Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2011, 2, 847-850 doi :1 0.4236/ aj ps.2011 .26100 Publ i s hed Online December 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 847 Total Phenolic Content and Antioxidant Activity of Standardized Extracts from Leaves and Cell Cultures of Three Callistemon Species Mohamed I. S. Abdelhady1,3*, Amira Abdel Motaal2, Ludger Beerhues3 1Pharmaco gnos y Dep artment, Facu lty o f Phar macy, Helwan Univer sity, Cairo , Egypt ; 2Pharma cogn osy Dep artmen t, F acult y of P harma cy, Cairo University, Cairo, Egypt; 3Institut für Pharmazeutische Biologie, Technische Universität Braunschweig, Braunschweig, Ger- many. Email: *mohibrahem@yahoo.com Received S eptember 26th, 2010; revised October 24th, 2011; accepted November 5th, 2011. ABSTRACT A comparative study was carried out with ethanolic (80%) extracts from leaves and cell cultures of three Callistemon species, namely C. lanceolatus (CL), C. viridiflorous (CV), and C. comboynensis (CC). Cell suspensions of the three species were grown in liquid Murashige and Skoog (MS) medium (100 ml) supplemented with 0.9 mg·g–1 kinetin in combination with 1.1 mg·g–1 NAA. The CL leaf extract was standardized to contain the highest amount of phenolics (104 ± 2.0 mg·g–1), followed b y CC (95.8 ± 1.2 mg·g–1) and CV (79.8 ± 4.6 mg·g–1). On the other hand, cell cultures of CV contained more phenolics (14.9 ± 0.6 mg·g–1) than those of the other two species, CL an d CC, which contained 12.2 ± 0.16 and 9.12 ± 0.16 mg·g–1, respectively. Nevertheless, CV leaf extract exhibited the highest antioxidant activity (91.4% ± 0.4%) at a concentration of 1000 µg·ml–1, comparable to 100 µg·ml–1 gallic acid (90.8% ± 1.5%). Keywords: Callistemon, Phenolic Content, Antioxidant Activity, Callus, Cell Cultures 1. Introduction The genus Callis temon (Myrtaceae) contains 34 species of beautiful evergreen shrubs and small trees. The majo- rity of the Callistemon species is endemic to the more temperate regions of Australia, four species are found in New Caledonia and seven species have been introduced to India as ornamental trees [1]. They are commonly kno- wn as bottle brushes because of their cylindrical brush- like flowers resembling the traditional bottle brush. C. lanceolatus, also named C. citrinus, is a well- known shrub. Leaves of this plant are used as a tea sub- stitute and have a refreshing flavor. Many phenolic com- pounds of this plant have been identified [2]. Due to the over-exploitation for its volatile oil and secondary me- tabolites, there is a great need to develop alternative stra- tegies of conservation and industrial production of the bioactive compounds from this plant [3]. No reports of works were found concerning the other two species, C. viridiflorou s and C. comboynensis. In vitro cultures have the potential to form secondary metabolites and to exhibit bioactivity comparable to the original plant [4,5]. Cultured cells may serve industrial purposes, e.g. by immobilization of cells in a matrix for use in bioreactors. Besides the genetic potential of the donor plant for callus induction and growth of this callus in in vitro cultures, a medium containing sufficient nu- trients, such as the preferred MS medium, is required [4]. Antioxidants play an important role in the prevention of human diseases. Antioxidant compounds may function as free radical scavengers, complexing agents for pro- oxidant metals, as well as reducing agents and quenchers of singlet oxygen formation [6-8]. Antioxidants are often used in oils and fatty foods to retard their autoxidation. Therefore, the importance of the search for natural anti- oxidants has greatly increased in recent years [9]. A fo- cus is on plant-derived polyphenols because of their po- tential antioxidant and antimicrobial properties. Phenolic compounds exhibit considerable free-radical scavenging activity, which is determined by their reactivity as hy- drogen- or electron- donating agents, their reactivity with other antioxidants and their metal chelating properties, as well as the stability of the resulting antioxidant-derived radicals [10,11]. Our present work is a comparative study of leaves and  Total Phenolic Content and Antioxidant Activity of Standardized Extracts from Leaves and Cell Cultures of 848 Three Callistemon Species cell cultures of three Callistemon species with respect to their potential as antioxidant agents in relation to their total content of phenolic compounds. 2. Materials and Methods 2.1. Plan t Ma teria l Plants of three Callistemon species, C. lanceolatus (CL), C. viridiflorus (CV), and C. comboynensis (CC), were collected from a cultivated area in Cairo, Alexandria Road, Egypt. They were kindly authenticated by Prof. Dr. M. Gebali (Plant Taxonomy and Egyptian Flora De- partment, National Research Center, Giza, Egypt). A voucher specimen of each was deposited at the herbar- ium of the Pharmacognosy Department, Faculty of Phar- macy, Helwan University, Cairo, Egypt. 2.2. Calli and Cell Cultures Callus of CL was induced by a combination of 0.9 mg·L–1 kinetin and 1.1 mg·L–1 NAA [12]. Calli of CV and CC were similarly induced (the detailed methodo- logy will be published later on). Calli material (0.3 g each) were collected in the active growth phase (after the 15th day of subculture) and placed in 250 ml flasks con- taining 100 ml liquid MS medium supplemented with 0.9 mg·L–1 kinetin in combination with 1.1 mg·L–1 NAA. The resulting cell cultures of the three Callistemon spe- cies were incubated in a horizontal shaker at 100 rpm and 25˚C for 21 days. 2.3. Preparation of the Extracts The three cell suspension cultures were aseptically fil- tered and the cells dried in a vacuum oven at 40˚C, to- gether with the leaves of the three species. They were then macerated in 80% ethanol for two days, filtered and macerated for another two days. After filtration, they were concentrated under vacuum at 50˚C. 2.4. Evaluation of the Antioxidant Activity Determination of the free radical scavenging activity of the different extracts was carried out using a modified quantitative DPPH (1,1-diphenyl-2-picrylhydrazyl; Sigma- Aldrich, St. Louis, MO, USA) assay [13]. Various con- centrations of sample extracts in methanol were prepared (1000, 500, 250, and 100 µg·ml–1). Gallic acid was used as a positive control at concentrations of 100, 50, 25, and 10 µg·ml–1. Blan k sample s were r un using 1 ml metha nol in place of the test extract. One ml of 0.2 mM DPPH in methanol was added to 1 ml of the test solution, or stan- dard, plus 1 ml of methanol for dilution and allowed to stand at room temperature in a dark chamber for 30 min. The change in colour from deep violet to light yellow was then measured at 517 nm. Inhibition of free radical in percent (I%) was calculated according to the following equation: I%A0A1 A0100 , with A0 being the absorbance of the control reaction (containing all re- agents except for the extract) and A1 the absorbance of the extract. Measurements were carried out in triplicates. 2.5. Determination of Total Phenolic Content A spectrophotometric method after MacDonald [14] was adopted for the determination of total polyphenols in the prepared extracts. Folin-Ciocalteu reagent from Merck (Darmstadt, Germany) was used and a standard calibra- tion curve was prepared using different concentrations of gallic acid in methanol (0.025 - 0.400 mg·ml–1). Cell cul- ture and leaf extracts were prepared in methanol at a concentration of 0.06 g/3 ml and 0.06 g/20 ml, respec- tively. Absorbance was measured at 765 nm. For each sample, three replicate assays were performed. The total phenolic content was calculated as gallic acid equivalent (GAE) by the following equation: TCVM . T is the total phenolic content in mg·g–1 of the extracts as GAE, C is the concentration of gallic acid established from the calibration curve in mg·ml–1, V is the volume of the extract solution in ml and M is the weight of the ex- tract in g. 3. Results and Discussion 3.1. Phenolic Content of the Extracts Ethanolic (80%) extracts from the leaves and cell cul- tures of the three Callistemon species were standardized for their contents of phenolic compounds. The calibra- tion curve showed linearity for gallic acid in the range of 25 - 400 µg·ml–1, with a correlation coefficient (R2) of 0.999 (Figure 1). Leaves of CL contained the highest content of phenolics (104 ± 2.0 mg·g–1), followed by CC (95.8 ± 1.2 mg·g–1) and CV (79.8 ± 4.6 mg·g–1). On the other hand, cell cultures of CV were standardized to contain more phenolics (14.9 ± 0.6) than the cell suspen- sions of the other two species, CL and CC, which con- tained 12.2 ± 0.16 and 9.12 ± 0.16 mg·g–1, respectively (Figure 2). 3.2. Antioxidant Activity of the Extracts It is well known that there is a strong relationship be- tween total phenol content and antioxidant activity, as phenols possess strong scavenging ability for free radi- cals due to their hydroxyl groups. Therefore, the pheno- lic content of plants may directly contribute to their an- tioxidant action [11,15,16]. The standardized Callistemon extracts were assessed for their capacity to scavenge DDPH free radical along with gallic acid as a positive control. The antioxidant activity data are presented as percent of free radical inhi- Copyright © 2011 SciRes. AJPS 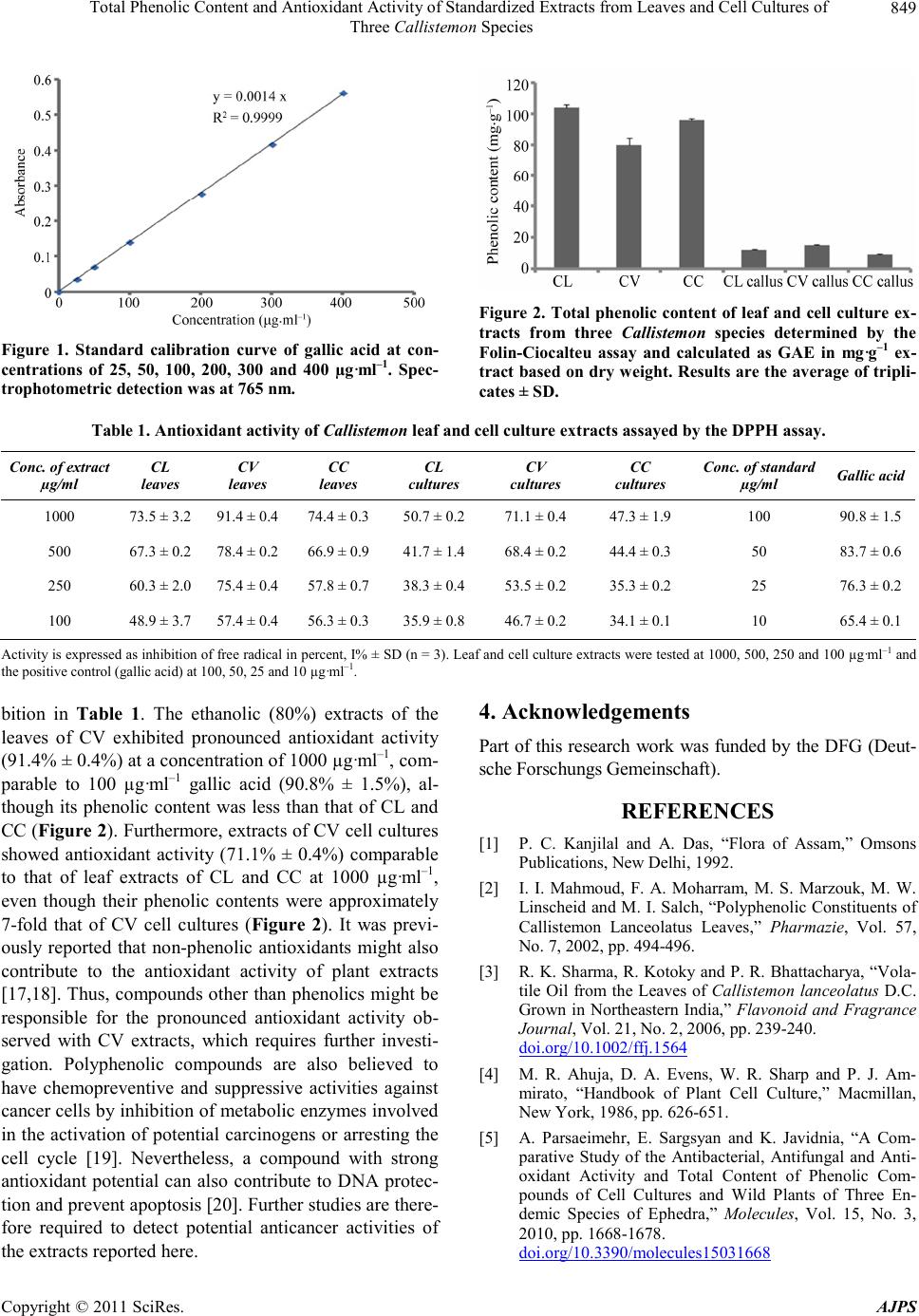 Total Phenolic Content and Antioxidant Activity of Standardized Extracts from Leaves and Cell Cultures of Three Callistemon Species Copyright © 2011 SciRes. AJPS 849 Figure 2. Total phenolic content of leaf and cell culture ex- tracts from three Callistemon species determined by the Folin-Ciocalteu assay and calculated as GAE in mg·g–1 ex- tract base d on dry we ight. Results are t he aver age of t ripl i- cates ± SD. Figure 1. Standard calibration curve of gallic acid at con- centrations of 25, 50, 100, 200, 300 and 400 µg·ml–1. Spec- trophotometric detection was at 765 nm. Table 1. Antioxidant activity of Callistemon leaf and cell culture extracts assayed by the DPPH assay. Conc. of ex tr a ct µg/ml CL leaves CV leaves CC leaves CL cultures CV cultures CC cultures Conc . of s tandard µg/ml Gallic acid 1000 73.5 ± 3.2 91.4 ± 0.4 74.4 ± 0.350.7 ± 0.2 71.1 ± 0.4 47.3 ± 1.9 100 90.8 ± 1.5 500 67.3 ± 0.2 78.4 ± 0.2 66.9 ± 0.941.7 ± 1.4 68.4 ± 0.2 44.4 ± 0.3 50 83.7 ± 0.6 250 60.3 ± 2.0 75.4 ± 0.4 57.8 ± 0.738.3 ± 0.4 53.5 ± 0.2 35.3 ± 0.2 25 76.3 ± 0.2 100 48.9 ± 3.7 57.4 ± 0.4 56.3 ± 0.335.9 ± 0.8 46.7 ± 0.2 34.1 ± 0.1 10 65.4 ± 0.1 Activity is expressed as inhibition of free radical in percent, I% ± SD (n = 3). Leaf and cell culture extracts were tested at 1000, 500 , 250 and 100 µg·ml–1 and the positive control (gallic acid) at 100, 50, 25 and 10 µg·ml–1. 4. Acknowledgements bition in Table 1. The ethanolic (80%) extracts of the leaves of CV exhibited pronounced antioxidant activity (91.4% ± 0.4%) at a concentration of 1000 µg·ml–1, com- parable to 100 µg·ml–1 gallic acid (90.8% ± 1.5%), al- though its phenolic content was less than that of CL and CC (Figure 2). Furthermore, extracts of CV cell cultures showed antioxidant activity (71.1% ± 0.4%) comparable to that of leaf extracts of CL and CC at 1000 µg·ml–1, even though their phenolic contents were approximately 7-fold that of CV cell cultures (Figure 2). It was previ- ously reported that non-phenolic antioxidants might also contribute to the antioxidant activity of plant extracts [17,18]. Thus, compounds other than phenolics might be responsible for the pronounced antioxidant activity ob- served with CV extracts, which requires further investi- gation. Polyphenolic compounds are also believed to have chemopreventive and suppressive activities against cancer cells by inhibition of metabo lic enzymes involved in the activation of potential carcinogens or arresting the cell cycle [19]. Nevertheless, a compound with strong antioxidant potential can also contribute to DNA protec- tion and prevent apoptosis [20]. Further studies are there- fore required to detect potential anticancer activities of the extracts reported here. Part of this research work was funded by the DFG (De ut- sche Forschungs G emeins chaft). REFERENCES [1] P. C. Kanjilal and A. Das, “Flora of Assam,” Omsons Publications, New Delhi, 1992. [2] I. I. Mahmoud, F. A. Moharram, M. S. Marzouk, M. W. Linscheid and M. I. Salch, “Polyphenolic Constituents of Callistemon Lanceolatus Leaves,” Pharmazie, Vol. 57, No. 7, 2002, pp. 494-496. [3] R. K. Sharma, R. Kotoky and P . R. Bhattach arya, “Vola- tile Oil from the Leaves of Callistemon lanceolatus D.C. Grown in Northeastern India,” Flavonoid and Fragrance Journal, Vol. 21, No. 2, 2006, pp. 239 -240. doi.org/10.1002/ffj.1564 [4] M. R. Ahuja, D. A. Evens, W. R. Sharp and P. J. Am- mirato, “Handbook of Plant Cell Culture,” Macmillan, New York, 1986, pp. 626-651. [5] A. Parsaeimehr, E. Sargsyan and K. Javidnia, “A Com- parative Study of the Antibacterial, Antifungal and Anti- oxidant Activity and Total Content of Phenolic Com- pounds of Cell Cultures and Wild Plants of Three En- demic Species of Ephedra,” Molecules, Vol. 15, No. 3, 2010, pp . 1668-167 8 . doi.org/10.3390/molecules15031668  Total Phenolic Content and Antioxidant Activity of Standardized Extracts from Leaves and Cell Cultures of 850 Three Callistemon Species [6] E. A. Bell, “The Possible Significance of Secondary Compounds in Plant,” In: E. A. Bell and B. V. Charlwood, Eds., Secondary Plant Products, Springer-Verlag, New York, 1980, pp.11-21. [7] F. Constable, O. L. Gamborg, W. G. W. Kurz and W. Steek, “Production of Secondary Metabolites in Plant Cell Cultures,” Planta Me dica, Vol. 25, 1974, pp. 158-165. do i.org/10. 1055/s-0028-1097926 [8] C. A. Rice-Evans, N. J. Miller and G. Paganaga, “Anti- oxidant Properties of Phenolic Compounds,” Trends in Plant Science, V ol. 2, No. 4, 1997, pp. 152-159. doi.org/10.1016/S1360-1385(97)01018-2 [9] C. Zollman and A. Vickers, “Complementary Medicine and the Patient,” British Medical Journal, Vol. 319, 1999, pp. 1486 -1494. [10] M. K. Ang-Lee, S . J. Moss and C. S. Yu an, “P.P . Herbal Medicines and Preoperative Care,” The Journal of the American Medical Association, Vol. 286, No. 2, 2001, pp. 208-216. doi.org/10.1001/jama.286.2.208 [11] A. Wojdylo, J. Oszmianski and R. Czemerys, “Antioxi- dant Activity and Phenolic Compounds in 32 Selected Herbs,” Food Chemistry, Vol. 105, No. 3, 2007, pp. 940- 949. doi.org/10.1016/j.foodchem.2007.04.038 [12] R. S. D. Paul Raj, S. M. Morais and K. Gopalakrishnan, “In Vitro Propagation of Callistemon citrinus. L.,” Indian Journal of Science and Technology, Vol. 3, No. 1, 2010, p. 67. [13] L. L. Mensor, F. S. Menezes, G. G. Leitão, A. S. Reis, T. C. dos Santos, C. S. Coube and S. G. Leitão, “Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method,” Phytotherapy Research, Vol. 15, No. 2, 2001, pp. 127-130. doi.org/10.1002/ptr.687 [14] S. Mcdonald, P. D. Prenzler, M. Autolovich and K. Ro- bards, “Phenolic Content and Antioxidant Activity of Olive Oil Extracts,” Food Chemistry, Vol. 73, No. 1, 2001, pp. 73-84. doi.org/10.1016/S0308-8146(00)00288-0 [15] A. Bendini, L. Cerretani, L. Pizzolante, T. Gallina-Toschi, F. Guzzo, S. Ceoldo, A. M. Marconi, F. Andreetta and M. Levi, “Phenol Content Related to Antioxidant and An- timicrobial Activity of Passiflora Spp. Extracts,” Euro- pean Food Research and Technology, Vol. 223, No. 1, 2006, pp . 102-109. doi.org/10.1007/s00217-005-0150-7 [16] A. Dlugosz, J. Lembas-Bogaczyk and E. Lamer-Zaraw- ska, “Antoxid Increases Ferric Reducing Antioxidant Po- wer (FRAP) even Stron ger than Vitamin C,” Acta Poloniae Pharmaceutica, Vol. 63, 2006, pp. 446- 4 48. [17] R. Harish and T. Shivanandappa, “Antioxidant Activity and Hepatoprotective Potential of Phyllanthus niruri,” Food Chemistry, Vol. 95, No. 2, 2006, pp . 18 0-185. doi.org/10.1016/j.foodchem.2004.11.049 [18] N. Hassimotto, M. Genovese and F. Lajolo, “Antioxidant Activity of Dietary Fruits, Vegetables, and Commercial Frozen Fruit Pulps,” Journal of Agricultural and Food Chemistry, Vol. 53 , No. 8 , 20 0 5 , pp. 29 28-2935. doi.org/10.1021/jf047894h [19] D. J. Newman, G. M. Gragg, S. Holbeck and E. A. Saus- ville, “Natural Products as Leads to Cell Cycle Pathway Targets in Cancer Ch emotherapy,” Current Cancer Drug Targets, V ol. 2, N o. 4, 20 02, pp. 279-308. doi.org/10.2174/1568009023333791 [20] V. Rajkumar, H. Guha and R. A. Kumar, “Antioxidant and Anticancer Potentials of Rheum Emodi Rhizome Ex- tracts,” Food and Chemical Toxicology, Vol. 49, No. 2, 2011, pp . 363-369 . Copyright © 2011 SciRes. AJPS |

