 American Journal of Plant Sciences, 2011, 2, 823-834 doi:10.4236/ajps.2011.26097 Published Online December 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 823 Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) Anete Teixeira Formiga1, Geraldo Luiz Gonçalves Soares2, Rosy Mary dos Santos Isaias1 1Departamento de Botânica, Campus da Pampulha, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; 2Departamento de Botânica, Campus do Vale, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. Email: aneteformiga@gmail.com Received September12th, 2010; revised October 9th, 2011; accepted October 22nd, 2011. ABSTRACT The ontogenetic characterization of the leaf galls induced in the internervural region and in the second and third order veins of A. spruceanum Müell Arg. (Apocynaceae) aims to evaluate the distinct levels of cell reaction during the process of gall fo rmation, and the relation between external gall morphology and the oviposition sites. The ground system had the most remarkable alterations, namely, the non differentiation of palisade parenchyma in both leaf sides, the hyper- plasia of the spongy parenchyma and the neoformation of fibersclereids, a cell type not observed in non galled leaves. Changes of the feeding sites inside the larval chamber reveal distinct levels of cell competence to respond to the insects stimuli and explain the variations in the shape of the larval chamber. Keywords: Aspidosperma, Cell Competence, Gall Development, Leaf Anatomy 1. Introduction The size and shape of galls are determined by the me- chanical injury, the salivary secretions, and the feeding activity of their related galling insects [1]. Consequently the morphology of a specific gall may be considered as an extended phenotype of its specific inducing insect [2,3], which must choose an adequate site of oviposition to guarantee the survival of its offspring. The eggs of the insects, as well as the secretions deposited with them at the site of oviposition, trigger gall induction. The exact location and number of eggs laid on the surface or inside the tissues of the host plant influence the final structure and size of the gall [3,4]. One of the most specialized groups of gall inducing insects is the Cecidomyiidae, whose galls complexity in- volves metabolical [5] and developmental patterns [6,7]. Processes of hyperplasia, hypertrophy, dedifferentiation or cell lysis [8] lead to the redifferentiation of specialized cells sensu Lev-Yadun [9] in the dermal, ground, and vascular systems. Such processes can occur in any plant cell that retains its nucleus at maturity [10] and are more intense the nearer the stimuli are. The number of cell divi- sions is intensely increased in the epidermis so as to ac- company the development of the structure. The paren- chyma adjacent to the larval chamber can be differen- tiated into a nutritive tissue that will nurture the insect [11]. New vascular bundles may differentiate and con- nect to the bundles of the host organ, forming a network for the translocation of substances. These bundles are directed to the larval chamber, and according to Mani [6] and Meyer and Maresquelle [7], commonly end up as sieve elements. The parenchyma adjacent to the vascular bundles is hyperplasic, with hypertrophied cells which may accumulate secondary metabolites, such as phenolic derivatives, which can be related with the occurrence of oxidative stress. The A spidosperma spruce anum Müell Arg. (Apocyna- ceae)-Cecidomyiidae system has been addressed in se- veral focuses [5,12,13]. These galls can be induced in young or mature leaves, and develop in the second or third order veins, or in the internervural region where they are commonly more numerous [12]. Through the anatomical analysis of the developmental stages of the galls of A. spruceanum, the present study aimed to relate the final gall phenotype with the distinct  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) 824 sites of oviposition, the internervural regions or on sec- ond and third order veins. At these sites, the levels of cell responses to the insects’ stimuli may vary. Moreover, the location of phenolic derivatives and reactive oxygen spe- cies (ROS) in gall tissues was established through histo- chemical tests. This location may be related to the ceci- dogenetic field established by the midge’s presence or to an investment in chemical defenses during the develop- ment of the galls. 2. Methodology 2.1. Collection and Fixation of Botanical Material Non galled leaves and leaves with galls at different deve- lopmental stages and with different oviposition sites were collected from October 2001 to October 2002 from individuals (n = 6) of A. spruceanum located at the Pam- pulha Campus of the Universidade Federal de Minas Gerais in Belo Horizonte, MG, Brazil. The voucher ma- terial is deposited at BHCB herbarium under the registra- tion number 46.274. The samples were fixed in FAA (37% formaldehyde, acetic acid and ethanol 50˚ GL, 1:1:18, v/v) [14] for analysis at light microscope, and with 2% ferrous sul- phate in 10% formalin [14] for detection of polyphenols. 2.2. Preparation of Histological Sections For permanent slides, some samples, fixed in FAA, were dehydrated in an ethanol series and included in historesin (Reichert-Jung®). Some other samples were embedded in Paraplast® [15], after dehydration in n- buthyl series [14]. The transverse sections (5 μm) were obtained in a rota- tory microtome (Jung-BIOCUT mod. 2035), and stained with 0.5% toluidine blue in 0.1% sodium carbonate at pH 11.0 [16]. The slides were mounted in water for immedi- ate observation. The material embedded in Paraplast® was sectioned (12 - 14 μm) in a rotative microtome (Jung-BIOCUT mod. 2035), and affixed to the slides with Bissing adhe- sive [15]. The staining was done with 0.5% safranin and astra blue (1:9 v/v) [15], and mounted with Entellan®. Transverse freehand sections of the non galled leaves, and of the galls in the 5 stages of development [12] in- duced in the internervural region and in the first and second order veins were done. These sections were clari- fied in 50% sodium hypochlorite, washed in distilled water, and stained in 0.5% safranin and astra blue (1:9 v/v) [15]. Sections were washed in distilled water, and mounted with jelly glycerin [15]. 2.3. Histochemical Tests The histochemical tests were done in freehand sections. The presence of lipids and starch was verified with Su- dan Black B in 70% ethanol [17], and with Lugol’s reagent (2% potassium iodide in 0.2% iodine) [18] for 15 minutes, respectively. The presence of polyphenols was verified with 2% ferrous sulphate in 10% formalin [14]. Proanthocyanidins and their oligomeric derivatives were tested by the fixation of the sections in 1% caffeine- sodium benzoate in 95% ethanol, for 5 minutes, and im- mersion in p-dimethylaminocynamaldehyde (DMACA) for 2 hours [19,20]. The slides were mounted in 50% gly-cerin. The detection of lignins was done with the rea- gent of Wiesner [21], in which the phloroglucinol in aci- dic conditions reacts with monomeric residues of the po- lymer (i.e. cinnamyl alcohol-derivatives) [22-24]. The sections were immersed for 5 minutes in phloroglucinol in 95% ethanol and mounted in 50% hydrochloric acid. Lignins were also detected with the reagent of Maule [22 -24], in which the sections were placed in 1% potassium permanganate for 5 minutes, washed in distilled water, transferred to hydrochloric acid for 1 minute, and mounted in 50% ammonia. This reagent causes the formation of colored derivatives by oxidation of the monomeric units of lignin. 2.4. Developmental Stages of the Galls To determine the occurrence of the various stages of gall development in A. sprucea n um, 25 leaves were randomly collected and the 137 galls found were classified accord- ing to the criteria described in Formiga et al. [12]. 2.5. Detection of Sites of Reactive Oxygen Species (ROS) The detection of the sites of ROS activity was performed using the DAB reagent (3-3’-diaminobenzidine). Free- hand cuts of fresh material were immersed in 0.5% DAB (Sigma) for 20 - 60 minutes in the dark [5,25]. The in- tensity of reaction was observed every 15 minutes. 3. Results 3.1. Non Galled Leaves The leaves of A. spruceanum are isobilateral, hyposto- matic, and hairy on the abaxial surface. The epidermis is uniseriate, covered by a thick cuticle. The palisade pa- renchyma is 3-layered at the adaxial, and 1-layered at the abaxial side of the lamina. The spongy parenchyma is 10 - 13 layered, with long armed cells. The vascular system consists of a first order vein, with initial cambial activity forming xylem and phloem in bicollateral arrangement. At the adaxial cortex, some small collateral vascular bundles are observed. In the second and third order veins, the bundles are collateral, involved by pericyclic fibers, parenchyma cells and the endodermis. Fibersclereids are common throughout the mesophyll. Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae)825 3.2. General Aspects of Galls The gall is closed, lenticular, verrucous, and green, no matter the site of oviposition (Figures 1(a)-(e)). The gall epidermis is similar to that of non galled leaf at all stages of gall development with apparently functional stomata. The main changes in relation to the non galled leaves are observed in the ground and vascular systems. The meso- phyll of the gall loses the distinction between palisade and spongy parenchyma characteristic of the non galled lamina. The vascular bundles are disorganized due to the hyperplasia and hypertrophy of the associated paren- chyma. The diverse orientation of the tracheary elements is evident. 3.3. Galls in the Internervural Region Induction. The first signs of gall induction are noticed by the bulging of the leaf blade (Figure 1(f)), with sites of hyperplasia at the epidermis. The epidermal cells are small and isodiametric (Figure 1(g)). In the adaxial epi- dermis, the cells are anticlinallly hypertrophied, and the cuticle is thin (Figure 1(h)). The cortex is composed of size-varied parenchyma cells, in which hyperplasia and cell hypertrophy are common. The insertion of the ovi- positor is noticed by a healing sheath (Figures 1(j)-(k)). In the central region, near the larval chamber, hypertro- phied, round, sometimes binucleated cells and hyperpla- sic sites occur (Figures 1(f), (j), (k)). The vascular bund- les of the second and third order veins are disorganized by hyperplasia of the associated parenchyma (Figure 1(l)). 3.4. Growth and Development There is an increase in the number of cell layers in the peripheral portions of the galls. The prominence of the gall on the leaf surface increases (Figure 2(a)). The epi- dermis becomes locally hypertrophied, its cells elongate anticlinally and present signs of metacutinization (Fig- ure 2(b)). In the peripheral portions of the gall cortex, the spongy parenchyma is hyperplasic, with long-armed hypertrophied cells (Figures 2(c)-(d)). Around the larval chamber, a sclerenchymatous ring limit the outer cortex and the nutritive tissue differentiates (Figure 2(f)). The larval chamber is elongated or circular (Figure 2 (e)). The circular shape occurs when the chamber is perpendicular to the proximal vascular bundle, and the vascularization is ensured by several bundles where the hyperplasia of the parenchyma leads to disorganization of the structure (Figure 2(f)). Fibersclereids occur adjacent to the epider- mis (Figures 2(b), (c)) and in the mid portion of the gall parenchyma occur (Figure 2 (f)). The vascular bundles immersed in the parenchyma of the gall are more altered the closer the larval chamber is (Figure 2(f)). 3.5. Maturation The dermal system is covered by a thick cuticle (Figure 3(a)). The ground system in the adaxial cortex is hyper- plasic, with no palisade differentiation (Figure 3(b)). Sclereids and fibersclereids occur throughout the cortex of the gall (Figure 3(b)). The spongy parenchyma is also hyperplasic, with hypertrophied cells. The larval cham- ber is round when sectioned perpendicular to the vein and is surrounded by a nutritive tissue (Figures 3(c), (d)). The sclereids around the nutritive tissue have thick walls. The vascularization maintains the characteristics of the earlier stages, particularly near the larval chamber (Fi- gure 3(d)). 3.6. Senescence The prominence of the gall on the leaf lamina reaches its maximum by the time the insect abandons the gall. The surface of the gall is verrucous and the larval chamber is more elongated horizontally when sectioned parallel to the vascular bundles. The dermal system maintains the characteristics of the maturation stage. The ground sys- tem consists of parenchyma cells interspersed with scle- reids and fibersclereids adjacent to the epidermis and more numerous than in the earlier stages of development. The larval chamber is covered with small portions of nu- tritive tissue, surrounded by a thicker ring of sclereids (Figure 3(c)). The vascularization of the gall is main- tained by one bundles which crosses the ring of sclereids (Figure 3(d)). The tracheary elements have spiral or pit- ted wall thickenings, and simple perforation plates (Fig- ure 3(e)). At the end of this phase, the gall has a series of fundamental cristarque (Figure 3(f)), brachisclereids, tra- cheoidal sclereids, and fibersclereids, interspersed to the spongy parenchyma. Reactions of cicatrization are ob- served in the cells of the nutritive tissue (Figure 3(g)). All developmental stages of the galls occurred all over the year (Table 2). 3.7. Galls in the Midrib Region, Second and Third Order Veins The galls developed on the veins have an asymmetrical increment of tissues (Figure 4(a)). The dermal system is formed by a uniseriate epidermis. This cell layer together with the exodermis has conspicuous wall thickening and metacutinization (Figure 4(b)). At the less developed side, the exodermis does not differentiate, and the epi- dermis has hypertrophied cells (Figure 4(c)). The cortex is parenchymatic with isodiametric and polygonal cells (Figure 4(d)), with long armed cells lateral to the larval chamber (Figure 4(e)). The larval chamber is placed within the vascular bundle and is surrounded by the nu- tritive tissue and the ring of sclereids (Figures 4(e)-(f)). Copyright © 2011 SciRes. AJPS 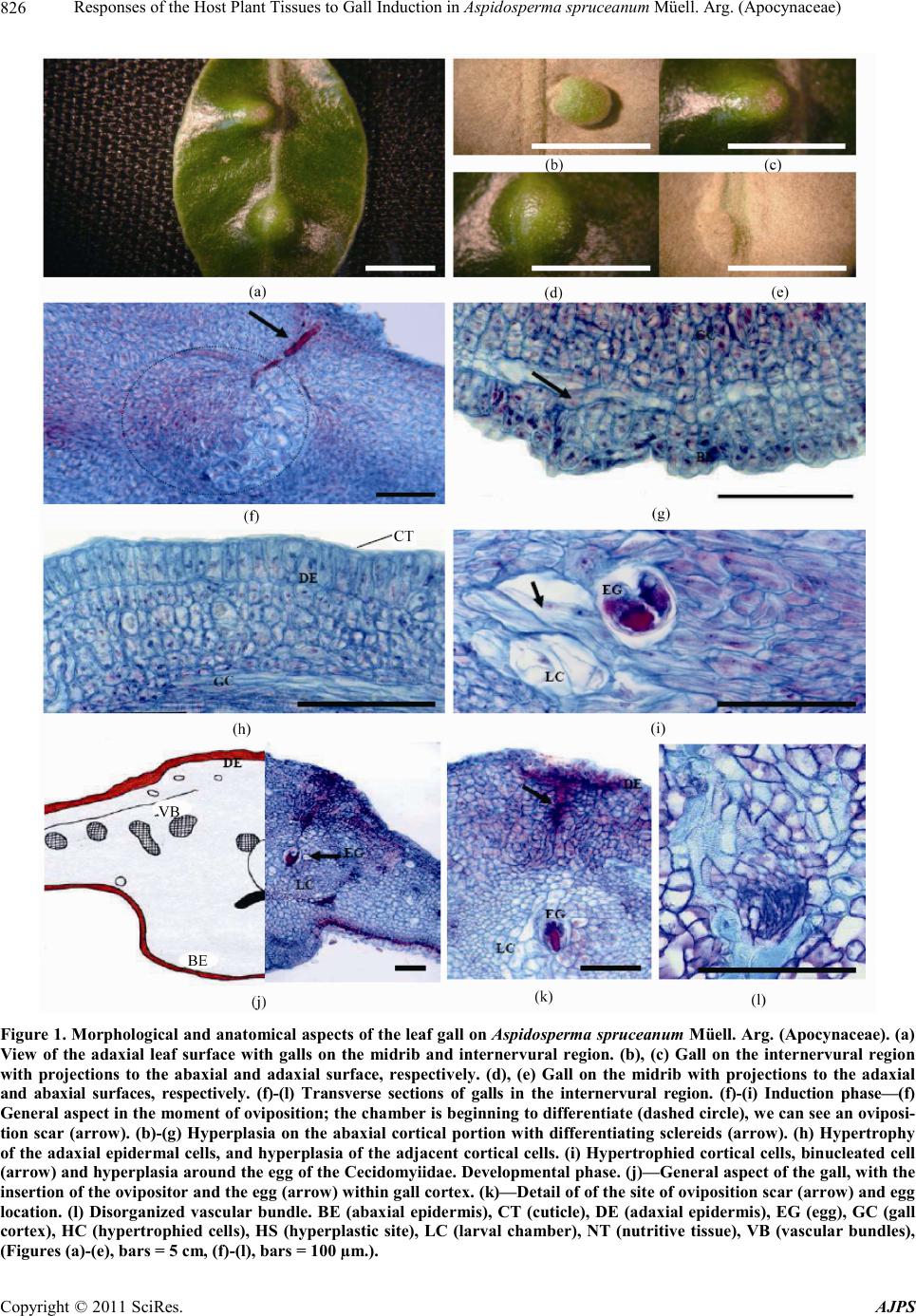 Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) Copyright © 2011 SciRes. AJPS 826 Figure 1. Morphological and anatomical aspects of the leaf gall on Aspidosperma spruceanum Müell. Arg. (Apocynaceae). (a) View of the adaxial leaf surface with galls on the midrib and internervural region. (b), (c) Gall on the internervural region with projections to the abaxial and adaxial surface, respectively. (d), (e) Gall on the midrib with projections to the adaxial and abaxial surfaces, respectively. (f)-(l) Transverse sections of galls in the internervural region. (f)-(i) Induction phase—(f) General aspect in the moment of oviposition; the chamber is beginning to differentiate (dashed circle), we can see an oviposi- tion scar (arrow). (b)-(g) Hyperplasia on the abaxial cortical portion with differentiating sclereids (arrow). (h) Hypertrophy of the adaxial epidermal cells, and hyperplasia of the adjacent cortical cells. (i) Hypertrophied cortical cells, binucleated cell (arrow) and hyperplasia around the egg of the Cecidomyiidae. Developmental phase. (j)—General aspect of the gall, with the insertion of the ovipositor and the egg (arrow) within gall cortex. (k)—Detail of of the site of oviposition scar (arrow) and egg location. (l) Disorganized vascular bundle. BE (abaxial epidermis), CT (cuticle), DE (adaxial epidermis), EG (egg), GC (gall cortex), HC (hypertrophied cells), HS (hyperplastic site), LC (larval chamber), NT (nutritive tissue), VB (vascular bundles), (Figures (a)-(e), bars = 5 cm, (f)-(l), bars = 100 µm.).  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae)827 Figure 2. Anatomical aspects of the leaf galls on the internervural region on Aspidosperma spruceanum Müell. Arg. (Apocy- naceae). 3. (a) Developmental phase with large larval chamber. (b) Detail of gall on the adaxial cortical portion with metacu- tinization, and sites of cell division on the epidermis. The parenchyma is in palisade arrangement with sclereids, and a pheno- lic idioblast. (c) Detail of the gall on the abaxial cortical portion with metacutinization and sclereids. (d) Gall cortex with hy- perplasic spongy parenchyma. (e) Detail of the nutritive tissue and sclerenchymatic zone around the larval chamber. (f)—Lateral portion of the gall with disorganized vascular bundles, some redirected to the larval chamber. BE (abaxial epi- dermis), CT (cuticle), DE adaxial epidermis), FS (fibersclereids), LC (larval chamber), NT (nutritive tissue), PP (palisade), S (sclereids), VB (vascular bundles). (In (a)-(g), bars = 100 μm. In h bar = 500 μm). The collenchyma and the 1 - 3 layered palisade paren- chyma are placed on both sides of the larval chamber. The vascular bundles of the second and third order veins diverge to the larval chamber. 3.8. Histochemistry of Galls The various stages of development of the galls on the leaves of Aspidosperma spruceanum are histochemically similar in relation to the production and storage of the analyzed metabolites (Table 1). The sites for lignins and ROS detection are similar, and there was no distinction between syringyl and Guaia- cyl lignin either in non galled or galled tissues. The ROS are detected in the palisade parenchyma and around the larval chamber in the border line of the inner cortex. A centrifugal gradient is visualized, which is more intense Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) 828 Figure 3. Anatomical aspects of the leaf galls on the internervural region on Aspidosperma spruceanum Müell. Arg. (Apocy- naceae). Stage 4. (a)—General aspect evidencing tissue zonation and round larval chamber. (b) Detail of the abaxial portion with numerous sclereids. (c) Detail of the larval chamber lined with nutritive tissue (arrow) and sclerenchymatic zone. (d)-(g) Maturation phase. (d)—Lateral portion of the gall with nutritive tissue surrounding the larval chamber and vascular bundle redirected towards it. (e) Tracheoidal sclereids with bordered pits. (f) Cristarque under polarized light (arrow). (g) Base of the larval chamber evidencing nutritive tissue, the sclerechymatic zone is interspersed by vascular tissues. Note early cica- triza- tion around the chamber. CT (cuticle), CL (larval chamber), EB (abaxial epidermis), ED (adaxial epidermis), ES (sclereids), FE (fibersclereids), FV (vascular bundle), NT (nutritive tissue), TC (scar tissue). (Bars = 100 µm). in the maturation phase (Figures 5(a)-(e)). 4. Discussion The Cecidomyiidae feeding activity caused alterations in dermal, ground and vascular systems of their host A. sp ru- ceanum leaves. The stimuli for gall development come from the insect, and the control of the growth and differ- entiation of the cells is directed to the morphogenesis of a new structure, as proposed by Dreger-Jauffret and Short- house [26]. This morphogenical redirection is directly de- pendent on the presence of the galling insect, and the changes cease after the senescence of the gall. Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae)829 Table 1. Histochemical analysis of the gall tissue layers in Aspidosperma spruceanum. Lipids Starch Phenolic derivatives Alcaloids Flavanols Cuticle + – – – – Epidermis + + +++ – – Outer cortex + ++ ++ – – Inner cortex – + – – Nutritive tissue – + – – – (+) positive reaction, (–) negative reaction. The number of signs indi- cates the intensity of the reaction. Table 2. Percentage of occurrence of the stages of the deve- lopment of the galls of Aspidosperma spruceanum. Stages Occurrence Induction 8.8% Development 24.8% Maturation 24.1% Senescence 42.3% Gall developmental phases were determined according to the criteria des- cribed in Formiga et al. (2009). N = 137 galls in 25 leaves. Figure 4. Anatomical aspects of leaf galls in the midrib region of Aspidosperma spruceanum Müell. Arg. (Apocynaceae). (a) General aspect. (b) Adaxial portion with metacutinization and hypertrophy of epidermal cells, and adjacent cortex with nu- merous sclereids. (c) Abaxial portion with metacutinization and hypertrophy of epidermal cells. (d) Hyperplasic spongy pa- renchyma. (e) General aspect of the gall with round larval chamber, surrounded by the nutritive tissue (arrow), the scler- enchymatic zone and sclereids interspersed within parenchymatic cells. (f) Detail of the larval chamber surrounded by nutri- tive tissue, and sclerenchymatic zone. CT (cuticle), CL (larval chamber), CP (parenchymal cells), E1 (epidermal hypertrophy and hyperplasia), E2 (epidermal hypertrophy), FE (fibersclereid), FI (fibers), FL (phloem), IC (isodiametric cells), PS (spongy parenchyma), XI (xylem). (In a, bar = 500 μm. In (b)-(f), bar = 100 μm). Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) 830 Figure 5. Histochemical tests for reactive oxygen species (ROS) on non galled leaves and galls of Aspidosperma spruceanum Müell. Arg. (Apocynaceae). (a)-(b) Non galled leaf. (a) General apect of leaf lamina. ROS concentrated on spongy paren- chyma. (b) Midrib region. ROS concentrated in palisade parenchyma. (c)-(e) Gall. (c) Inner cortex with larval chamber. ROS concentrated in the inner cells of the nutritive tissue and around the sclerenchymatic zone. (e) Adaxial cortical portion. ROS concentrated on epidermis and cortex. (e) Abaxial cortical portion with ROS concentrated on the epidermis and parenchyma. (Bars = 100 μm) . All the four basic developmental stages of the Cecidomy- iidae galls se nsu Rohfritsch [27] occur simultaneously in A. spru c e n um (Table 2), indicating the multivoltinism of the insect, as proposed by Campos et al. [13]. The induction phase seems to start with oviposition, which occurs inside the leaf tissues, indicating that the female of this species has a strong and long ovipositor, called terebra [28], able to pierce the thick cuticle, the epidermis and the parenchyma. Moreover, the different degrees of cell hypertrophy imme- diately around the egg and the hyperplasia of the tissue fac- ing the adaxial leaf surface indicate the concomitant stimuli of a fluid injected at the time of oviposition, and the me- chanical injury caused by the ovipositor. This is an ana- tomical evidence of the insects’ activity, difficult to visual- ize in nature due to its diminutive dimension. The occurrence of galls in the induction phase in young and mature leaves denotes a wide range of oviposition sites for the galling herbivore. This behavior is relatively uncommon in gall inducing insects, which are referred to prefer to lay eggs on meristematic tissues [27]. In fact, the parenchymatic cells that react to the behavior of the Cecidomyiidae in A. spruceanum are considered to be partially differentiated or less specialized sensu Buvat [29]. The dedifferentiation of mature cells requires so- phisticated changes in cellular morphogenetical programs, and in the case of the galls must proceed through the growth and developmental phase. At this phase, a con- spicuous feature is the redifferentiaton of columnar scle- reids, not observed in non galled leaves of the host spe- cies. Galling herbivores, in general, are not capable of in- ducing a new structure or tissue strange to the morpho- genetical program of their host plant cells [6,30]. Never- theless, this feature had been reported in some other dip- teran [27,31-33], and may help in the mechanical support spongy tissue differentiated in the inner cortex of the gall. Fibersclereids may develop from parenchyma cells, with the growth and elongation of the structure of the gall. This is only possible if the cells are still alive when the gall starts its development, as previously described by Arduin et al. [34] in Cecidomyiidae galls induced in Struthanthus vulgaris. In the galls of A. spruceanum, the number of fibersclereids increases from the induction th- rough the growth and development, and maturation phases. Also, the fibersclereids increase in the vicinity of the larval chamber. The lignification plus the accumulation of phe- nolic compounds in gall parenchyma commonly occur in Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae)831 Table 3. ROS analysis on the tissue layers of non galled leaves and of distinct gall developmental phases in Aspidosperma spruceanum. Gall Developmental Phases Non Galled Leaves Induction Development Maturation Senescence Cuticle – – – – – Epidermis – – – – – Palisade parenchyma ++++ abs abs abs abs Outer cortex abs ++ ++ ++ ++ Spongy parenchyma ++ abs abs abs abs Inner cortex abs +++ +++ ++++ +++ Nutritive tissue abs ++ ++ ++ ++ (+) positive reaction, (–) negative reaction. abs = absent. The number of signs indicates the intensity of the reaction. response to different types of injuries [35], and consists in a high mechanical resistance against the attack of predators and parasitoids. The sclerenchymatic layer, com- mon in many midge induced galls and located between the vascular and the nutritive tissues [27], is evidenced in these galls since the phase of growth and development. Another feature of the sclerenchyma in the galls of A. spruceanum is the numerous connections with the sur- rounding layers, facilitating the transport of substances, in an acessory function to the vascular system. In the maturation phase, the gall tissues are fully dif- ferentiated, and the final shape and dimensions are built. The most striking alterations are the non-differentiation of palisade parenchyma on both leaf surfaces, the hyper- plasia of the spongy parenchyma and the increased for- mation and diversification of lignified cells. The inhibi- tion of differentiation of palisade and spongy paren- chyma is commonly cited in galls. Arduin et al. [34] and Isaias [36] demonstrated it in midge induced gall in Struthanthus vulgaris and Machaerium spp., and Vecchi [37], in galls induced by a microlepidoptera in Tibou- china pulchra. Kraus et al. [38] affirmed that these reac- tions are a convergent pattern in galls of several species of Brazilian flora. In fact, the variety of gall morphotypes induced in leaves seems to be mainly a result of changes in the ground system, whose cells respond more readily to the stimuli of the insect. Even in the vascular system, the remarkable change is the hyperplasia of the vascular parenchyma, with some xylem bundles redirected to the larval chamber. The helical thickening of their cells is common in organs in primary growth, and should allow the elongation of the gall during the growth and development phase. The vas- cular system maintained the common pattern of the bun- dles of the non galled organs, with the maintenance of the formation of cristarque. Alterations to a greater or lesser extent in the vascular system, and the neoformation of bundles were described by Meyer and Maresquelle [7] in various types of galls. According to Isaias [36], galls that develop on the first order veins have an increased translocation of assimilates towards to the area of the gall, and therefore should not have neoformation of bundles. Similarly, in the galls of A. spruceanum induced at the second and third order veins no new bundles are differentiated. Also, since the vascu- lar system includes the highest levels of cell differentia- tion [39], the inducing Cecidomyiidae seems to have little ability to manipulate the vascular tissues. The ability of the Cecidomyiidae to change the site of feeding inside de chamber can explain the different shapes of the larval chamber in the gall of A. spruceanum. It is elongated when parallel to the vein and round when perpendicular to the vascularization. This may be indica- tive of the rotational movements of the body of a Ceci- domyiidae inside the gall, as observed by Arduin et al. [34] in the galls of Struthanthus vulgaris. Another possi- ble cause of the variation in the shape of the larval chamber is the responsiveness of the tissues involved in the formation of gall, consisting of partially differenti- ated parenchyma cells sensu Buvat [29]. When the galls develop in the region of the veins, the differentiation of collenchyma in the abaxial portion is inhibited, mostly because the presence of a temporary tissue of support, common in growing organs [29], are not necessary in these galls. Considering that the gall is a temporary and fast growing structure, with limited size and shape, its support is given directly by the neoforma- tion of numerous sclereids and fibersclereids. Considering that the cells of the vascular system are highly specialized and anucleated, the feeding activity of the gall inducing insect should be restricted to the pa- renchyma cells. The cecidogenetic field is interrupted by the cells whose ontogenetical fate is reached, and there- fore, the larval chamber necessarily have to stretch fol- lowing the direction of the bundles, i.e., the axial paren- chyma cells, the pericycle, and the endodermis. This di- Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) 832 rection explains the variations in the shape of the larval chamber. In opposition to the structural changes, few metabo- lites accumulate in gall site. The reaction for lipids evi- denced the metacutinization, and may be related to the protection against desiccation [40]. The absence of lipids as a reserve substance is consistent with the studies of Bronner [11,41] which proposes the formation of nutri- tive and reserve tissue rich in carbohydrates in galls of Cecidomyiidae. The accumulation of polyphenols in the peripheral layers are usually related to an effective che- mical defense [1,42], however, this hypothesis does not apply to the galls of A. spruceanum since these galls have many natural enemies [5]. The lignification can re- strict water loss inside the gall, defining a microenviron- ment that has enough moisture for the survival of the gall inducer. The sites of positive reaction to ROS were similar to those of lignins (Table 3), a coincidence already detected by Hückelhoven [43]. Cell wall lignification and the ac- cumulation of ROS were also intense around the larval chamber. This region has intense cell divisions and dif- ferentiation, and is in direct contact with the gall induc- ing insect, which can explain its high level of oxidative stress. It is relevant to mention that the coincidence of the sites for lignification and ROS detection reinforces the antioxidant role of lignin biosynthesis since this proc- ess consumes large amounts of hydroxyl radicals [44,45]. By the time the galls reach the phase of senescence, the feeding activity of the insect ends up, and the suberi- zation of the cells lining the larval chamber is anatomi- cally evidenced. This phase can occur even after the fal- ling of the leaves, when the insects pupate in the soil and the imago emerges to start a new set of inductions [27]. This is true for galls induced either in the internervural or in the veins. 5. Conclusions The development of the galls of A. spru ceanum corrobo- rates the pattern previously established for the galls of midges, with the oviposition in parenchyma layers, and significant changes in the three plant tissue systems. In this gall, the shape of the larval chamber followed the direction of the vascular parenchyma cells, the pericycle and the endodermis, which are very responsive tissues. The neoformation of fibersclereids deserves attention for this kind of cells is not observed in the host non galled leaves. Also they provide structural protection for the Ce- cidomyiidae, and may function as an accessory transport system. The lignification of the cells at the same site of ROS accumulation is a further indication of protection to the gall inducer, which generates a safe microenvironment protected from pathogens such as fungi and bacteria, and effective against environmental factor such as dryness and diffusion of toxic free-radicals inside the gall. These galls are also efficient in nutrient supply, and their ana- tomical features evidence their adaptive value for the gall inducer. 6. Acknowledgements The authors would like to thank the undergraduate stu- dent Ariane Chagas de Castro of the Universidade Fe- deral de Minas Gerais (UFMG) for helping with the col- lections, CNPq and CAPES for financial support and scholarship. REFERENCES [1] T. Nyman and R. Julkunen-Titto, “Manipulation of the Phenolic Chemistry of Willows by Gall-Inducing Saw- flies,” Proceedings of the National Academy of Sciences, Vol. 97, No. 24, 2000, pp. 13184-1318. doi:10.1073/pnas.230294097 [2] R. Dawkins, “The Extended Phenotype the Gene as the Unit of Selection,” Oxford University Press, New York, 1982. [3] J. D. Shorthouse, D. Wool and A. Raman, “Gall-Inducing Insect—Nature’s Most Sophisticated Herbivores,” Basic and Applied Ecology, Vol. 6, No. 5, 2005, pp. 407-411. doi:10.1016/j.baae.2005.07.001 [4] M. Z. D. Moura, R. M. S. Isaias and G. L. G. Soares, “Species-Specific Changes in Tissue Morphogenesis In- duced by Two Arthropod Leaf Gallers in Lantana cama- ra L. (Verbenaceae),” Australian Journal of Botany, Vol. 56, No. 2, 2008, pp. 153-160. doi:10.1071/BT07131 [5] D. C. Oliveira and R. M. S. Isaias, “Cytological and His- tochemical Gradients Induced by a Sucking Insect in Galls of Aspidosperma australe Arg. Muell (Apocyna- ceae),” Plant Science, Vol. 178, No. 4, 2010, pp. 350- 358. doi:10.1016/j.plantsci.2010.02.002 [6] M. S. Mani, “Ecology of Plant Galls,” Dr. W. Junk Pub- lish, Hague, 1964. [7] J. Meyer and H. J. Maresquelle, “Anatomie des Galles,” Gerbrüder Borntraeger, Berlin, 1983. [8] L. A. Rey, “Developmental Morphology of Two Types of Hymenopterous Galls,” In: J. D. Shorthouse and O. Roh- fritsch. Eds., Biology of Insect Induced Galls, Oxford University, Oxford, 1992. [9] S. Lev-Yadun, “Stem Cells in Plants are Differentiated Too,” Cur ren t Opin ion in Pl ant Bi ol ogy, Vol. 4, 2003, pp. 93-100. [10] D. E. Fosket, “Plant Growth and Development. A Mole- cular Approach,” Academic Press, London. 1994. [11] R. Bronner, “The Role of Nutritive Cells in the Nutrition of Cynipids and Cecidomyiids,” In: J. D. Shorthouse and O. Rohfritsch, Eds., Biology of Insect Induced Galls, Oxford University, Oxford, 1992. Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae)833 [12] A. T. Formiga, S. J. M. R. Gonçalves, G. L. G. Soares and R. M. S. Isaias, “Relações Entre o Teor de Fenólicos e o Ciclo das Galhas de Cecidomyiidae em Aspidosperma spruceanum Müell Arg. (Apocynaceae),” Acta Botanica Brasilica, Vol. 23, No. 1, 2009, pp. 93-99. doi:10.1590/S0102-33062009000100012 [13] P. T. Campos, M. C. D. Costa, R. M. S. Isaias, A. S. F. P. Moreira, D. C. Oliveira and J. P. Lemos-Filho, “Pheno- logical Relationships between Two Insect Galls and Their Host Plants: Aspidosperma australe and A. spruceanum (Apocynaceae),” Acta Botanica Brasilica, Vol. 24, No. 3, 2010, pp. 727-733. doi:10.1590/S0102-33062010000300016 [14] D. A. Johansen, “Plant Microtechnique,” McGraw-Hill Book, New York, 1940. [15] J. E. Kraus and M. Arduin, “Manual Básico de Métodos em Morfologia Vegetal,” Ed. Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, 1997. [16] T. P. O’Brien and M. E. McCully, “The Study of Plant Structure Principles and Selected Methods,” Termarcarphi PTX, Mellrime, 1981. [17] A. G. Pearse, “Histochemistry, Theoretical and Applied,” Churchill-Livingstone, Edinburgh, Vol. 1, 1968. [18] W. A. Jensen, “Botanical Histochemistry: Principles and Practice,” W. H. Freeman and Company, San Francisco, 1962. [19] W. Feucht, P. P. S. Schmid and E. Christ, “Distribution of Flavanols in Meristematic and Mature Tissues of Prunus avium Shoots,” Journal of Plant Physiology, Vol. 125, No. 1-2, 1986, pp. 1-8. [20] W. Feucht, D. Treutter and E. Christ, “Role of Flavanols in Yellowish Beech Trees of the Black Forest,” Tree Ph y- siology , Vol. 17, No. 5, 1997, pp. 335-340. [21] J. E. Sass, “Botanical Microtechnique,” The Iowa State University Press, Ames, 1958. [22] C. Vallet, B. Chabbert, Y. Czaninski and B. Monties, “His- tochemistry of Lignin Deposition during Sclerenchyma Differentiation in Alfalfa Stems,” Annals of Botany, Vol. 78, No. 5, 1996, pp. 625-632. doi:10.1006/anbo.1996.0170 [23] J. Lin, X. He, Y. Hu, T. Kuang and R. Ceulemans, “Lig- nification and Lignin Heterogeneity for Various Age Cla- sses of Bamboo (Phyllostachys pubescens) Stems,” Phy- siol ogia Plantarum, Vol. 114, No. 2, 2002, pp. 296-302. doi:10.1034/j.1399-3054.2002.1140216.x [24] A. Mansour and E. De Fays, “Rhythmic Growth Rings of Wood and Their Relationship with the Foliage in Oak Seedlings Grown in a Favourable Environment,” Annals of Botany, Vol. 82, No. 1, 1998, pp. 89-96. doi:10.1006/anbo.1998.0648 [25] S. Rosseti and P. M. Bonnatti, “In Situ Histochemical Monitoring of Ozone- and TMV Induced Reactive Oxy- gen Species in Tobacco Leaves,” Plant Physiology and Biochemistry, Vol. 39, No. 5, 2001, pp. 433-442. doi:10.1016/S0981-9428(01)01250-5 [26] F. Dreger-Jauffret and J. D. Shorthouse, “Diversity of Gall-Inducing Insects and Their Galls,” In: J. D. Shorth- ouse and O. Rohfritsch, Eds., Biology of Insect-Induced Galls, Oxford University, Oxford. 1992. [27] O. Rohfritsch, “Patterns in Gall Development,” In: J. D. Shorthouse and O. Rohfritsch, Eds., Biology of Insect In- duced Galls, Oxford University, Oxford. 1992. [28] J. Meyer, “Plant Gall and Gall Inducers,” Gerbrüder Born- traeger, Berlin, 1987. [29] R. Buvat, “Ontogeny, Cell Differentiation, and Structure of Vascular Plants,” Springer-Verlag, Berlin, 1989. doi:10.1007/978-3-642-73635-3 [30] M. S. Mani, “Introduction to Cecidology,” In: J. D. Shor- thouse and O. Rohfritsch, Eds. Biolog y of Insect-Induced Galls, Oxford University Press, New York, 1992, pp. 3-7. [31] A. Raman and C. Devadas, “Morphology, Anatomy and Development of the Midrib Galls on the Leaflets of Lan- nea coromandelica (Hoult.) Merrill (Anacardiaceae) Caused by Odinadiplosis odinae Mani (Diptera),” Pro- ceedings of the Indian National Science Academy, Vol. 86, No. 3, 1977, pp. 159-166. [32] J. E. Kraus, H. C. Sugiura and S. Cutrupi, “Morfologia e Ontogenia em Galhas Entomógenas de Guarea macro- phylla subsp. Tuberculata (Meliaceae),” Fitopatologia Brasileira, Vol. 21, No. 3, 1996, pp. 349-356. [33] L. DeBruyn, I. Vandevyvere, D. Jaminé and E. Prinsen, “The Effects of Gall Formation by Lipara lucens (Diptera, Choropidae) on its Host Phragmites australis (Poaceae),” In: G. Csóka, W. J. Mattson, G. N. Stone and P. W. Price, Eds., The Biology of Gall-Inducing Arthropods, Forest Service USDA, St. Paul, 1998. [34] M. Arduin, J. E. Kraus and M. Venturelli, “Estudo Mor- fológico de Galha Achatada em Folha de Struthanthus vulgaris Mart. (Loranthaceae),” Revista Brasileira de Botânica, Vol. 14, 1991, pp. 47-156. [35] S. F. Fink, “Pathological and Regenerative Plant Anatomy,” Gebrüder Borntraeger, Berlin, 1999. [36] R. M. S. Isaias, “Galhas Entomógenas em Machaerium (Leguminosae-Papilionoidae): Anatomia e Histoquímic,” Tese de Doutorado, Universidade de São Paulo, São Paulo, 1998. [37] C. Vecchi, “Galha Foliar em Tibouchina pulchra (Cham.) Cogn. (Melastomataceae): Morfo- Anatomia e Ontoge- nia,” Dissertação de Mestrado, Universidade de São Pau- lo, São Paulo, 1999. [38] J. E. Kraus, J. A. Solórzano Filho, M. Arduin and R. M. S. Isaias, “Respostas Morfogenéticas de Plantas Brasileiras à Insetos Galhadores,” In: R. Fortunato and N. Bacigalupo, Eds., Monographs in Systematic Botany from the Missouri Botanical Garden, Missouri Botanical Garden, Sr. Louis, 1998. [39] D. A. Herms and W. J. Mattson, “The Dilemma of Plants: To Grow or Defend,” Quarterly Review of Botany, Vol. 67, No. 3, 1992, pp. 283-335. [40] B. J. Deverall, “Defense Mechanisms of Plants,” Cam- bridge Press, New York, 1977. [41] R. Bronner, “Contribution l´etude Histochimique des Ti- Copyright © 2011 SciRes. AJPS  Responses of the Host Plant Tissues to Gall Induction in Aspidosperma spruceanum Müell. Arg. (Apocynaceae) Copyright © 2011 SciRes. AJPS 834 ssus Nourriciers des Zoocecidies,” Marcellia, Vol. 40, 1977, pp. 1-134. [42] S. Coruh and S. Ercisli, “Interactions between Galling Insects and Plant Total Phenolic Contents in Ro sa canina L. Genotypes,” Scientific Research and Essays, Vol. 5, No. 14, 2010, pp. 1935-1937. doi:10.1146/annurev.phyto.45.062806.094325 [43] R. Hückelhoven, “Cell Wall—Associated Mechanisms of Disease Resistance and Susceptibility,” Annual Review of Phytopathology, Vol. 45, 2007, pp. 101-127. [44] K. Apel and H. Hirt, “Reactive Oxygen Species: Metabo- lism, Oxidative Stress, and Signal Transduction,” Annual Review of Plant Biology, Vol. 55, 2004, pp. 373-399. doi:10.1146/annurev.arplant.55.031903.141701 [45] O. R. Gottlieb, M. A. C. Kaplan and M. R. M. B. Borin, “Biodiversidade. Um Enfoque Químico-Biológico,” Edi- tora da UFRJ, Rio de Janeiro, 1996.
|