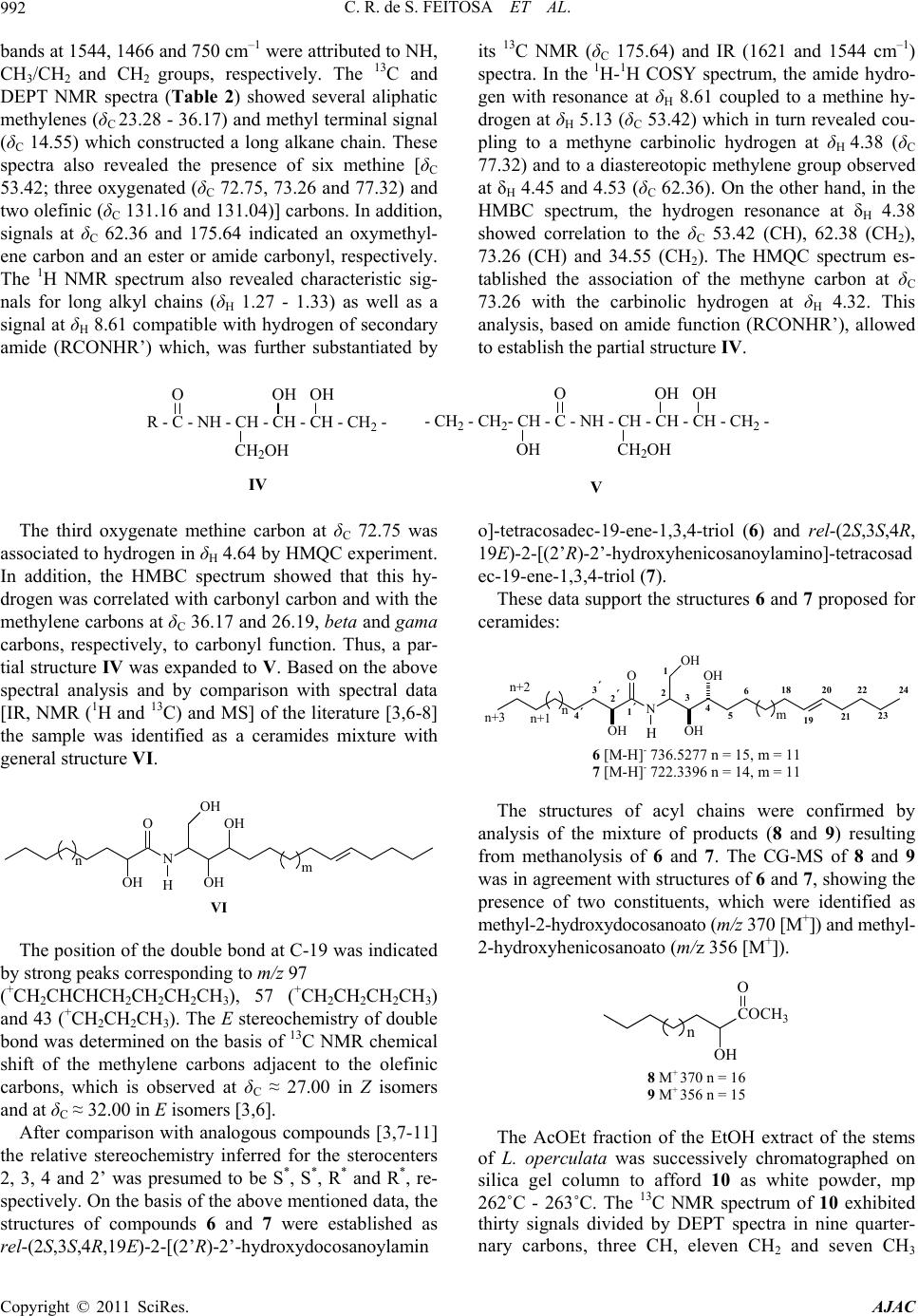 C. R. de S. FEITOSA ET AL. 992 bands at 1544, 1466 and 750 cm–1 were attributed to NH, CH3/CH2 and CH2 groups, respectively. The 13C and DEPT NMR spectra (Table 2) showed several aliphatic methylenes (δC 23.28 - 36.17) and methyl terminal signal (δC 14.55) which constructed a long alkane chain. These spectra also revealed the presence of six methine [δC 53.42; three oxygenated (δC 72.75, 73.26 and 77.32) and two olefinic (δC 131.16 and 131.04)] carbons. In addition, signals at δC 62.36 and 175.64 indicated an oxymethyl- ene carbon and an ester or amide carbonyl, respectively. The 1H NMR spectrum also revealed characteristic sig- nals for long alkyl chains (δH 1.27 - 1.33) as well as a signal at δH 8.61 compatible with hydrogen of secondary amide (RCONHR’) which, was further substantiated by its 13C NMR (δC 175.64) and IR (1621 and 1544 cm–1) spectra. In the 1H-1H COSY spectrum, the amide hydro- gen with resonance at δH 8.61 coupled to a methine hy- drogen at δH 5.13 (δC 53.42) which in turn revealed cou- pling to a methyne carbinolic hydrogen at δH 4.38 (δC 77.32) and to a diastereotopic methylene group observed at δH 4.45 and 4.53 (δC 62.36). On the other hand, in the HMBC spectrum, the hydrogen resonance at δH 4.38 showed correlation to the δC 53.42 (CH), 62.38 (CH2), 73.26 (CH) and 34.55 (CH2). The HMQC spectrum es- tablished the association of the methyne carbon at δC 73.26 with the carbinolic hydrogen at δH 4.32. This analysis, based on amide function (RCONHR’), allowed to establish the partial structure IV. R - C - NH - CH - CH - CH - CH2 - O CH2OH OH OH IV - CH2 - CH2- CH - C - NH - CH - CH - CH - CH2 - O CH2OH OH OH OH V The third oxygenate methine carbon at δC 72.75 was associated to hydrogen in δH 4.64 by HMQC experiment. In addition, the HMBC spectrum showed that this hy- drogen was correlated with carbonyl carbon and with the methylene carbons at δC 36.17 and 26.19, beta and gama carbons, respectively, to carbonyl function. Thus, a par- tial structure IV was expanded to V. Based on the above spectral analysis and by comparison with spectral data [IR, NMR (1H and 13C) and MS] of the literature [3,6-8] the sample was identified as a ceramides mixture with general structure VI. N O OH OH OH OH H m n H VI The position of the double bond at C-19 was indicated by strong peaks corresponding to m/z 97 (+CH2CHCHCH2CH2CH2CH3), 57 (+CH2CH2CH2CH3) and 43 (+CH2CH2CH3). The E stereochemistry of double bond was determined on the basis of 13C NMR chemical shift of the methylene carbons adjacent to the olefinic carbons, which is observed at δC ≈ 27.00 in Z isomers and at δC ≈ 32.00 in E isomers [3,6]. After comparison with analogous compounds [3,7-11] the relative stereochemistry inferred for the sterocenters 2, 3, 4 and 2’ was presumed to be S*, S*, R* and R*, re- spectively. On the basis of the above mentioned data, the structures of compounds 6 and 7 were established as rel-(2S,3S,4R,19E)-2-[(2’R)-2’-hydroxydo cosanoylamin o]-tetracosadec-19-ene-1,3,4-triol (6) and rel-(2S,3S,4R, 19E)-2-[(2’R)-2’-hydroxyhenicosanoylamino]-tetracosad ec-19-ene-1,3,4-triol (7). These data support the structures 6 and 7 proposed for ceramides: 2´ 1 23419 20 21 22 23 24 1´N OH O OH OH OH H nm n+1 n+2 5 618 3´ 4´ n+3 H 6 [M-H]- 736.5277 n = 15, m = 11 7 [M-H]- 722.3396 n = 14, m = 11 The structures of acyl chains were confirmed by analysis of the mixture of products (8 and 9) resulting from methanolysis of 6 and 7. The CG-MS of 8 and 9 was in agreement with structures of 6 and 7, showing the presence of two constituents, which were identified as methyl-2-hydroxydocosanoato (m/z 370 [M+]) and methyl- 2-hydroxyhenicosanoato (m/z 356 [M+]). COCH3 OH O n 8 M+ 370 n = 16 9 M+ 356 n = 15 The AcOEt fraction of the EtOH extract of the stems of L. operculata was successively chromatographed on silica gel column to afford 10 as white powder, mp 262˚C - 263˚C. The 13C NMR spectrum of 10 exhibited thirty signals divided by DEPT spectra in nine quarter- nary carbons, three CH, eleven CH2 and seven CH3 Copyright © 2011 SciRes. AJAC 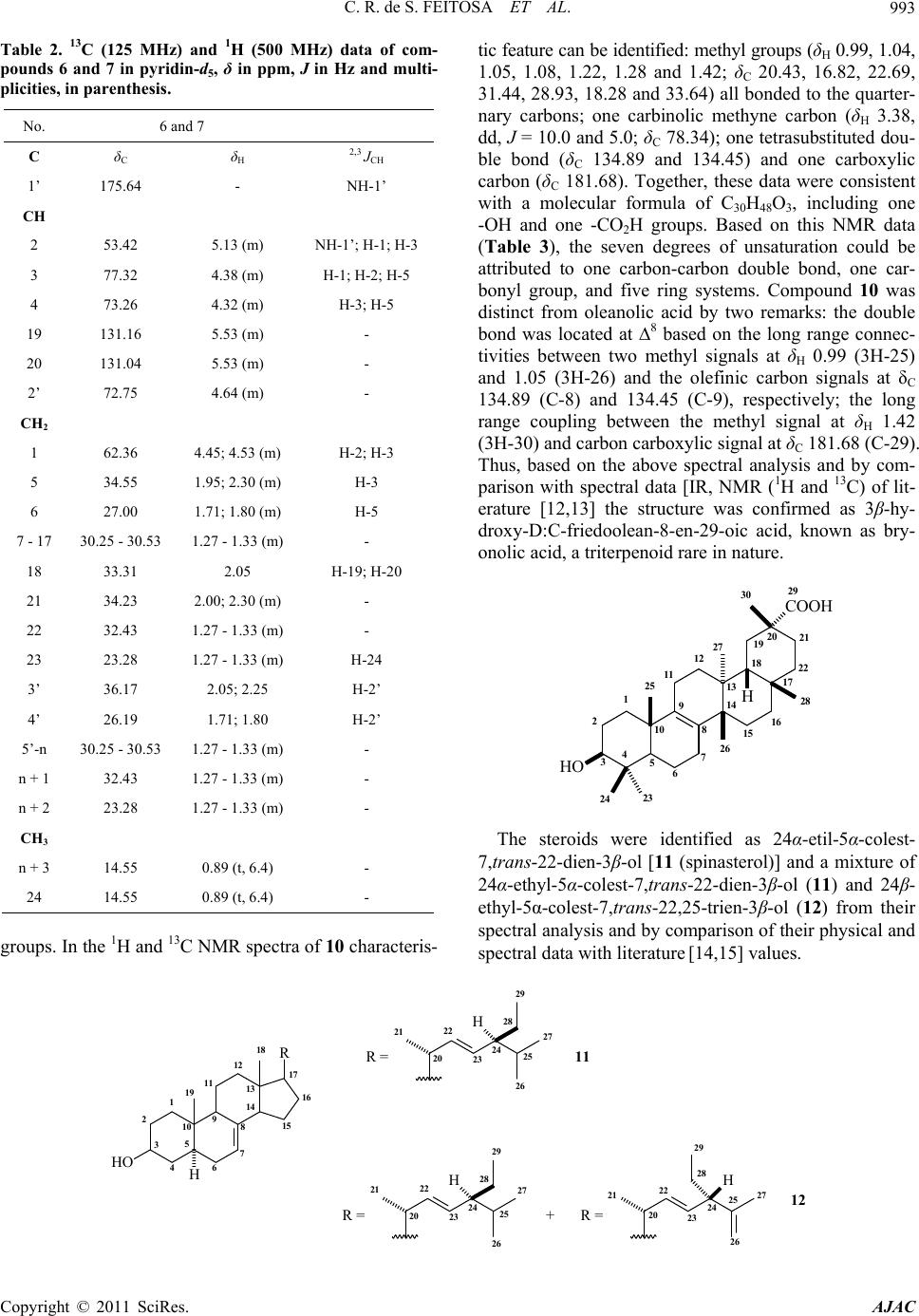 C. R. de S. FEITOSA ET AL.993 Table 2. 13C (125 MHz) and 1H (500 MHz) data of com- pounds 6 and 7 in pyridin-d5, δ in ppm, J in Hz and multi- plicities, in parenthesi s. No. 6 and 7 C δC δH 2,3 JCH 1’ 175.64 - NH-1’ CH 2 53.42 5.13 (m) NH-1’; H-1; H-3 3 77.32 4.38 (m) H-1; H-2; H-5 4 73.26 4.32 (m) H-3; H-5 19 131.16 5.53 (m) - 20 131.04 5.53 (m) - 2’ 72.75 4.64 (m) - CH2 1 62.36 4.45; 4.53 (m) H-2; H-3 5 34.55 1.95; 2.30 (m) H-3 6 27.00 1.71; 1.80 (m) H-5 7 - 17 30.25 - 30.53 1.27 - 1.33 (m) - 18 33.31 2.05 H-19; H-20 21 34.23 2.00; 2.30 (m) - 22 32.43 1.27 - 1.33 (m) - 23 23.28 1.27 - 1.33 (m) H-24 3’ 36.17 2.05; 2.25 H-2’ 4’ 26.19 1.71; 1.80 H-2’ 5’-n 30.25 - 30.53 1.27 - 1.33 (m) - n + 1 32.43 1.27 - 1.33 (m) - n + 2 23.28 1.27 - 1.33 (m) - CH3 n + 3 14.55 0.89 (t, 6.4) - 24 14.55 0.89 (t, 6.4) - groups. In the 1H and 13C NMR spectra of 10 characteris- tic feature can be identified: methyl groups (δH 0.99, 1.04, 1.05, 1.08, 1.22, 1.28 and 1.42; δC 20.43, 16.82, 22.69, 31.44, 28.93, 18.28 and 33.64) all bonded to the quarter- nary carbons; one carbinolic methyne carbon (δH 3.38, dd, J = 10.0 and 5.0; δC 78.34); one tetrasubstituted dou- ble bond (δC 134.89 and 134.45) and one carboxylic carbon (δC 181.68). Together, these data were consistent with a molecular formula of C30H48O3, including one -OH and one -CO2H groups. Based on this NMR data (Table 3), the seven degrees of unsaturation could be attributed to one carbon-carbon double bond, one car- bonyl group, and five ring systems. Compound 10 was distinct from oleanolic acid by two remarks: the double bond was located at ∆8 based on the long range connec- tivities between two methyl signals at δH 0.99 (3H-25) and 1.05 (3H-26) and the olefinic carbon signals at δC 134.89 (C-8) and 134.45 (C-9), respectively; the long range coupling between the methyl signal at δH 1.42 (3H-30) and carbon carboxylic signal at δC 181.68 (C-29). Thus, based on the above spectral analysis and by com- parison with spectral data [IR, NMR (1H and 13C) of lit- erature [12,13] the structure was confirmed as 3β-hy- droxy-D:C-friedoolean-8-en-29-oic acid, known as bry- onolic acid, a triterpenoid rare in nature. HO COOH H 1 2 3456 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The steroids were identified as 24α-etil-5α-colest- 7,trans-22-di en-3β-ol [11 (spinasterol)] and a mixture of 24α-ethyl-5α-colest-7,trans-22-dien-3β-ol (11) and 24β- ethyl-5α-colest-7,trans-22,25-trien-3β-ol (12) from their spectral analysis and by comparison of their physical and spectral data with literature [14,15] values. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 HO R H 27 20 21 22 23 24 25 26 28 29 H R = 29 28 20 21 22 23 24 25 26 27 R = H + 29 20 21 22 23 24 25 26 27 28 H R = 11 12 Copyright © 2011 SciRes. AJAC 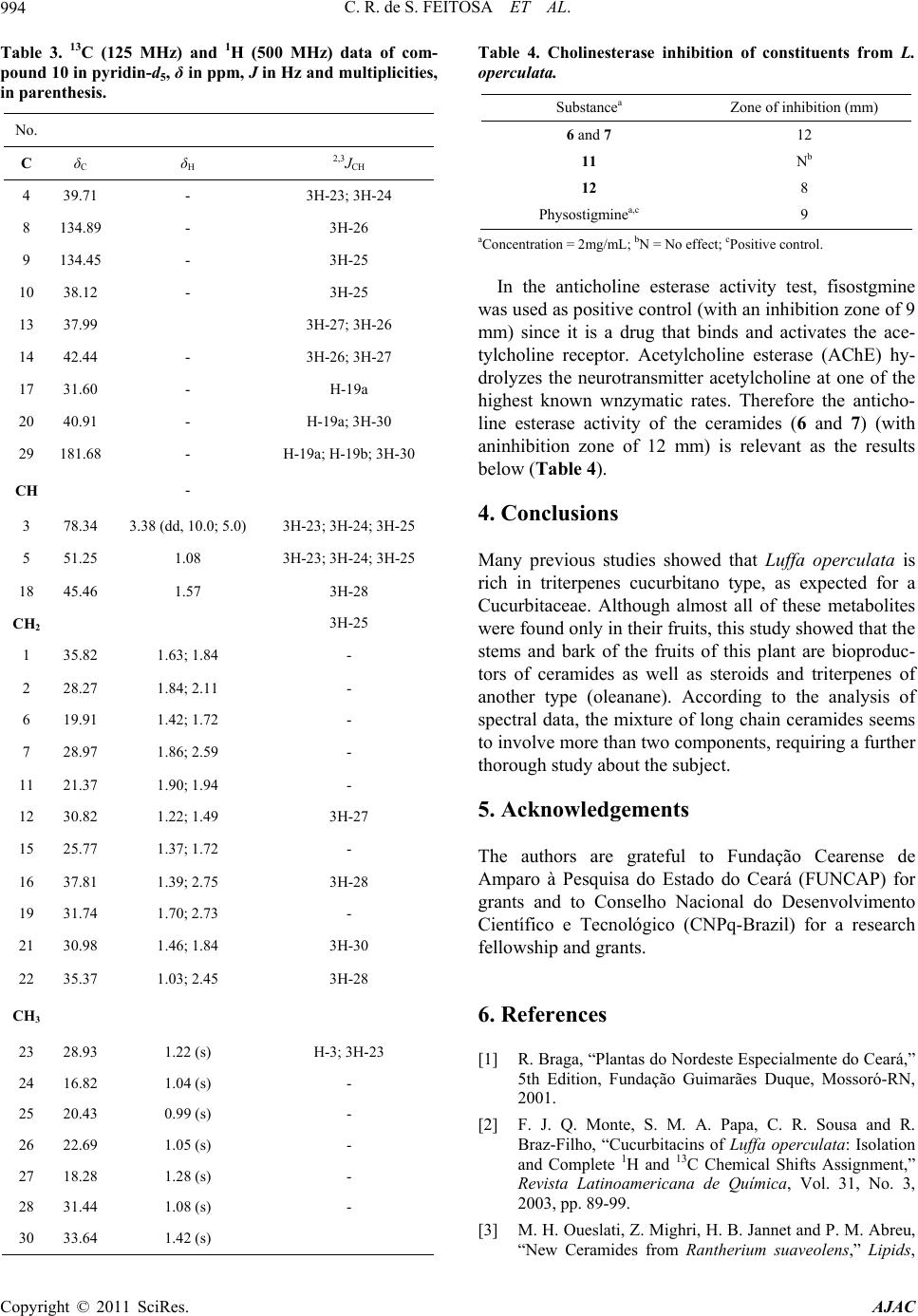 C. R. de S. FEITOSA ET AL. 994 Table 3. 13C (125 MHz) and 1H (500 MHz) data of com- pound 10 in pyridin-d5, δ in ppm, J in Hz and multiplicities, in parenthesis. No. C δC δH 2,3JCH 4 39.71 - 3H-23; 3H-24 8 134.89 - 3H-26 9 134.45 - 3H-25 10 38.12 - 3H-25 13 37.99 3H-27; 3H-26 14 42.44 - 3H-26; 3H-27 17 31.60 - H-19a 20 40.91 - H-19a; 3H-30 29 181.68 - H-19a; H-19b; 3H-30 CH - 3 78.34 3.38 (dd, 10.0; 5.0) 3H-23; 3H-24; 3H-25 5 51.25 1.08 3H-23; 3H-24; 3H-25 18 45.46 1.57 3H-28 CH2 3H-25 1 35.82 1.63; 1.84 - 2 28.27 1.84; 2.11 - 6 19.91 1.42; 1.72 - 7 28.97 1.86; 2.59 - 11 21.37 1.90; 1.94 - 12 30.82 1.22; 1.49 3H-27 15 25.77 1.37; 1.72 - 16 37.81 1.39; 2.75 3H-28 19 31.74 1.70; 2.73 - 21 30.98 1.46; 1.84 3H-30 22 35.37 1.03; 2.45 3H-28 CH3 23 28.93 1.22 (s) H-3; 3H-23 24 16.82 1.04 (s) - 25 20.43 0.99 (s) - 26 22.69 1.05 (s) - 27 18.28 1.28 (s) - 28 31.44 1.08 (s) - 30 33.64 1.42 (s) Table 4. Cholinesterase inhibition of constituents from L. operculata. Substancea Zone of inhibition (mm) 6 and 7 12 11 Nb 12 8 Physostigminea,c 9 aConcentration = 2mg/mL; bN = No effect; cPositive control. In the anticholine esterase activity test, fisostgmine was used as positive control (with an inhibition zone of 9 mm) since it is a drug that binds and activates the ace- tylcholine receptor. Acetylcholine esterase (AChE) hy- drolyzes the neurotransmitter acetylcholine at one of the highest known wnzymatic rates. Therefore the anticho- line esterase activity of the ceramides (6 and 7) (with aninhibition zone of 12 mm) is relevant as the results below (Table 4). 4. Conclusions Many previous studies showed that Luffa operculata is rich in triterpenes cucurbitano type, as expected for a Cucurbitaceae. Although almost all of these metabolites were found only in their fruits, this study showed that the stems and bark of the fruits of this plant are bioproduc- tors of ceramides as well as steroids and triterpenes of another type (oleanane). According to the analysis of spectral data, the mixture of long chain ceramides seems to involve more than two components, requiring a further thorough study about the subject. 5. Acknowledgements The authors are grateful to Fundação Cearense de Amparo à Pesquisa do Estado do Ceará (FUNCAP) for grants and to Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq-Brazil) for a research fellowship and grants. 6. References [1] R. Braga, “Plantas do Nordeste Especialmente do Ceará,” 5th Edition, Fundação Guimarães Duque, Mossoró-RN, 2001. [2] F. J. Q. Monte, S. M. A. Papa, C. R. Sousa and R. Braz-Filho, “Cucurbitacins of Luffa operculata: Isolation and Complete 1H and 13C Chemical Shifts Assignment,” Revista Latinoamericana de Química, Vol. 31, No. 3, 2003, pp. 89-99. [3] M. H. Oueslati, Z. Mighri, H. B. Jannet and P. M. Abreu, “New Ceramides from Rantherium suaveolens,” Lipids, Copyright © 2011 SciRes. AJAC  C. R. de S. FEITOSA ET AL.995 Vol. 40, No. 10, 2005, pp. 1075-1079. doi:10.1007/s11745-005-1472-3 [4] Viegas Junior, V. S. Bolzani and M. Furian, “Produtos Naturais Como Canditatos a Fármacos Úteis no Trata- mento do Mal de Alzheimer,” Química Nova, Vol. 27, No. 4, 2004, pp. 655-660. doi:10.1590/S0100-40422004000400021 [5] I. K. Rhee, M. V. Meent, K. Ingkaninan and R. Verpoorte, “Screening for Acetylcholinesterase Inhibitors from Amaryllidaceae Using Silica Gel Thin-Layer Chroma- togramphy in Combination with Bioactivity Staining,” Journal of Chromatography, Vol. 915, No. 1, 2001, pp. 217-223. doi:10.1016/S0021-9673(01)00624-0 [6] F. Cateni, J. Zilic, G. Falsone, G. Scialino and E. Banfi, “New Cerebrosides from Euphorbia peplis, L.: Antim- icrobial Activity Evaluation,” Bioorganic & Medicinal Chemistry Letters, Vol. 13, No. 24, 2003, pp. 4345-4350. doi:10.1016/j.bmcl.2003.09.044 [7] M. L. Veras, “Estudo Químico e Farmacológico de Acnistus arborescens L. Schlecht e Physalis angulata L.,” Thesis, Programa de Química, DQOI, Universidade Federal do Ceará, Fortaleza, 2006. [8] A. I. V. Maia, M. L. Veras, R. Braz-Filho, N. P. Lopes, E. R. Silveira and O. D. L. Pessoa, “New Ceramides from Acnistus arborescens,” Journal of Brazilian Chemical Society, Vol. 21, No. 5, 2010, pp. 867-871. doi:10.1590/S0103-50532010000500014 [9] F. Ramos, Y. Takaishi, K. Kawazoe, C. Osorio, C. Duque, R. Acuña, Y. Fujimoto, M. Sato, M. Okamoto, T. Oshi- kawa and S. U. Ahmed, “Immunosuppressive Diace- tylenes, Ceramides and Cerebrosides from Hydrocotyle leucocephala,” Phytochemistry, Vol. 67, No. 11, 2006, pp. 1143-1150. doi:10.1016/j.phytochem.2006.03.004 [10] L. P. Sandjo, P. Hannewald, M. Yemloul, G. Kirsch and B. T. Ngadjui, “ Triumfettamide and Triumfettoside Ic, Two Ceramides and Other Secondary Metabolites from the Stems of Wild Triumfetta cordifolia A. Rich. (Tili- aceae),” Helvetica Chimica Acta, Vol. 91, No. 7, 2008, pp. 1326-1335. doi:10.1002/hlca.200890144 [11] C. F. S. Christophe, F. S. Kouam, S. F. Kouam, M. P. P. Herve, I. K. Simo, B. T. Ngadjui, I. R. Green and K. Krohn, “Benjaminamide: A New Ceramide and Other Com- pounds from the Twigs of Ficus benjamina (Moraceae),” Biochemical Systematics and Ecology, Vol. 36, No. 3, 2008, pp. 238-243. doi:10.1016/j.bse.2007.08.014 [12] F. Khallouki, W. E. Hull and R. W. Owen, “Characteriza- tion of a Rare Triterpenoid and Minor Phenolic Com- pounds in the Root Bark of Anisophyllea dichostyla R. Br.,” Food and Chemical Toxicolgy, Vol. 47, No. 8, 2009, pp. 2007-2012. doi:10.1016/j.fct.2009.05.018 [13] C. Honda, K. Suwa, S. Takeyama and W. Kamisako, “Relative Population of S-Form and F-Form Conformers of Bryonolic Acid and Its Derivates in Equilibrium in CDCl3 Solutions,” Chemical & Pharmaceutical Bulletin, Vol. 50, No. 4, 2002, pp. 467-474. doi:10.1248/cpb.50.467 [14] N. Jahan, W. Ahmed and A. Malik, “New Steroidal Gly- cosides from Mimusops elengi,” Journal of Natural Pro- ducts, Vol. 58, No. 8, 1995, pp. 1244-1247. doi:10.1021/np50122a014 [15] W. Y. Kang and X. J. Xu, “Structure of a New Xanthone from Securidaca inappendicuata,” Chemistry of Natural Compounds, Vol. 44, No. 4, 2008, pp. 432-434. doi:10.1007/s10600-008-9089-9 Copyright © 2011 SciRes. AJAC
|