 American Journal of Plant Sciences, 2011, 2, 790-807 doi:10.4236/ajps.2011.26094 Published Online December 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes M. A. K. Shaddad1, M. Hamdia Abd El-Samad2, H. T. Mohammed1 1Botany Department, Faculty of Science, Assiut University, Assiut, Egypt, 2Department of Botany and Microbiology, Faculty of Science, Minia University, Minia, Egypt. Email: hamdia10@yahoo.com Received November 19th, 2010; revised June 25th, 2011; accepted July 8th, 2011. ABSTRACT Two maize genotypes (Nefertiti and Bashaier) were picked up from nine maize genotypes during the early vegetative growth (25 days) to be cultivated in open field upon the crop yield under the different drought stress levels (90,70,50,30) or under the interaction effect of drought stress and phytohormones or polyamines. According to the data of growth criteria, the maize genotype Nefertiti was found to be the most drought sensitive genotype, while the genotype Bashaier was found to be the most drought resistant genotype. Additionally while the photosynthetic pigments remained more or less unchanged in genotype Bashaier, their biosynthesis destroyed earlier in the drought sensitive genotype (Nefertiti). Also while the genotype Bashaier absorbed and accumulated a sufficient amount of mono and divalent cations (K+, Ca++ and Mg++), the genotype Nefertiti did not. Accordingly while the genotype Bashaier gave a crop yield up to 50% field capacity, the genotype Nefertiti gave a crop yield only up to 70% field capacity and failed to give a crop yield be- yond this level. The interaction effect of drought stress and phytohormones and polyamines improved the all above characteristics. Interestingly each of these activators considerably improved the production of crop yield only in geno- type Bashaier specially polyamines they produced more than 60% field capacity and at the level of 30% field capacity (the level which did not give crop yield in this genotype). However, phytohormones in generally did not make an im- portant effect on the crop yield in genotype Nefertiti although they improved the dry matter production during the ve- getative stages. Such situation seemed to be complicated and borne many questions to be studied in the future. Keywords: Drought, Maize (Zea maize L.), Phytohormones, Poly ami nes, Leaf Area, Dry Weight, Yield 1. Introduction Drought is the most important limiting factor for crop production and it is becoming an increasingly severe pro- blem in many regions of the world [1,2]. Drought is still a serious agronomic problem and one of the most impor- tant factors contributing to crop yield loss. According to statistics, the percentage of drought affected land areas more than doubled from the 1970s to the early 2000s in the world [3]. Maize is one of the major summer crops in the irrigated areas of Egypt. However, maize is very sen- sitive to water stress [4-6]. The effects of water stress on maize include the visible symptoms of reduced growth, delayed maturity and reduced biomass and crop grain yield. For example, water stress on maize has been shown to reduce plant height [6], leaf area index [7] and root growth [8]. Grain yield can be reduced by decreasing yield components like grain number and grain weight [5,9]. Many researchers have evaluated the effect of timing of water stress on maize yield [6,10-12]. Flowering has been found to be the most sensitive stage to water deficit, with reductions in biomass, yield and harvest index [4,5,9,13, 14]. The high sensitivity of maize to water stress means that under water limiting conditions it is difficult to im- plement irrigation management strategies without incur- ring significant yield losses [4,15]. Much of the past re- search on water stress on maize has consisted of full withholding of irrigation and conditions of severe water stress [11,12]. It is clear from the literature that cessation of irrigation in maize causes significant yield losses. Therefore, it is important to know the crop response to moderate water deficit at different growth stages and un- der cropping and irrigation conditions similar to the one experienced by farmers in the area.  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 791 Phytohormones such as IAA, gibberellic acid (GA3), and kinetin are known to be involved in the regulation of plant response to the adverse effect of stress conditions [16-18] show that plant hormones can be defined as or- ganic substances that are produced in one part of plant and trans located to another parts, where at very low con- centration, they stimulate physiological response. Plants are usually subjected to environmental factors such as drought or water salinity. Also, exogenously applied phytohormones or poly- amines have multiple roles in improving drought tole- rance of maize. These functions are improved cell water status and alleviation of oxidative damage on the bio- logical membranes. This suggests that maintenance of water economy through stabilized cellular structure is an important mechanism of drought tolerance in maize. Es- tablishment of similar roles of polyamines are likely to be a great step in improving drought tolerance in high water requiring plant species. Polyamines are now being regarded as plant growth regulators and secondary messenger in signaling path- ways [19-21], and play an array of physiological roles in plant growth and development [22]. Although, they in- duce tolerance against several abiotic stresses in plants [23-25], mechanisms of their action during exogenous application in modulating physiological phenomena and improving drought stress tolerance are not fully under- stood.). Thus the aim of the present work was to test the effect of exogenous treatments with some phytohor- mones and polyamines in counteracting the adverse ef- fects of drought on growth, photosynthetic pigments, some mineral contents and yield production, of the two maize genotypes, which has been recorded to be differed in their drought stress tolerance according to the data of short duration experiment [26]. 2. Materials and Methods A field experiment was conducted using two maize ge- notypes Bashaier and Nefartiti selected for drought tole- rance in the growth chamber experiments. Grains were obtained from the breeding program of seeds station, Beni-suef, Egypt to be used in the field Work of Minia governorate. In view of the nature of the field experiment, Minia represented an ideal location since it is virtually rainless in the summer. A randomized split plot design was used [27]. Soil plots (50 m2) were prepared by add- ing manure 25 m3 Feddan-1 (60 m3·ha–1), plowing twice (once perpendicular to the other), leveling, and lining (7 m-long lines, 60 cm apart). Sowing (early June) on lines in small holes (Goras, 25 - 30 cm apart, two seeds per hole). Only one seedling per Gora was left after seedling establishment. Fertilizers included 200 kg. Feddan–1 Su- perphosphate (15% P2O5), and 120 units nitrogen Fed- dan–1 (200 kg Feddan-1 46% urea, in three installments once after sowing, once after second irrigation, and once after third irrigation). Plots were flood-irrigated after sow- ing, and seeds were sown in each plot and soil was brought to field capacity, then plots are classified into four groups according to the amount of moisture content in each plot. Plants were grown with further irrigation at 90%, 70%, 50%, and 30% field capacity, plants was ir- rigated every 12 day with these field capacities. Irriga- tion water was thoroughly measured by introducing a water meter on the irrigation line. Irrigation was stopped three weeks before harvest to allow drying. Plants were harvested 120 days after sowing. Plants in each soil plots are classified into eight groups the first group was the control, the rest groups were treated with 10 ml 200 μ. mol of one of the polyamines (putrescine, spermine or mixture of putrescine and sper- mine polyamines) or 20 μ. mol of one of the phytohor- mones (IAA, GA3, Kinetin and mixture of these phyto- hormones). Plants are treated with these phytohormones or polyamines two time during the age of maize the first at 30 old-days and the second at 45 old-day. At the end of the experimental period plant height, dry matter yield of the different organs (stem and leaves) were deter- mined. Plant height was determined by direct measure- ment from soil surface to the tip of the flag leaf. Deter- mination of the dry matter involved harvesting and care- ful separation of fresh organs. Fresh organs were then dried in an oven at 80˚C. Successive weighing was car- ried out until a constant dry weight was recorded. Leaf area was determined by measuring leaf length and maxi- mum width and applying the formula; Leaf area = k (leaf length * leaf maximum width) cm2·plant–1 This formula provided a simple way for determination of leaf area particularly in the field where large leaves had to be measured. The coefficient k was calculated and assigned different values for different grasses [28,29], and was recently reviewed and given a value of 0.75 for maize [30]. The photosynthetic pigments, chlorophyll a, chlorophyll b and carotenoids, were determined using the spectrophotometric method recommended by [31]. The flame emission technique was chosen for determination of potassium due to its simplicity, precision, and sensi- tivity. A flame photometer (Corning 410, Corning Sci- ence Products, Halstead, Essex, England) was used for this purpose. The EDTA titration method was employed for calcium and magnesium of different plant organs [32]. Harvest was carried out 120 days after sowing and cobs were left to dry (air dry for 2 weeks). Yield was determined as  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 792 Ardab Feddan–1 (Ardab = 120 kg) of air-dry grains. Yield attributes determined included the 1000 grain weight, and number of grains in each row. 3. Statistical Analysis The experimental data were subjected to the one way analysis of variances (ANOVA test) using the SPSS ver- sion 11.0 to quantify and evaluate the source of variation and the means were separated by the least significant differences, L.S.D. at P level of 0.05% and 0.01% [33]. 4. Results The data in Table 1 reveal that, the dry weight of stem and leaves of maize genotype Nefertiti decreased highly significantly by increasing the drought stress level in the soil. This reduction in the dry weight of stem and leaves was highly significant even at the highest soil moisture content level used when compared with the relative con- trol values (90% F.C.). At the level of 30% F.C the per- cent of reduction in the dry weight in stem and leaves was 75%, and 62% respectively, which indicate more deleterious effect of sever drought stress was recorded in dry weight of maize genotype Nefertiti. The stimulatory effect of the growth promoters varied considerably from one to another. Polyamines alone or in combination in- crease the dry matter yield at 50% F.C over those of the relative control values, (the level which reduce dry mat- ter yield to about 50% in only drought stresses plants). Also IAA promoted the dry matter production in plants irrigated with 50% F.C by about 56% over those of re- lated control value. Kinetine and mixture phytohormones induce a slight increase in these values at 50% F.C (about 27%). GA3 alleviated the inhibitory effect of drought at 50% F.C. In general polyamines seemed to be the superior up to 50% F.C, while phytohormones were the superior at 30% F.C (most of them nearly alleviated the inhibitory effect of drought stress. Drought stress up to 70% F.C induced a slight effect in the stem dry weight of maize genotype Bashaier, then it reduced gradually by the further increase in the level of drought stress. On the other hand the dry matter yield of leaves remained more or less unchanged up to the level of 50% F.C, and then a minute reduction was obtained. At the level of 30% F.C the percent of reduction in the dry weight of stem and leaves was 51% and 20% respec- tively in compared with the relative control (90% F.C). Phytohormones or polyamines treatments considerably increased the values of dry weight of stem and leaves of maize genotype Bashaier as compared with those of plants subjected only to the various level of drought stress. According to the data of dry matter of stem and leaves the maize genotype Bashaie r considered the drought tolerant genotype, while the maize genotype Ne- fertiti was the drought sensitive genotype. At the level of 30% F.C, the percent of reduction in the stem dry weight in maize genotype. Bashaier was 51% while in maize ge- notype Nefertiti the percent of reduction in the stem dry weight was 75%. Thus the data of field experiment rec- ommended the data of growth chamber experiment where maize genotype Ba shaier still the most drought tolerant genotype and the maize genotype Nefertiti was the most drought sensitive genotype. The leaf area of the maize genotype Nefertiti decreased highly significant by decreasing the soil moisture content, this reduction was more pronounced at the lowest field capacity used (30% F.C). At this level the percent of reduction was about 45% as compared with the relative control. The inhibitory effect of drought stress on the values of leaf area was completely alleviated as a result of phytohormones and polyamines treatments. Spermine was the most effective in the production of leaf area especially at the level of 90% F.C when com- pared with putrescine. The mixture of putrescine & spe- rmine was much more obvious in production of leaf area at all soil moisture content used. It is worthy to mention that, the highest values of leaf area obtained at the level of 30% F.C were recorded in plants treated with IAA or the mixture of polyamines (Ta ble 2). The leaf area of maize genotype Bashaier decreased smoothly by increasing the drought stress level in the soil. It reduced by only 24% at the level of 30% F.C. Phyto- hormones treatment considerably increased the values of leaf area, whatever the level of the soil moisture content used as compared with the corresponding drought stressed plants. Interestingly kinetin seemed to be the most effec- tive phytohormones in the two maize cultivars at the sever drought than the other phytohormones and or the mixture of them. Also, while IAA was the least effective phytohormones at mild drought it on the other hand the most effective at sever drought stress. Polyamines treat- ment resulted in a considerable increase in these values over those of the relative control up to the level of 70%, which was much more obvious in plants sprayed with mixture of polyamines. Thereafter these polyamines con- siderably elevated the values of leaf area, when com- pared with those of plants subjected to the 50% and 30% F.C without polyamines treatment (Table 2). One of the especial interest in this work is that the number of leaves remained constant in the two maize genotypes Nefer titi, and Bashaier whatever the treatment used which means that, the differences might located in the growth of leaves (area), rather than in the number of leaves which means that at least in maize external envi- ronmental conditions is not a limiting factor in the num- 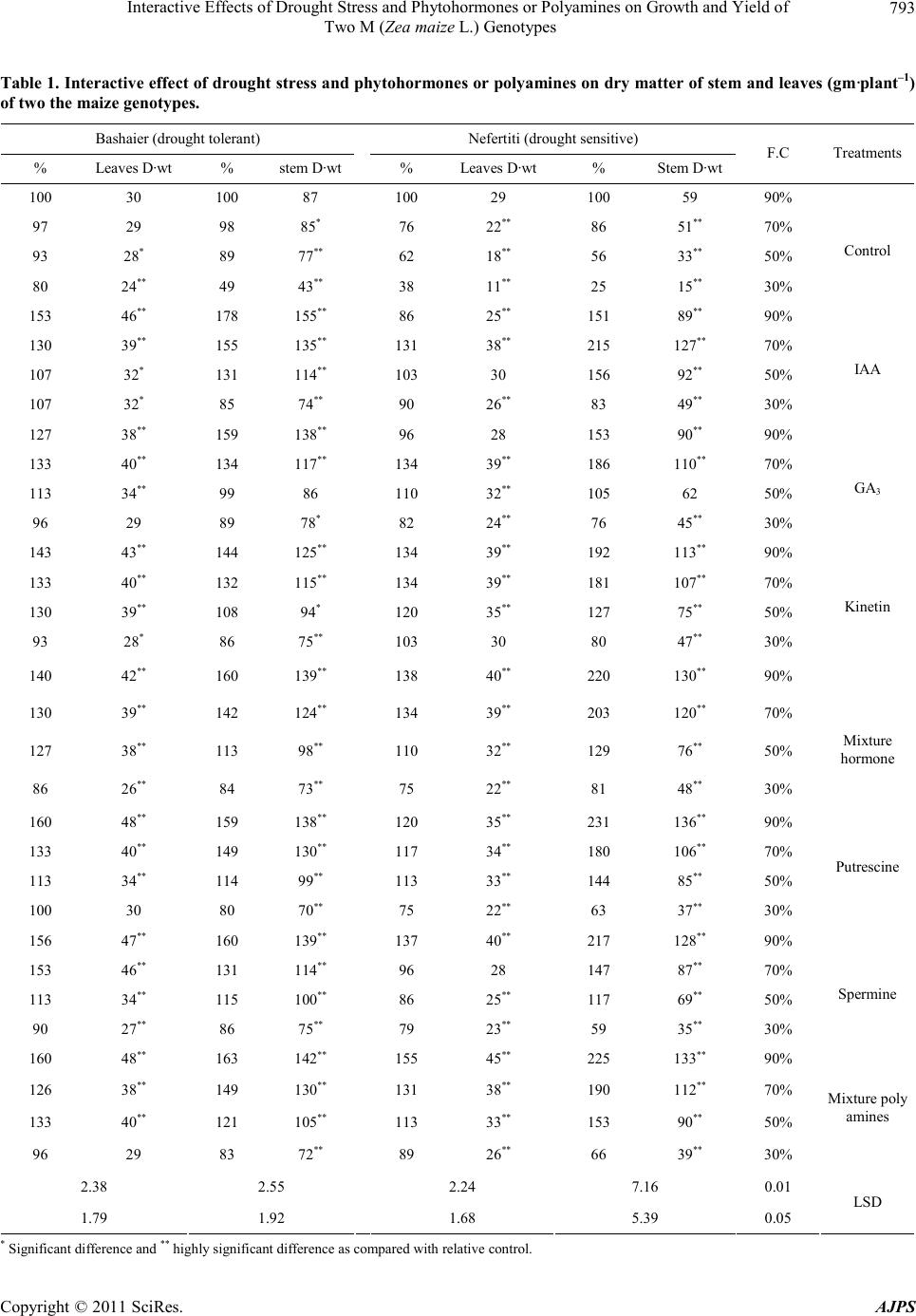 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 793 Table 1. Interactive effect of drought stress and phytohormones or polyamines on dry matter of stem and leaves (gm·plant–1) of two t he maize genotypes. Bashaier (drought tolerant) Nefertiti (drought sensitive) % Leaves D·wt % stem D·wt % Leaves D·wt % Stem D·wt F.C Tr eat m ent s 100 30 100 87 100 29 100 59 90% 97 29 98 85* 76 22** 86 51** 70% 93 28* 89 77** 62 18** 56 33** 50% 80 24** 49 43** 38 11** 25 15** 30% Control 153 46** 178 155** 86 25** 151 89** 90% 130 39** 155 135** 131 38** 215 127** 70% 107 32* 131 114** 103 30 156 92** 50% 107 32* 85 74** 90 26** 83 49** 30% IAA 127 38** 159 138** 96 28 153 90** 90% 133 40** 134 117** 134 39** 186 110** 70% 113 34** 99 86 110 32** 105 62 50% 96 29 89 78* 82 24** 76 45** 30% GA3 143 43** 144 125** 134 39** 192 113** 90% 133 40** 132 115** 134 39** 181 107** 70% 130 39** 108 94* 120 35** 127 75** 50% 93 28* 86 75** 103 30 80 47** 30% Kinetin 140 42** 160 139** 138 40** 220 130** 90% 130 39** 142 124** 134 39** 203 120** 70% 127 38** 113 98** 110 32** 129 76** 50% 86 26** 84 73** 75 22** 81 48** 30% Mixture hormone 160 48** 159 138** 120 35** 231 136** 90% 133 40** 149 130** 117 34** 180 106** 70% 113 34** 114 99** 113 33** 144 85** 50% 100 30 80 70** 75 22** 63 37** 30% Putrescine 156 47** 160 139** 137 40** 217 128** 90% 153 46** 131 114** 96 28 147 87** 70% 113 34** 115 100** 86 25** 117 69** 50% 90 27** 86 75** 79 23** 59 35** 30% Spermine 160 48** 163 142** 155 45** 225 133** 90% 126 38** 149 130** 131 38** 190 112** 70% 133 40** 121 105** 113 33** 153 90** 50% 96 29 83 72** 89 26** 66 39** 30% Mixture poly amines 2.38 2.55 2.24 7.16 0.01 1.79 1.92 1.68 5.39 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control. 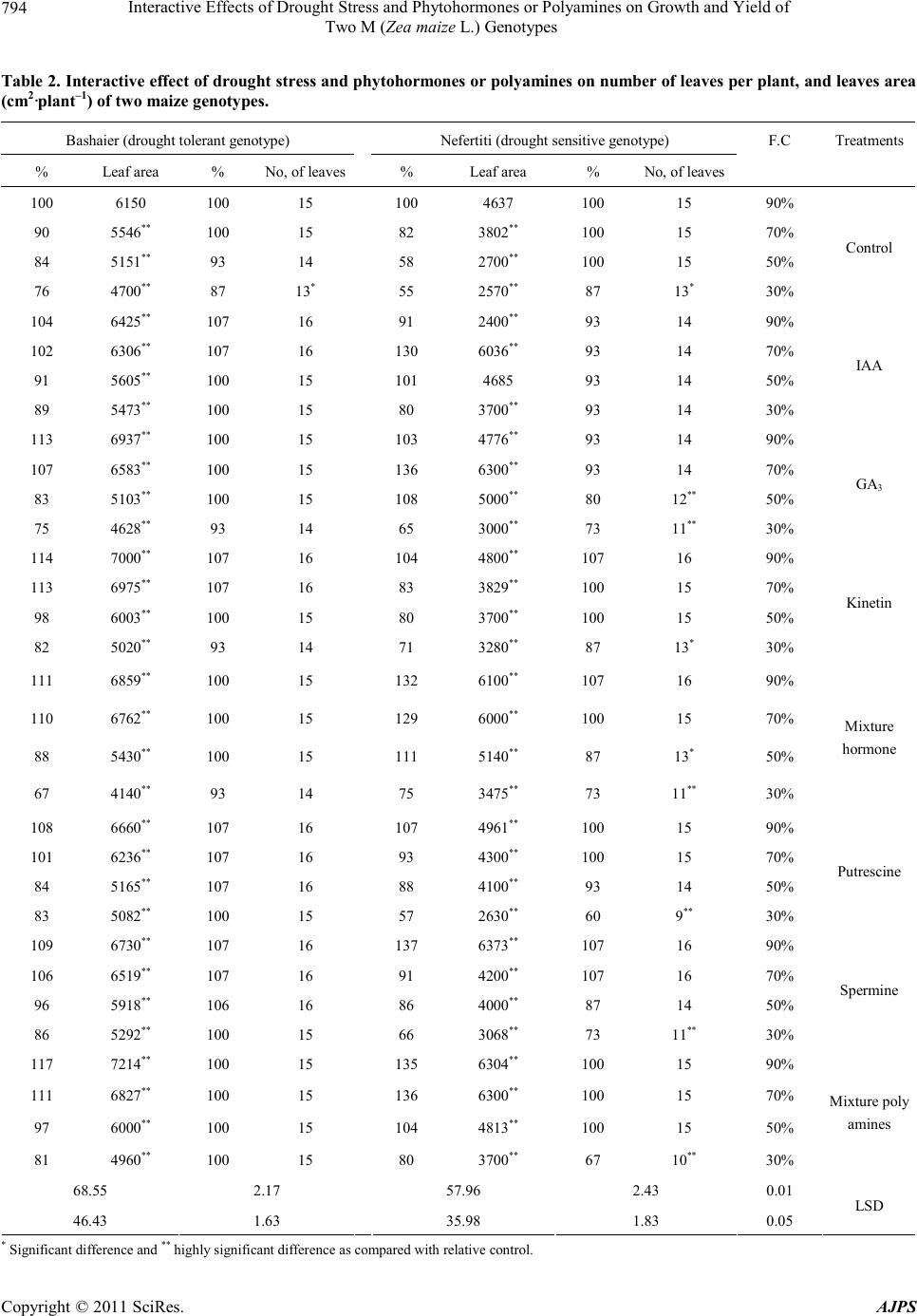 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 794 Table 2. Interactive effect of drought stress and phytohormones or polyamines on number of leaves per plant , and leaves area (cm2·plant–1) of two maize genotypes. Bashaier (drought tolerant genotype) Nefertiti (drought sensitive genotype) F.C Tr eat m ent s % Leaf area % No, of leaves % Leaf area % No, of leaves 100 6150 100 15 100 4637 100 15 90% 90 5546** 100 15 82 3802** 100 15 70% 84 5151** 93 14 58 2700** 100 15 50% 76 4700** 87 13* 55 2570** 87 13* 30% Control 104 6425** 107 16 91 2400** 93 14 90% 102 6306** 107 16 130 6036 ** 93 14 70% 91 5605** 100 15 101 4685 93 14 50% 89 5473** 100 15 80 3700** 93 14 30% IAA 113 6937** 100 15 103 4776 ** 93 14 90% 107 6583** 100 15 136 6300 ** 93 14 70% 83 5103** 100 15 108 5 000 ** 80 12** 50% 75 4628** 93 14 65 3000** 73 11** 30% GA3 114 7000** 107 16 104 4800 ** 107 16 90% 113 6975** 107 16 83 3829** 100 15 70% 98 6003** 100 15 80 3700** 100 15 50% 82 5020** 93 14 71 3280** 87 13* 30% Kinetin 111 6859** 100 15 132 6100 ** 107 16 90% 110 6762** 100 15 129 6000 ** 100 15 70% 88 5430** 100 15 111 5 140 ** 87 13* 50% 67 4140** 93 14 75 3475** 73 11** 30% Mixture hormone 108 6660** 107 16 107 4961 ** 100 15 90% 101 6236** 107 16 93 4300** 100 15 70% 84 5165** 107 16 88 4100** 93 14 50% 83 5082** 100 15 57 2630** 60 9** 30% Putrescine 109 6730** 107 16 137 6373 ** 107 16 90% 106 6519** 107 16 91 4200** 107 16 70% 96 5918** 106 16 86 4000** 87 14 50% 86 5292** 100 15 66 3068** 73 11** 30% Spermine 117 7214** 100 15 135 6304 ** 100 15 90% 111 6827** 100 15 136 6300 ** 100 15 70% 97 6000** 100 15 104 4 813 ** 100 15 50% 81 4960** 100 15 80 3700** 67 10** 30% Mixture poly amines 68.55 2.17 57.96 2.43 0.01 46.43 1.63 35.98 1.83 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control.  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 795 ber of leaves. The data of maize genotype Nefertiti in Table 3 reveal that chlorophyll “a, b, and carotenoids” contents de- creased by decreasing the soil moisture content, which was much more obvious at the sever drought A marked and progressive increase in the photosynthetic pigments concentration was exhibited when the drought stressed plants sprayed with phytohormones or polyamines, which was much more obvious in IAA treated plants. The data of maize genotype Bashaier in Table 4 ob- served that, the photosynthetic pigments (chlorophyll “a, b, and carotenoids) concentration remained almost more or less unchanged at most drought stress level and a slight reduction was recorded in these values only at the level of 30% F.C. Using of the growth regulators (phy- tohormones or polyamines) considerably activated the biosynthesis of the photosynthetic pigments. This stimu- latory effect was much more obvious in polyamines treated plants than in phytohormones treated plants. Also, the mixture of polyamines was the superior especially at the sever drought when compared with the other activa- tors. The data of maize genotype Nefertiti in Table 5 re- veals that, The contents of potassium in shoots and roots slightly increased up to the level of 50% F.C, then it re- mained more or less unchanged even at the lowest field capacity used (30% F.C). A marked and progressive ac- tivation in absorption of K+ in shoots and roots has been observed as a result of phytohormones or polyamines treatment. There is no, observable differences among the different growth promoting substances used (Ta ble 5) The data of maize genotype Nefertiti in Table 5 re- veals that, there is a highly significant reduction in the absorption and accumulation of Ca++ and Mg ++ in shoots and roots, as the drought stress level increased in the soil the highest reduction was obtained at the highest drought stress level used. On the other hand the absorption and accumulation of Ca++ and Mg++ improved as a result of the interaction effect of phytohormones or polyamines in shoots and roots of the maize genotype Nefertiti. This stimulatory effect was more pronounced in shoots than in roots and in polyamines treated plants than in phytohor- mones treated plants. The data of the maize genotype Bashaier in Table 6 reveals that, the contents of potassium in shoots and roots increased progressively by increasing drought stress level in the soil, therefore the highest increase in K+ content was found to be at 30% F.C. At this level the percent of in- crease in K+ content in shoots and roots was 124% and 128% respectively in relation to the relative control val- ues. The amount of K+ in shoot was much higher than in roots whatever the drought stress level used. Additional activation in K+ content in shoots and roots of drought stressed maize genotype Bashaier was observed as re- sults of the phytohormones or polyamines treatments. This seemed to be more observable in shoots than in roots. The data of maize genotype Bashaier in Table 6 reveal that, there is a general promotion in the absorption and accumulation of calcium and magnesium in stems and roots of maize genotype Bashaier by drought stress. This was more pronounced in roots than in stems. Treatment with growth regulators resulted in most cases in a general activation in calcium and magnesium in the two tested plant organs. The data of maize genotype Nefertiti in Table 7 re- veals that, the number of grains per ear and the 1000 grain weight reduced slightly in up to the level of 70% F.C. On the other hand the number of grains and the 1000 grain weight increased progressively at the level of 90% F.C, and to some extent at the level of 70% F.C as a result of phytohormones or polyamines treatment The data of maize genotype Nefertiti in Table 7 re- veals that, it is worthy to mention that, the drought sensi- tive cultivar produce crop yield only up to the level of 70% F.C, while treatment with polyamines produce crop yield up to 50% F.C. The crop yield at the level of 50% in plants treated with putrescine, spermine or mixture polyamines was 13, 15, and 12.8 Ardab/feddan in rela- tion to 21.14 Ardab/feddan produced in normally treated plants (90% F.C). Also IAA is the only phytohormone which produce crop yield at the level of 50% F.C (5.23 Ardab/feddan). Additionally all of the used polyamines increased the crop yield at the normal condition (90% F.C) as compared with untreated plants. The crop yield of plants treated with putrescine, spermine or mixture polyamines was 26.5, 24, and 26 Ardab/feddan com- pared to 21.14 (at the normal level). Moreover plants treated with mixture of polyamines produced 25 Ardab/ feddan; at the level of 70% F.C. GA3, Kinetin and mix- ture of hormones retarded the crop yield even at the level of 90% F.C, although they considerably enhance the plant growth during the vegetative stage. This unex- pected phenomenon seemed to be complicated and needs a numerous and further studies, Why those phytohor- mones stimulated the vegetative growth and inhibit crop yield? Is there a negative correlation between the exoge- nous application of these phytohormones and the group of phytohormones responsible for the flowering and fruit- ing or associated with the exogenous dose of the applied phytohormones???,, Is a concentration affect,, or what???? The data of maize genotype Bashaier in Table 8 re- veals that, imposing drought stress induce a very minute reduction in a number of grains per ear (12% only at 50% F.C) and the 1000 grain weight. On the other hand 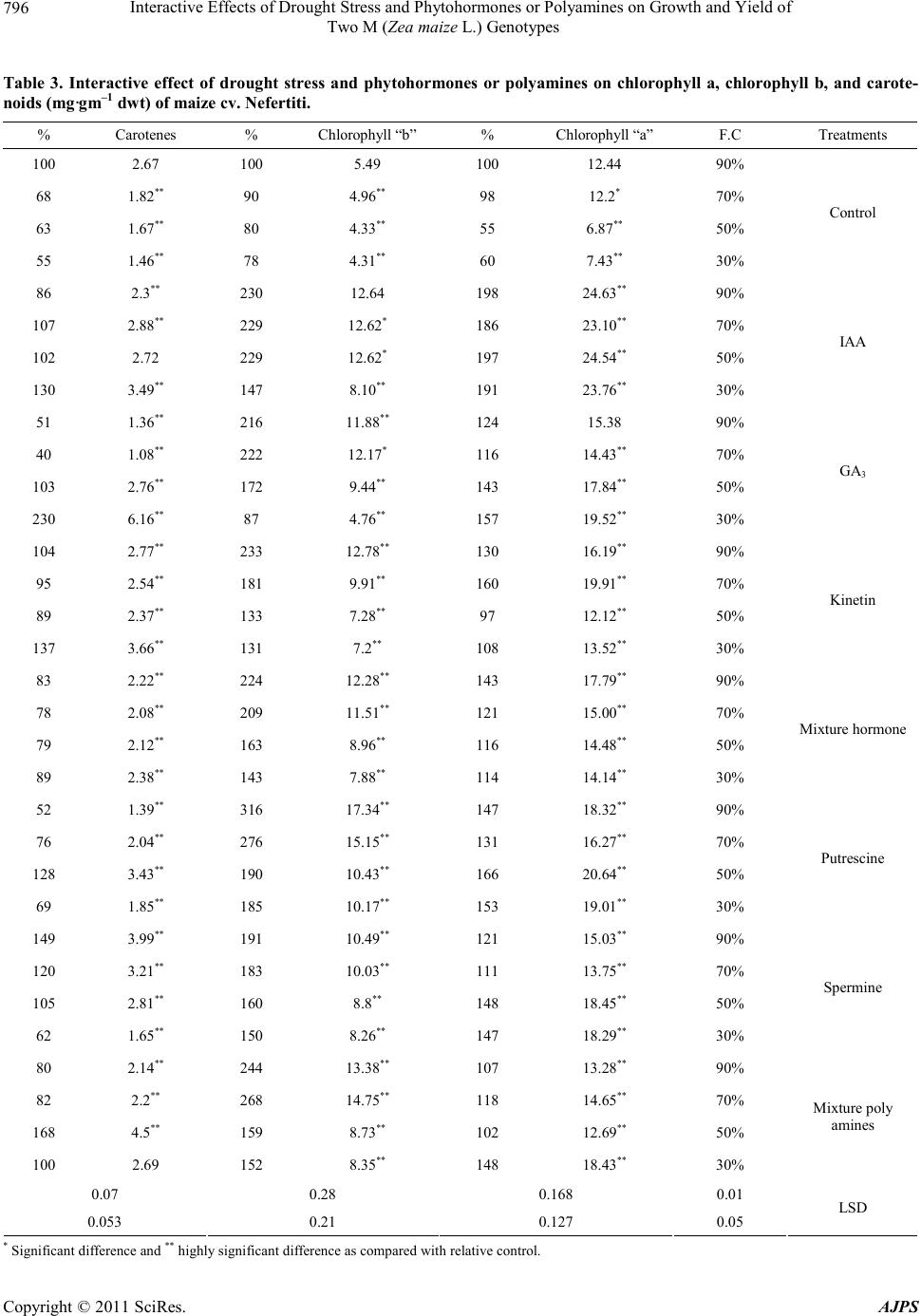 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 796 Table 3. Interactive effect of drought stress and phytohormones or polyamines on chlorophyll a, chlorophyll b, and carote- noids (mg·gm–1 dwt) of maize cv. Nefertiti. % Carotenes % Chlorophyll “b” % Chlorophyll “a” F.C Trea tments 100 2.67 100 5.49 100 12.44 90% 68 1.82** 90 4.96** 98 12.2* 70% 63 1.67** 80 4.33** 55 6.87** 50% 55 1.46** 78 4.31** 60 7.43** 30% Control 86 2.3** 230 12.64 198 24.63** 90% 107 2.88** 229 12.62* 186 23.10** 70% 102 2.72 229 12.62* 197 24.54** 50% 130 3.49** 147 8.10** 191 23.76** 30% IAA 51 1.36** 216 11.88** 124 15.38 90% 40 1.08** 222 12.17* 116 14.43** 70% 103 2.76** 172 9.44** 143 17.84** 50% 230 6.16** 87 4.76** 157 19.52** 30% GA3 104 2.77** 233 12.78** 130 16.19** 90% 95 2.54** 181 9.91** 160 19.91** 70% 89 2.37** 133 7.28** 97 12.12** 50% 137 3.66** 131 7.2** 108 13.52** 30% Kinetin 83 2.22** 224 12.28** 143 17.79** 90% 78 2.08** 209 11.51** 121 15.00** 70% 79 2.12** 163 8.96** 116 14.48** 50% 89 2.38** 143 7.88** 114 14.14** 30% Mixture hormone 52 1.39** 316 17.34** 147 18.32** 90% 76 2.04** 276 15.15** 131 16.27** 70% 128 3.43** 190 10.43** 166 20.64** 50% 69 1.85** 185 10.17** 153 19.01** 30% Putrescine 149 3.99** 191 10.49** 121 15.03** 90% 120 3.21** 183 10.03** 111 13.75** 70% 105 2.81** 160 8.8** 148 18.45** 50% 62 1.65** 150 8.26** 147 18.29** 30% Spermine 80 2.14** 244 13.38** 107 13.28** 90% 82 2.2** 268 14.75** 118 14.65** 70% 168 4.5** 159 8.73** 102 12.69** 50% 100 2.69 152 8.35** 148 18.43** 30% Mixture poly amines 0.07 0.28 0.168 0.01 0.053 0.21 0.127 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control. 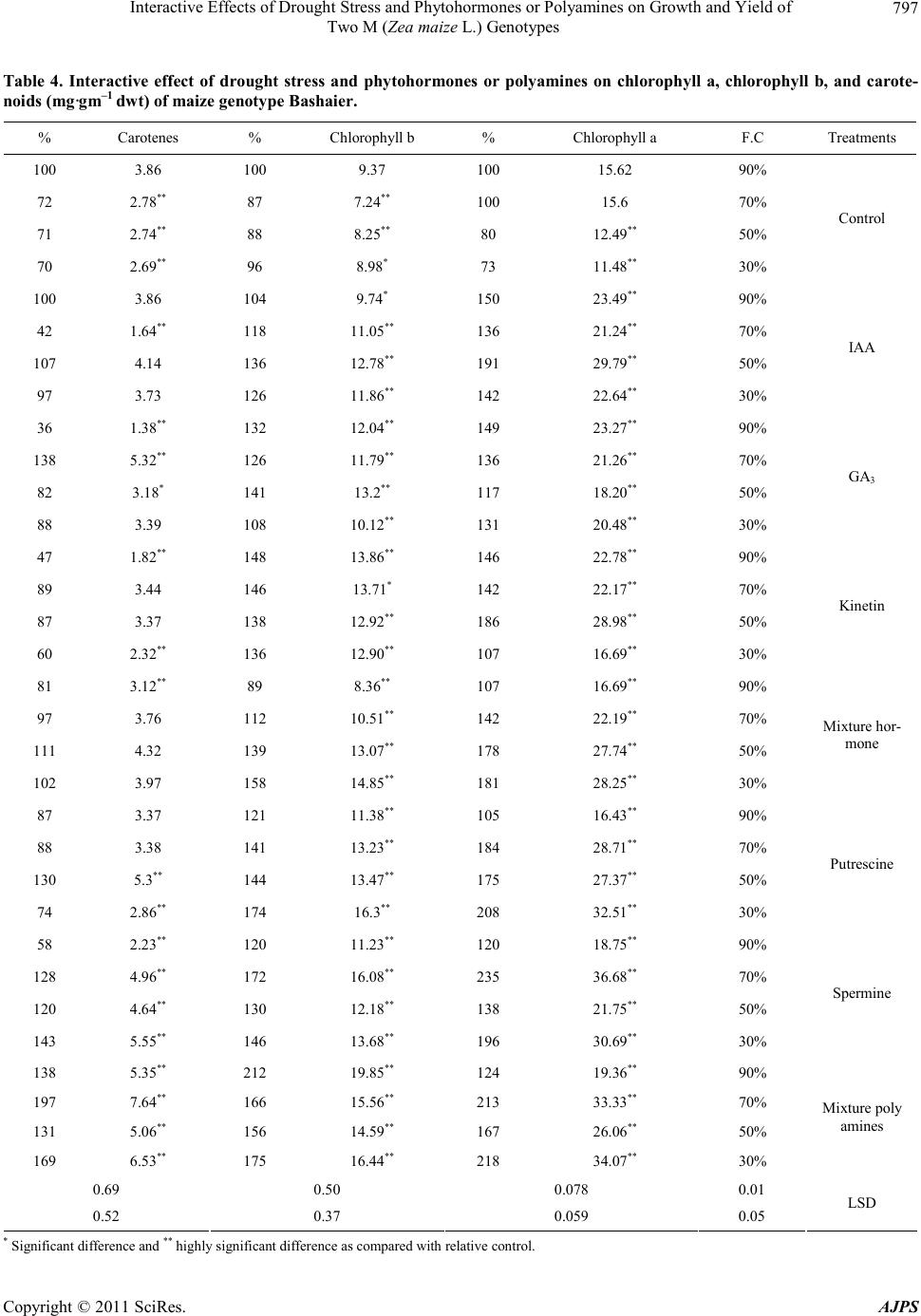 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 797 Table 4. Interactive effect of drought stress and phytohormones or polyamines on chlorophyll a, chlorophyll b, and carote- noids (mg·gm–1 dwt) of maize genotyp e Bashaier. % Carotenes % Chlorophyll b % Chlorophyll a F.C Treatm ent s 100 3.86 100 9.37 100 15.62 90% 72 2.78** 87 7.24** 100 15.6 70% 71 2.74** 88 8.25** 80 12.49** 50% 70 2.69** 96 8.98* 73 11.48** 30% Control 100 3.86 104 9.74* 150 23.49** 90% 42 1.64** 118 11.05** 136 21.24** 70% 107 4.14 136 12.78** 191 29.79** 50% 97 3.73 126 11.86** 142 22.64** 30% IAA 36 1.38** 132 12.04** 149 23.27** 90% 138 5.32** 126 11.79** 136 21.26** 70% 82 3.18* 141 13.2** 117 18.20** 50% 88 3.39 108 10.12** 131 20.48** 30% GA3 47 1.82** 148 13.86** 146 22.78** 90% 89 3.44 146 13.71* 142 22.17** 70% 87 3.37 138 12.92** 186 28.98** 50% 60 2.32** 136 12.90** 107 16.69** 30% Kinetin 81 3.12** 89 8.36** 107 16.69** 90% 97 3.76 112 10.51** 142 22.19** 70% 111 4.32 139 13.07** 178 27.74** 50% 102 3.97 158 14.85** 181 28.25** 30% Mixture hor- mone 87 3.37 121 11.38** 105 16.43** 90% 88 3.38 141 13.23** 184 28.71** 70% 130 5.3** 144 13.47** 175 27.37** 50% 74 2.86** 174 16.3** 208 32.51** 30% Putrescine 58 2.23** 120 11.23** 120 18.75** 90% 128 4.96** 172 16.08** 235 36.68** 70% 120 4.64** 130 12.18** 138 21.75** 50% 143 5.55** 146 13.68** 196 30.69** 30% Spermine 138 5.35** 212 19.85** 124 19.36** 90% 197 7.64** 166 15.56** 213 33.33** 70% 131 5.06** 156 14.59** 167 26.06** 50% 169 6.53** 175 16.44** 218 34.07** 30% Mixture poly amines 0.69 0.50 0.078 0.01 0.52 0.37 0.059 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control. 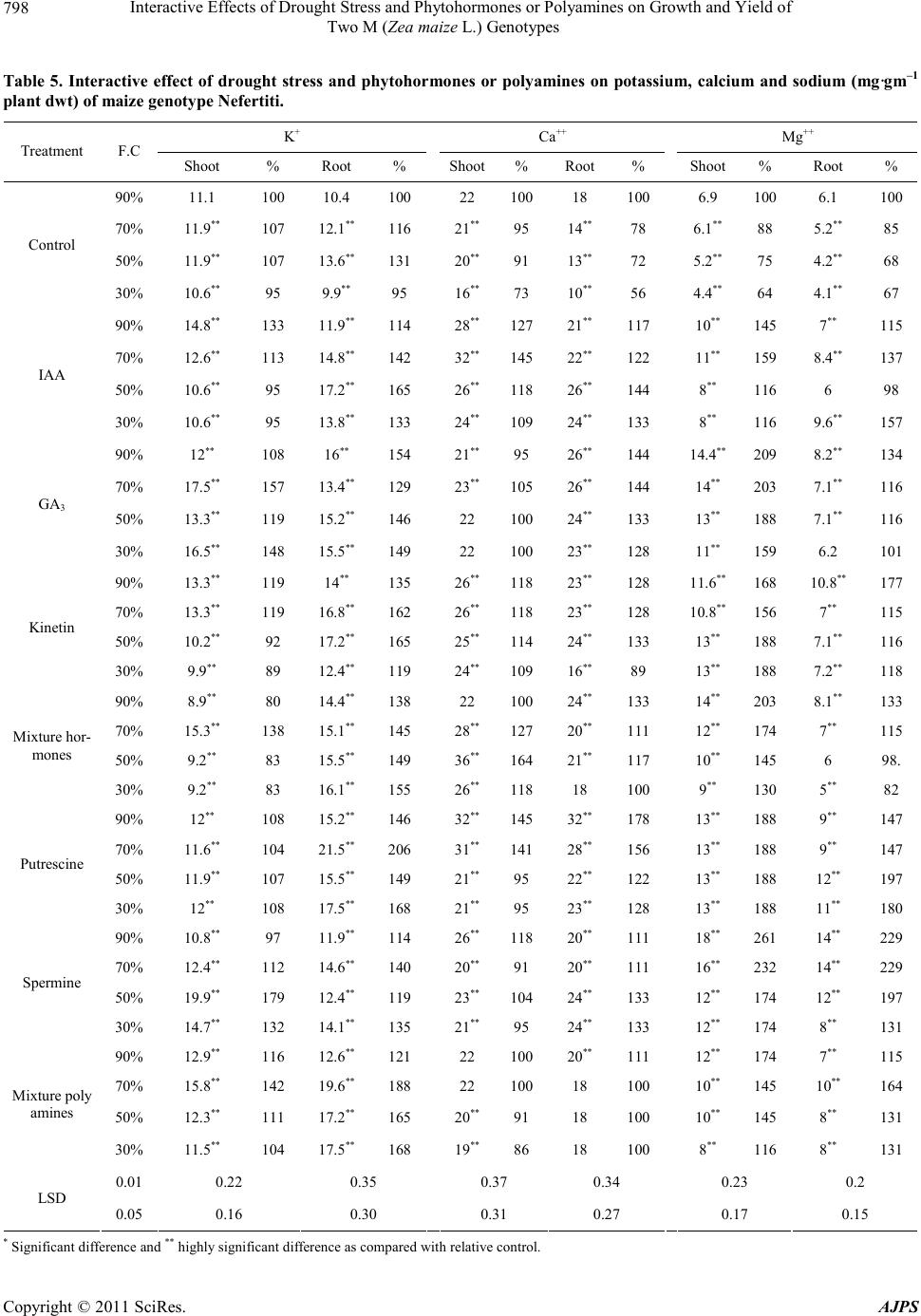 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 798 Table 5. Interactive effect of drought stress and phytohormones or polyamines on potassium, calcium and sodium (mg·gm–1 plant dwt) of maize genotype N efertiti. K+ Ca++ Mg++ Treatm ent F.C Shoot % Root % Shoot % Root % Shoot % Root % 90% 11.1 100 10.4 10022 10018 1006.9 100 6.1 100 70% 11.9** 107 12.1** 116 21** 95 14** 78 6.1** 88 5.2** 85 50% 11.9** 107 13.6** 131 20** 91 13** 72 5.2** 75 4.2** 68 Control 30% 10.6** 95 9.9** 95 16** 73 10** 56 4.4** 64 4.1** 67 90% 14.8** 133 11.9** 114 28** 127 21** 117 10** 145 7** 115 70% 12.6** 113 14.8** 142 32** 145 22** 122 11** 159 8.4** 137 50% 10.6** 95 17.2** 165 26** 118 26** 144 8** 116 6 98 IAA 30% 10.6** 95 13.8** 133 24** 109 24** 133 8** 116 9.6** 157 90% 12** 108 16** 154 21** 95 26** 144 14.4** 209 8.2** 134 70% 17.5** 157 13.4** 129 23** 105 26** 144 14** 203 7.1** 116 50% 13.3** 119 15.2** 14622 10024** 133 13** 188 7.1** 116 GA3 30% 16.5** 148 15.5** 14922 10023** 128 11** 159 6.2 101 90% 13.3** 119 14** 135 26** 118 23** 128 11.6** 168 10.8** 177 70% 13.3** 119 16.8** 162 26** 118 23** 128 10.8** 156 7** 115 50% 10.2** 92 17.2** 165 25** 114 24** 133 13** 188 7.1** 116 Kinetin 30% 9.9** 89 12.4** 119 24** 109 16** 89 13** 188 7.2** 118 90% 8.9** 80 14.4** 13822 10024** 133 14** 203 8.1** 133 70% 15.3** 138 15.1** 145 28** 127 20** 111 12** 174 7** 115 50% 9.2** 83 15.5** 149 36** 164 21** 117 10** 145 6 98. Mixture hor- mones 30% 9.2** 83 16.1** 155 26** 11818 1009** 130 5** 82 90% 12** 108 15.2** 146 32** 145 32** 178 13** 188 9** 147 70% 11.6** 104 21.5** 206 31** 141 28** 156 13** 188 9** 147 50% 11.9** 107 15.5** 149 21** 95 22** 122 13** 188 12** 197 Putrescine 30% 12** 108 17.5** 168 21** 95 23** 128 13** 188 11** 180 90% 10.8** 97 11.9** 114 26** 118 20** 111 18** 261 14** 229 70% 12.4** 112 14.6** 140 20** 91 20** 111 16** 232 14** 229 50% 19.9** 179 12.4** 119 23** 104 24** 133 12** 174 12** 197 Spermine 30% 14.7** 132 14.1** 135 21** 95 24** 133 12** 174 8** 131 90% 12.9** 116 12.6** 12122 10020** 111 12** 174 7** 115 70% 15.8** 142 19.6** 18822 10018 10010** 145 10** 164 50% 12.3** 111 17.2** 165 20** 91 18 10010** 145 8** 131 Mixture poly amines 30% 11.5** 104 17.5** 168 19** 86 18 1008** 116 8** 131 0.01 0.22 0.35 0.37 0.34 0.23 0.2 LSD 0.05 0.16 0.30 0.31 0.27 0.17 0.15 * Significant difference and ** highly significant difference as compared with relative control. 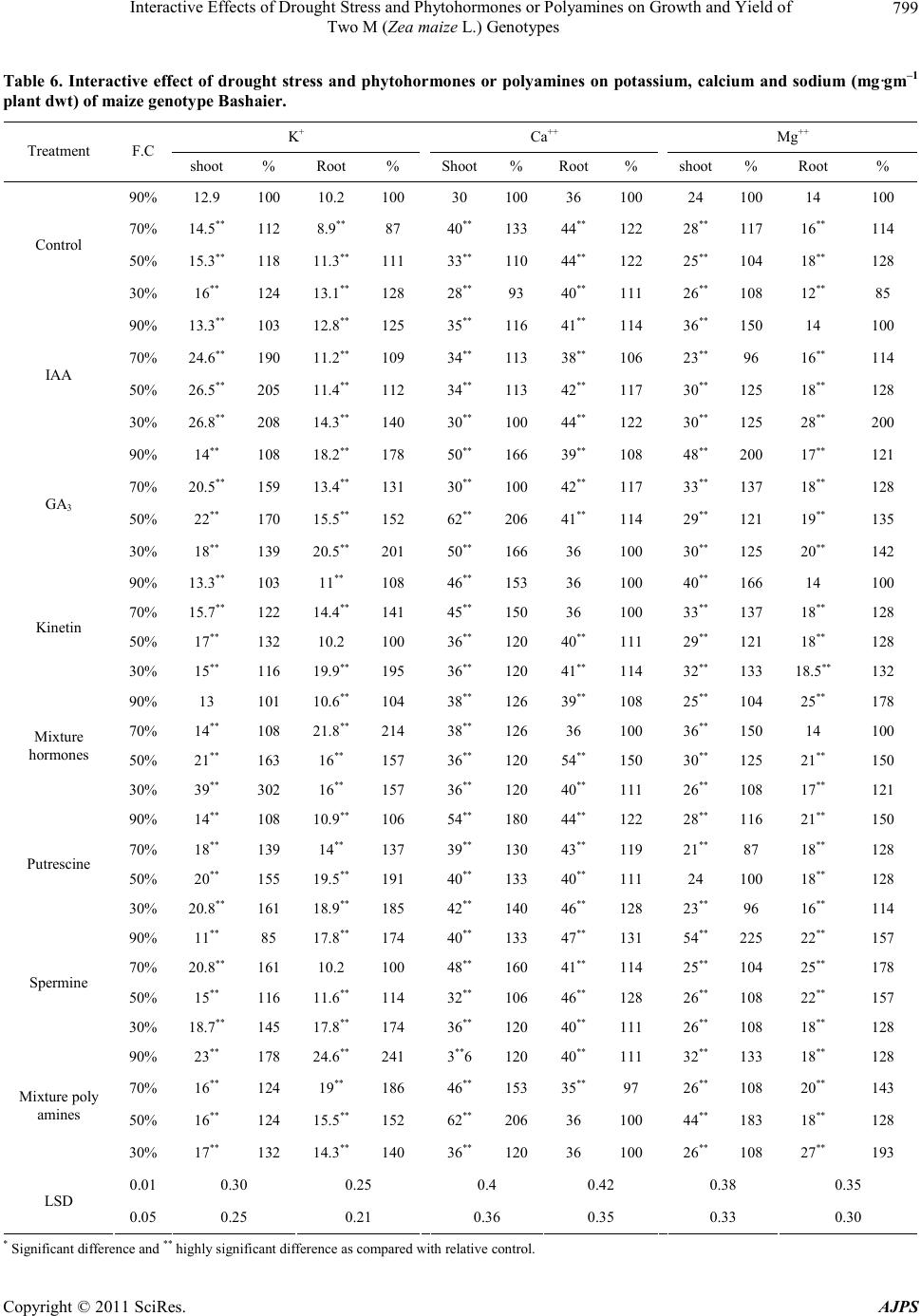 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 799 Table 6. Interactive effect of drought stress and phytohormones or polyamines on potassium, calcium and sodium (mg·gm–1 plant dwt) of maize genotype Bashaie r. K+ Ca++ Mg++ Treatm ent F.C shoot % Root % Shoot % Root % shoot % Root % 90% 12.9 100 10.2 100 30 100 36 100 24 100 14 100 70% 14.5** 112 8.9** 87 40** 133 44** 122 28** 117 16** 114 50% 15.3** 118 11.3** 111 33** 110 44** 122 25** 104 18** 128 Control 30% 16** 124 13.1** 128 28** 93 40** 111 26** 108 12** 85 90% 13.3** 103 12.8** 125 35** 116 41** 114 36** 150 14 100 70% 24.6** 190 11.2** 109 34** 113 38** 106 23** 96 16** 114 50% 26.5** 205 11.4** 112 34** 113 42** 117 30** 125 18** 128 IAA 30% 26.8** 208 14.3** 140 30** 100 44** 122 30** 125 28** 200 90% 14** 108 18.2** 178 50** 166 39** 108 48** 200 17** 121 70% 20.5** 159 13.4** 131 30** 100 42** 117 33** 137 18** 128 50% 22** 170 15.5** 152 62** 206 41** 114 29** 121 19** 135 GA3 30% 18** 139 20.5** 201 50** 166 36 100 30** 125 20** 142 90% 13.3** 103 11** 108 46** 153 36 100 40** 166 14 100 70% 15.7** 122 14.4** 141 45** 150 36 100 33** 137 18** 128 50% 17** 132 10.2 100 36** 120 40** 111 29** 121 18** 128 Kinetin 30% 15** 116 19.9** 195 36** 120 41** 114 32** 133 18.5** 132 90% 13 101 10.6** 104 38** 126 39** 108 25** 104 25** 178 70% 14** 108 21.8** 214 38** 126 36 100 36** 150 14 100 50% 21** 163 16** 157 36** 120 54** 150 30** 125 21** 150 Mixture hormones 30% 39** 302 16** 157 36** 120 40** 111 26** 108 17** 121 90% 14** 108 10.9** 106 54** 180 44** 122 28** 116 21** 150 70% 18** 139 14** 137 39** 130 43** 119 21** 87 18** 128 50% 20** 155 19.5** 191 40** 133 40** 111 24 100 18** 128 Putrescine 30% 20.8** 161 18.9** 185 42** 140 46** 128 23** 96 16** 114 90% 11** 85 17.8** 174 40** 133 47** 131 54** 225 22** 157 70% 20.8** 161 10.2 100 48** 160 41** 114 25** 104 25** 178 50% 15** 116 11.6** 114 32** 106 46** 128 26** 108 22** 157 Spermine 30% 18.7** 145 17.8** 174 36** 120 40** 111 26** 108 18** 128 90% 23** 178 24.6** 241 3**6 120 40** 111 32** 133 18** 128 70% 16** 124 19** 186 46** 153 35** 97 26** 108 20** 143 50% 16** 124 15.5** 152 62** 206 36 100 44** 183 18** 128 Mixture poly amines 30% 17** 132 14.3** 140 36** 120 36 100 26** 108 27** 193 0.01 0.30 0.25 0.4 0.42 0.38 0.35 LSD 0.05 0.25 0.21 0.36 0.35 0.33 0.30 * Significant difference and ** highly significant difference as compared with relative control. 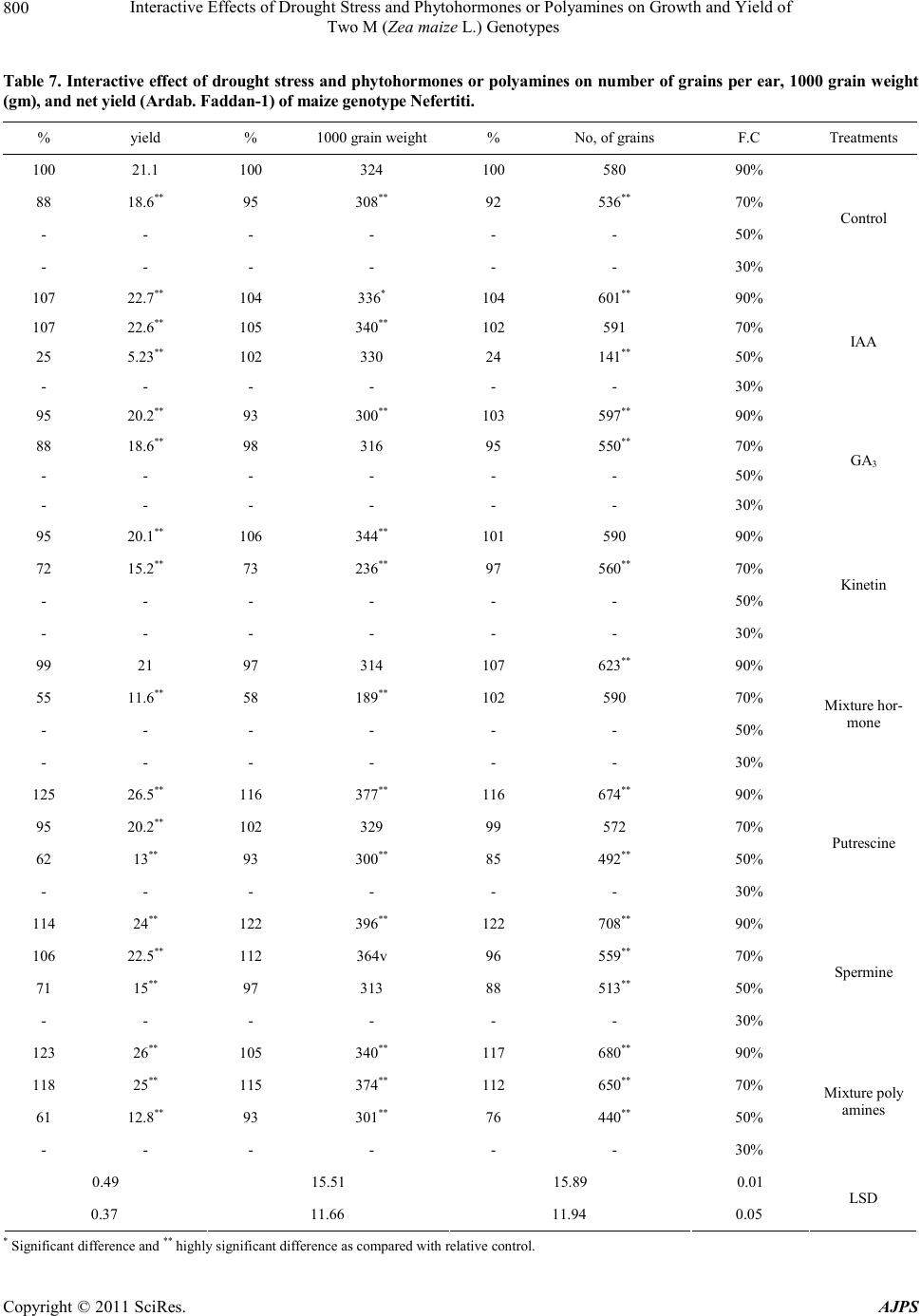 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 800 Table 7. Interactive effect of drought stress and phytohormones or polyamines on number of grains per ear, 1000 grain weight (gm), and ne t yie ld (Arda b. Faddan-1) of ma ize geno type Ne f ert iti. % yield % 1000 grain weight % No, of grains F.C Treat ments 100 21.1 100 324 100 580 90% 88 18.6** 95 308** 92 536** 70% - - - - - - 50% - - - - - - 30% Control 107 22.7** 104 336* 104 601** 90% 107 22.6** 105 340** 102 591 70% 25 5.23** 102 330 24 141** 50% - - - - - - 30% IAA 95 20.2** 93 300** 103 597** 90% 88 18.6** 98 316 95 550** 70% - - - - - - 50% - - - - - - 30% GA3 95 20.1** 106 344** 101 590 90% 72 15.2** 73 236** 97 560** 70% - - - - - - 50% - - - - - - 30% Kinetin 99 21 97 314 107 623** 90% 55 11.6** 58 189** 102 590 70% - - - - - - 50% - - - - - - 30% Mixture hor- mone 125 26.5** 116 377** 116 674** 90% 95 20.2** 102 329 99 572 70% 62 13** 93 300** 85 492** 50% - - - - - - 30% Putrescine 114 24** 122 396** 122 708** 90% 106 22.5** 112 364v 96 559** 70% 71 15** 97 313 88 513** 50% - - - - - - 30% Spermine 123 26** 105 340** 117 680** 90% 118 25** 115 374** 112 650** 70% 61 12.8** 93 301** 76 440** 50% - - - - - - 30% Mixture poly amines 0.49 15.51 15.89 0.01 0.37 11.66 11.94 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control. 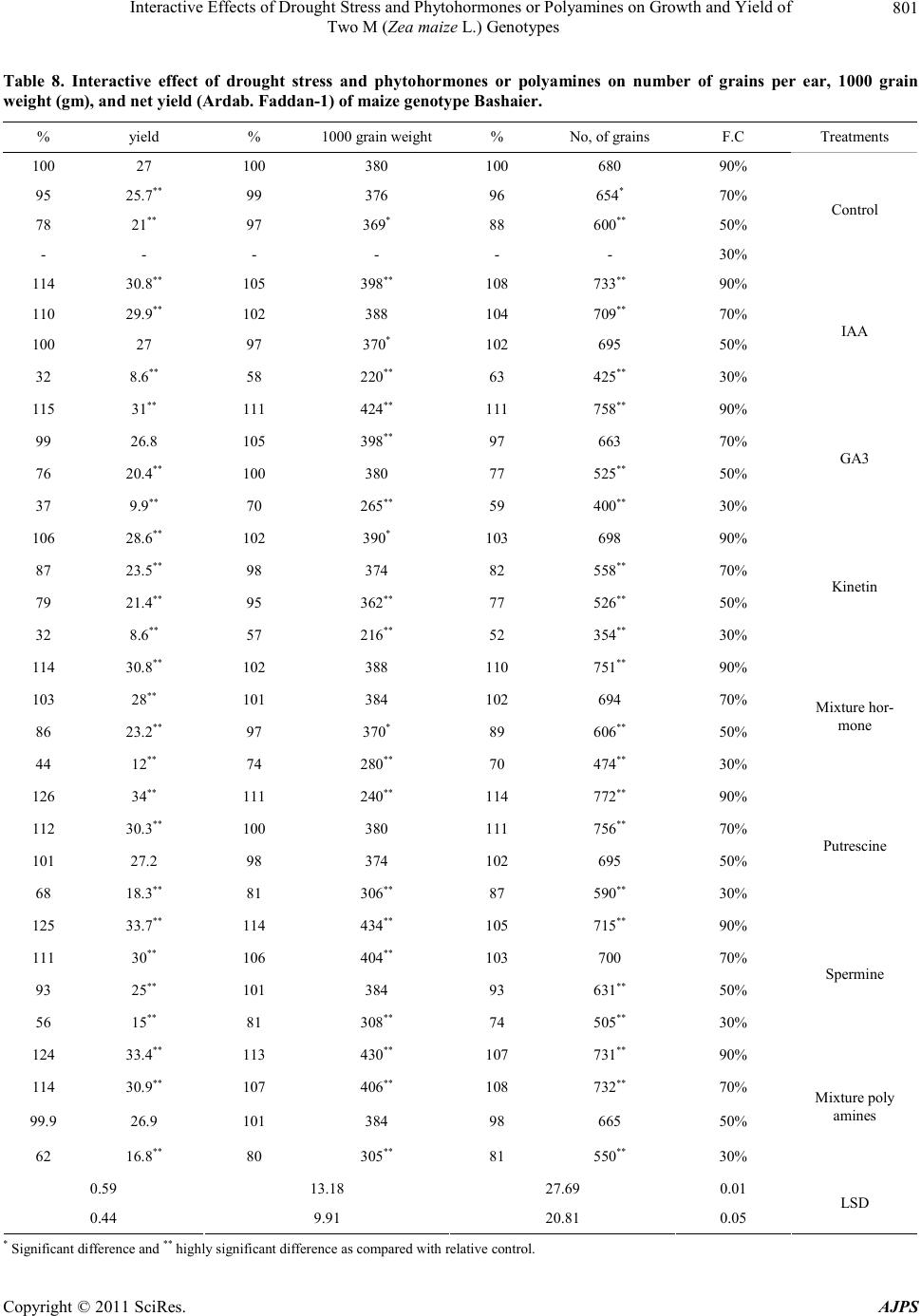 Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 801 Table 8. Interactive effect of drought stress and phytohormones or polyamines on number of grains per ear, 1000 grain weight (g m), and net yield ( Ardab. F addan-1) of maiz e genotype B a shaier. % yield % 1000 grain weight % No, of grains F.C Treatm ent s 100 27 100 380 100 680 90% 95 25.7** 99 376 96 654* 70% 78 21** 97 369* 88 600** 50% - - - - - - 30% Control 114 30.8** 105 398** 108 733** 90% 110 29.9** 102 388 104 709** 70% 100 27 97 370* 102 695 50% 32 8.6** 58 220** 63 425** 30% IAA 115 31** 111 424** 111 758** 90% 99 26.8 105 398** 97 663 70% 76 20.4** 100 380 77 525** 50% 37 9.9** 70 265** 59 400** 30% GA3 106 28.6** 102 390* 103 698 90% 87 23.5** 98 374 82 558** 70% 79 21.4** 95 362** 77 526** 50% 32 8.6** 57 216** 52 354** 30% Kinetin 114 30.8** 102 388 110 751** 90% 103 28** 101 384 102 694 70% 86 23.2** 97 370* 89 606** 50% 44 12** 74 280** 70 474** 30% Mixture hor- mone 126 34** 111 240** 114 772** 90% 112 30.3** 100 380 111 756** 70% 101 27.2 98 374 102 695 50% 68 18.3** 81 306** 87 590** 30% Putrescine 125 33.7** 114 434** 105 715** 90% 111 30** 106 404** 103 700 70% 93 25** 101 384 93 631** 50% 56 15** 81 308** 74 505** 30% Spermine 124 33.4** 113 430** 107 731** 90% 114 30.9** 107 406** 108 732** 70% 99.9 26.9 101 384 98 665 50% 62 16.8** 80 305** 81 550** 30% Mixture poly amines 0.59 13.18 27.69 0.01 0.44 9.91 20.81 0.05 LSD * Significant difference and ** highly significant difference as compared with relative control.  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 802 phytohormones or polyamines treatment considerably in- creased the number of grains per ear and the 1000 grain weight up to 70% F.C. Additionally putrescine and IAA increase this number at the level of 50% F.C. The data of the crop yield of maize genotype Bashaier in Table 8 was interesting: 1) It produced a suitable amount of crop yield up to 50% F.C (It produce 21 Ardab/feddan compared to 27 Ardab/feddan at 90% F.C) note that the sensitive geno- type did not produce any crop yield at 50% F.C. 2) Phytohormones or polyamines give a crop yield more or less similar to that recorded in the relative con- trol up to 50% F.C. This was more pronounced in poly- amines than in phytohormones. Moreover most of these activators enhanced the crop yield to 70% F.C in relation to the relative control. This was also more pronounced in polyamines & IAA treated plants. 3) Phytohormones or polyamines give a suitable amount of crop yield at the level of 30% F.C (The level which did not give a crop yield in this genotype) 5. Discussion The phenological characteristics (dry matter yield and leaf area) of the field experiment recommended the drought tolerance of maize genotype Bashaier and the susceptibility of maize genotype Nefertiti. Accordingly the growth of maize genotype Bashaier remained mostly unchanged at mild drought stress whereas the growth of maize genotype Nefertiti dropped even at the lower level of drought stress, also the percent of reduction of the studied growth parameter at sever drought was much more higher in maize genotype Nefertiti than maize ge- notype Bashaier. Accordingly, at the level of 30% F.C. The percent of reduction in the dry matter of leaves and stems of maize genotype Nefertiti was 62% & 75% re- spectively, while in maize genotype Bashaier it was 20% & 51% respectively. The differences in the growth crite- tria among species and cultivars might be used as a suit- able selection criterion for the drought tolerance of these species and genotypes. The inhibitory effect of drought on growth parameters could be attributed to the osmotic effect of water stress [26,34,35]. Also, the reduction of yield may be ascribed to the harmful effect of soil mois- ture stress and nutrient balance disorder in root media [36], or reduced rate of new cell production may be make additional contribution to the inhibition of growth [37-39]. The reduction in growth criteria due to drought stress might be related to disturbance of water flow from root to shoot [40], decrease in water potential of cell sap [41], or inhibition of cell division [42]. The values of leaf area are varied among the two drought stressed maize genotypes. They were much higher in mai ze genotype Bashaier than in maize genotyp e Nefer- titi. Also the reduction in the leaf area was much more pronounced in maize genotype Nefertiti than in maize genotype Bashaier. Along with this the photosynthetic pigments remained more or less unchanged in maize ge- notype Bashaier and decreased highly significantly in maize genotype Nefertiti, which might improved the pho- tosynthetic apparatus in maize genotype Bashaier than in maize genotype Nefertiti. The ability of the plants to in- crease its green area could be increased its drought tol- erance [26]. This was found to be linked with the effi- ciency of photosynthesis apparatus and consequently the production of photoassimilates in the two maize geno- types. [43,44] reported that, the reduction in leaves area by drought stress may be due to a reduction in leaf ex- pansion, probably due to the effect of drought stress on cell division or cell expansion or both. [45] reported that, the reduction observed in the leaf area and dry weight of the drought stressed plants can be attributed to the chan- ges in plant water relations under drought stress which cause a reduction in meristem activity as well as cell elongation [46], thereby inhibiting leaf expansion [47]. The observed decrease in dry weight of the drought- stressed plants can be traced to the scanty recovery of leaves following limited photosynthesis production [45]. [48] proposed that, the death of old leaves due to the drought stress in the tissue would prevent the supply of nutrients or hormones to emerging leaves, and reducing leaf area. [49] concluded that a part from decreased growth might be attributed to the reduction in the leaf area and leaf number. The death of expanded leaves leading to decrease in photosynthetic leaf area [50]. Leaf area reduced signifi- cantly under water stress [51]. Reduction in the leaf area by water stress is an important cause of reduced crop yield through reduction in photosynthesis [51,52]. In recommendation when this plants sprayed with poly- amines or phytohormones, the dry matter yield, leaf area, and photosynthetic pigments enhanced markedly, which indicated the complete correlation among the three pa- rameters. [53] study the effect of drought stressed maize, cowpea and broad bean plants and found that while the photosynthetic pigments decreased significantly in maize they remained more or less unchanged in cowpea and broad bean plants, consequently the dry matter produc- tion in maize was much more affected by drought stress than in cowpea and broad bean plants. They also re- ported that when these plants sprayed with phytohor- mones or polyamines there is a marked stimulatory effect in green area and consequently the photosynthetic pig- ments which consequently accumulated the dry mass of the three tested species [54] working with drought stressed wheat cultivars and found that, the reduction in photo-  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 803 synthetic pigments was more pronounced in drought sus- ceptible than in drought resistant cultivars. Other inves- tigators reported a reduction in chlorophyll contents un- der drought conditions [55,56]. The reduction in total photosynthetic pigments has been reported to be related to the activation of chlorophyllase, which catalyses the catabolism of chlorophyll [57]. [58,59] reported that the degradation of thylakoid can occur in response to water stress. The enhancement of chlorophyll degradation in leaves of stressed plants can probably be due to the dis- turbance in hormonal balance. Such disturbance may be manifested by dimensioned kinetin biosynthesis and in- creased abscisic acid. The former is known to inhibit chlorophyllase activity whereas the letter is known to accelerate it [60]. The differences in the responses to drought stress be- tween the two selected maize genotypes were mirrored by the differences in the absorption, accumulation and compartmentation of K+, Ca++ and Mg++ in the different organs of the two genotypes. Our data reveal that while drought stress had a marked stimulatory effect in the absorption and accumulation of K+, Ca++ and Mg++ in different organs of maize genotype Bashaier it on other hand, significantly inhibited the accumulation of these cations in the different organs of maize genotype Nefer- titi. It has been well known that osmotic regulators in- clude many important small molecules such as potassium, soluble sugars, proline and petaine [61-63]. These small molecules are also important physiological indicators for evaluating osmotic adjustment ability [64]. The maize genotype Nefertiti produced a crop yield (number of grains per ear, 1000 grain weight and the total yield per feddan) up to only 70% F.C, and failed completely to give crop yield at the levels of 50% & 30% F.C. while treatment with polyamines produce crop yield up to 50% F.C. Additionally all of used polyamines in- creased the crop yield at the normal condition (90% F.C) as compared with untreated plants. The crop yield of plants treated with putrescine, spermine or mixture po- lyamines was 26.5, 24, or 26 Ardab/feddan compared to 21.14 (at the normal level). It is worthy to mention that IAA is the only phytohormone which produced a crop yield up to 50% F.C (5.23 Ardab/feddan), although the other phytohormones considerably enhanced the plant growth during the vegetative stage. This unexpected phe- nomenon seemed to be complicated and need a numer- ous and further studies. On the other hand the production of crop yield in ma- ize genotype Bashaier under different treatments was interesting. This genotype produce 21 Ardab/feddan of maize grain at the level of 50% F.C, which considered an excellent results (note that the sensitive cultivar produce the same amount at the normal condition), on the oppo- site to the sensitive cultivar, phytohormones treatment increased the crop yield over the control value of the this drought tolerant cultivar up to 70% F.C, also the pro- duced crop yield of IAA treated plants at 50% F.C was similar to that of control plants. Also a pronounced in- creased the crop yield was obtained up to the level of 70% F.C as a results of polyamines treatment in com- parison with the relative control values. Moreover Putre- scine increase the crop yield by 1.24 Ardab/feddan over the control at the level of 50% F.C. Interestingly at the level of 30% F.C. while the only drought stressed plants did not give a crop yield, the phytohormones or poly- amines yielded a suitable amount of maize grain at the level of 30% F.C. The data also reveal that the improve- ment of crop yield was much more pronounced in poly- amines treated plants than phytohormone ones. Putre- scine was also superior in produce 18.3 Ardab/feddan at 30% F.C in relation to 27 Ardab/feddan in relative con- trol plants (A very interesting results). Stress during dif- ferent growth stages might decrease translocation of as- similates to the grains, which lowered grain weight and increased the empty grains. There are some reports indi- cated that lower soil moisture might inhibit photosynthe- sis and decrease translocation of assimilates to the grain which lowered grain weight [65,66]. Moreover, water stress might lead to a considerable increase in secondary rachis branch abortion and resulted in a reduction in spikelets number per panicle [67]. In addition, drought stress could curtail the kernel sink potential by reducing the number of endosperm cells and amyloplasts formed [68,69]. Therefore, the rate of reducing in grain weight is correlated to the reduction in the capacity of the en- dosperm to accumulate starch, in terms of both rate and duration [69]. On the other hand, some studies show that there would be significantly higher gain in biomass (dry Ministry weight) after stress imposed. This dry weight would be associated to the cell division and new material synthesis [67,70]. Increasing the number of filled grains might be due to the contribution of carbohydrates from current photosynthesis which have been more and efficiently would translocated into the grain and thus increased the grain yield [71]. Usually, water stress at grain filling in- duces early senescence and shortens the grain filling pe- riod but increases remobilization of assimilates from the straw to the grains [72,73]. The early senescence induced by a moderate water-deficit during grain filling can en- hance the remobilization of stored assimilates and accel- erate grain filling of rice [69,71]. Other experiences show that plants could cope with stress condition exhibiting morphological alteration such  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 804 as root characteristic in which affect grain formation [74]. The slow grain-filling rate and low grain weight of infe- rior spikelets have often been attributed to a limitation in carbohydrate supply [69]. It’s important to stress on the differential effects of the using of phytohormones or polyamines from the vegeta- tive stage to the fruiting stage among the two selected maize genotypes. Exogenous application of any of the growth promoters “phytohormones and polyamines”. Im- proved the drought tolerance of the two maize genotypes during vegetative stage through the improvement of pho- nological characteristics and the physiological behavior which consequently and interestingly induce a surprising improvement in the crop yield especially in maize geno- type Bashaier (the drought tolerant cultivar), They pro- duce a suitable amount of crop at the level of 30% F.C (the level which did not give any crop yield in plants subjected only to 30% F.C), also IAA is the only phyto- hormone which give a crop yield in maize genotype Ne- fertiti up to 50% F.C. Why the phytohormone did not give a crop yield in maize geno type Nefertiti beyond 70% F.C Although they improved Interactive Effects of Drou- ght Stress and Phytohormones or Polyamines on Growth and Yield of Two M(Zea maize L) Genotypes. The growth and the physiology of this cultivar during the vegetative stage and also why these phytohormones make this considerable improvement in crop yield only in maize cv. Bashaier? The answer seemed to be com- plicated which might associated with the genotypic va- riation rather than the growth activators it self which might indicated the great variation in the interaction be- tween the response of the two maize genotypes with the different growth activators. The situation was very com- plicated and might include many physiological processes and also may be associated with induction of phlorogen and phytochrome (phytohormones responsible for flow- ering and fruiting stage). So, and according to our data in maize genotype Nefertiti the role played by these activa- tors was differed from vegetative to flowering stage and the use of these activators only up to vegetative growth is not a sign for improving of crop yield. The diversity and complexity between the stress factors and gene action among the different species and genotypes as well as the different growth stages, still open question. In conclusion maize genotype Nefertiti in addition to its sensitivity to drought stress, is also not responded enough to exogenous application of the used growth pro- moters especially phytohormones except for IAA to some extent. On the other hand the drought stressed maize ge- notype Bashaier was also strongly responded to phyto- hormones or polyamines treatments. Exogenous applica- tion of polyamines seemed to be more effective than phytohormones. Putrescine might be the superior poly- amine. Finally and according to these results, this study opened many fields of studies in the future, this fields might linked with the breading programs (the con- trast- ing behaviors of the produced cultivars and lines), from one hand and the differential responses of the added plant promoters. Also it’s difficult to recommend the role of any of these plant promoters before the flowering and fruiting stages. REFERENCES [1] J. B. Passioura, “Drought and Drought Tolerance,” Plant Growth Regulation, Vol. 20, No. 2, 1996, pp. 79-83. doi:10.1007/BF00024003 [2] J. B. Passioura, “The Drought Environment: Physical, Biological and Agricultural Perspectives,” Journal of Ex- perimental Botany, Vol. 58, No. 2, 2007, pp. 113-117. doi:10.1093/jxb/erl212 [3] N. Isendahl and G. Schmidt, “Drought in the Mediterra- nean-WWF Policy Proposals,” A. WWF Report, Madrid, 2006. [4] F. M. Rhoads and J. M. Bennet, “Irrigation of Agricul- tural Crops,” In: B. A. Stewart and D. R. Nielsen, Eds., American Society of Agronomy, Agronomy Madison30, Crop Science Society of America and Soil Science Society of America, 1991, pp. 569-596. [5] R. K. Pandey, J. W. Maranville and A. Admou, “Deficit Irrigation and Nitrogen Effects on Maize in a Sahelian Environment,” Agriculture Water Management, Vol. 46, No. 1, 2000, pp. 1-13. doi:10.1016/S0378-3774(00)00073-1 [6] R. Çakir, “Effect of Water Stress at Different Develop- ment Stages on Vegetative and Reproductive Growth of Corn,” Field Crops Research, Vol. 89, No. 1, 2004, pp 1-6. doi:10.1016/j.fcr.2004.01.005 [7] S. B. Traore, R. E. Carlson, C. D. Pilcher and M. E. Rice, “Bt and Non-Bt Maize Growth and Development as Af- fected by Temperature and Drought Stress,” Agronomy Journal, Vol. 92, No. 5, 2000, pp. 1027-1035. doi:10.2134/agronj2000.9251027x [8] A. O. Jama and M. J. Ottman, “Timing of the First Irriga- tion in Corn and Water Stress Conditioning,” Agronomy Journal, Vol. 85, No. 6, 1993, pp. 1159-1164. doi:10.2134/agronj1993.00021962008500060013x [9] M. E. Otegui, F. H. Andrade and E. E. Suero, “Growth, Water Use and Kernel Abortion of Maize Subjected to Drought at Silking,” Field Crops Research, Vol. 40, No, 2, 1995, pp. 87-94. doi:10.1016/0378-4290(94)00093-R [10] O. T. Denmead and R. H. Shaw, “The Effects of Soil Moisture Stress at Different Stages of Growth on the De- velopment and Yield of Corn,” Agronomy Journal, Vol. 52, No. 5, 1960, pp. 272-274. doi:10.2134/agronj1960.00021962005200050010x [11] E. C. Stegman, “Corn Grain Yield as Influenced by Tim- ing of Evapotranspiration Deficits,” Irrigation Science,  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 805 Vol. 3, No. 1, 1982, pp. 75-87. doi:10.1007/BF00264851 [12] S. M. Scheierling, G. E. Cardon and R. A. Young, “Im- pact of Irrigation Timing on Simulated Water-Crop Pro- duction Functions,” Irrigation Science, Vol. 18, No. 1, 1997, pp. 23-31. doi:10.1007/s002710050041 [13] D. S. NeSmith and J. T. Ritchie, “Effects of Soil Water- Deficits during Tassel Emergence on Development and Yield Component of Maize (Zea mays),” Field Crops Research, Vol. 28, No. 3, 1992, pp. 251-256. do i:1 0. 10 16 / 03 78 -42 90 ( 92 )90 04 4- A [14] D. S. NeSmith and J. T. Ritchie, “Maize (Zea mays L.) Response to a Severe Soil Water-Deficit during Grain Filling,” Field Crops Research, Vol. 29, No. 1, 1992, pp. 23-35. doi :1 0.10 16 / 03 78 - 42 90 (92) 9 00 73 - I [15] F. R. Lamm, D. H. Rogers and H. L. Manges, “Irrigation Scheduling with Planned Soil Water Depletion,” T. ASAE, Vol. 37, No. 5, 1994, pp. 1491-1497. [16] M. A. Walker and B. Dumbroff, “Effect of Salt Stress on Abscisic and Cytokinin Levels,” Zeitschrift für Pflanzen Physiologie, Vol. 101, 1981, p. 661. [17] M. A. Hamdia, “The Counteraction Effect of GA3 or IAA with Endogenous Ethylene of Wheat Plants under Salt Stress Conditions,” Sixth Egyptian Botanical Con- ference Cairo University, No. 1, 1998, pp. 97-110. [18] C. Kaya, A. L. Tuna and I. Yokas, “The Role of Plant Hormones in Plants under Salinity Stress,” Book Salinity and Water Stress, Vol. 44, No. 1, 2009, pp. 45-50. do i:1 0. 10 07 / 97 8 -1- 40 20 - 90 65 -3_5 [19] P. J. Davies, “The Plant Hormones: Their Nature, Occur- rence and Function,” In: P. J. Davies, Eds., Plant Hor- mones, Biosynthesis, Signal Transduction, Action, Klu- wer, Dordrecht, 2004. [20] J. H. Liu, H. Kitashiba, J. Wang, Y. Ban and T. Morigu- chi, “Polyamines and Their Ability to Provide Environ- mental Stress Tolerance to Plants,” Plant Biotechnology, Vol. 24, No. 12, 2007, pp. 117-126. [21] T. Kusano, T. Berberich, C. Tateda and Y. Takahashi, “Po- lyamines: Essential Factors for Growth and Survival,” Plan- ta, Vol. 228, No. 3, 2008, pp. 367-381. do i:1 0. 10 07 / s00 42 5 -0 08 -07 72 - 7 [22] R. M. Ali, “Role of Putrescine in Salt Tolerance of At- ropa Belladonna Plant,” Plant Science, Vol. 52, No. 2, 2000, pp. 173-179. doi:10.1016/S0168-9452(99)00227-7 [23] H. Nayyar, S. Kaur, S. S. Kumar, K. J. Singh and K. K. Dhir, “Involvement of Polyamines in the Contrasting Sen- sitivity of Chickpea (Cicer arietinum L.) and Soybean (Glycine max (L.),” Botanical Bulletin Academia Sinica, Vol. 46, No. 4, 2005, pp. 333-338. [24] J. Yang, J. Zhang, K. Liu, Z. Wang and L. Liu, “Involve- ment of Polyamines in the Drought Resistance of Rice,” Journal of Experimental Botany, Vol. 58, No. 6, 2007, pp. 1545-1555. doi:10.1093/jxb/erm032 [25] M. Farooq, S. M. A. Basra, M. H. Rehman and B. A. Saleem, “Incorporation of Polyamines in the Priming Me- dia Enhances the Germination and Early Seedling Growth in Hybrid Sunflower (Helianthus annuus L.),” Interna- tional Journal of Agricultural Biology, Vol. 9, No. 6, 2007, pp. 868-872. [26] M. A. K. Shaddad, M. A. Hamdia and H. T. Mohammed, “Drought Tolerance of Some Zea mays L. Genotypes at the Early Growth Stage,” Assiut University Journal of Botany, Vol. 38, No. 2, 2009, pp. 135-147. [27] M. J. Roberts, S. P. Long, L. L. Tieszen and C. L. Beadle, “Measurement of Plant Biomass and Net Production of Herbaceous Vegetation,” In: D. O. Hall, J. M. Scurlock, H. R. Bolhar-Nordenkampf, R. C. Leegoood and S. P. Long, Eds., Photosynthesis and Production in a Chang- ing Environment, Chapman & Hall, London, 1993, pp. 1-21. doi:10.1007/978-94-011-1566-7_1 [28] G. W. McKee, “A Coefficient for Computing Leaf Area in Hybrid Corn,” Agronomy Journal, Vol. 56, 1964, pp. 240-241. [29] R. Bonhomme, M. Varlet, C. Grancher and P. Chartier, “The Use of Hemispherical Photographs for Determining Leaf Area Index of Young Crops,” Photosynthetica, Vol. 8, No. 3, 1974, pp. 299-301. [30] J. M. Norman and G. S. Campbell, “Canopy Structure,” In: R. W. Pearcy, J. Ehleringer, H. A. Moony and P. W. Rundel, Eds., Plant Physiological Ecology, Chapman & Hall, London, 1994, pp. 301-326,. [31] H. Metzner, H. Rauand and H. Senger, “Untersuchungen Zur Synchronsiserbakeit Einzelner Pigment-Mangel Mu- tanten von Chlorella,” Planta, Vol. 65, No. 2, 1996, pp. 86-194. [32] D. Schwarzenbach and H. Biederman, “Komplexone X- Erdoliukomplex von 0, 0-Diazofarbstoffen,” Helvetia Chemica Acta, Vol. 31, No. 3, 1948, pp. 678-687. doi:10.1002/hlca.19480310303 [33] R. G. Steel and J. H. Torrie, “Principles and Procedures of Statistics,” McGraw-Hill Book Co., New York, 1960. [34] R. Munns, D. P. Szhachtmanand and A. G. Condon, “The Significance of Two-Phase Growth Response to Salinity in Wheat and Barley,” Austri an Jou rnal Pl ant Ph ysiolog y, Vol. 22, No. 4, 1995, pp. 561-569. do i:1 0. 10 71 /P P 9 95 05 61 [35] J. Salter, K. Morris, P. C. E. Bailey and P. I. Boon, “In- teractive Effects of Salinity and Water Depth on the Growth of Melaleuca Ericifolia Sm. (Swamp Paper Bark) Seedlings,” Aquetic Botany, Vol. 86, No. 3, 2007, pp. 213-222. doi:1 0.10 16 / j .aqu a bo t. 20 0 6. 10 .00 2 [36] A. L. Saleh, M. M. Hussien, S. Y. El-Faham, M. S. Abo- El-Kier and A. A. Abd-El-Kader, “Mineral Status in Bar- ley Grains as Affected by Benzyladenine and Salinity,” Thailand 17th WCSS, Bangkok, 14-21 August 2002, pp. 14-21. [37] M. E. Younis, O. A. Shahaby, S. A. Abo-Hamed and A. H. Ibrahim, “Effects of Water on Growth, Pigments and 14 CO2: Assimilation in Three Sorghum Cultivars,” Jour- nal of Agronomy and Crop Science, Vol. 10, No. 2, 2000, pp. 918-924.  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 806 [38] Z. Zhu, G. Wei, J. Li, Q. Qian and J. Yu, “Silicon Allevi- ates Salt Stress and Increases Antioxidant Enzymes Ac- tivity in Leaves of Salt—Stressed Cucumber (Cucumis sativus L.),” Plant Science, Vol. 167, No. 3, 2004, pp. 527-533. doi:10.1016/j.plantsci.2004.04.020 [39] M. H. Baek, J. H. Kim, B. Y. Chung, J. S. Kim and I. S. Lee, “Alleviation of Salt Stress by Low Dose-Irradiation in Rice,” Biologia Planarum, Vol. 49, No. 2, 2005, pp. 273-276. doi:1 0.10 07 / s10 53 5-005- 3 27 6 -3 [40] F. C. Meinzer, J. H. Fownes and R. A. Harrington, “Grow- th Indices and Stomatal Control of Transpiration in Acacia Koa Stands Planted at Different Densities,” Tree Physi- ology, Vol. 16, No. 7, 1996, pp. 607-615. [41] J. M. Ribaut and P. E. Pilet, “Effect of Water Stress on the Growth, the Osmotic Potential and Abscisic Acid Con- tent of Maize Roots,” Physiologia Plantarum, Vol. 81, No. 2, 1991, pp. 156-162. doi:10.1111/j.1399-3054.1991.tb02123.x [42] M. Bracale, M. L. Christian-Sauini, W. Dicordo and M. Grazia-Galli, “Water Deficit in Pea Root Tips: Effect on the Cell Cycle and on the Production of Dehydrin-Like Protein,” Annual Botany, Vol. 6, No. 2, 1997, pp. 593- 600. doi:10.1006/anbo.1996.0356 [43] R. Munns, H. Greenway, R. Delane and J. Gibbs, “Ion Concentration and Carbohydrate Status of the Elongation Leaf Tissue of Hordeum Vulgaris Growing at High Ex- ternal NaCl. II-Cause of the Growth Reduction,” Journal of Experimental Botany, Vol. 33, No. 4, 1982, pp. 574- 583. doi:10.1093/jxb/33.4.574 [44] M. Drew, C. Guenther and T. Läuchi, “The Combined Effect of Salinity and Root Anoxia on Growth and Net Na+ and K+ Accumulation in Zea Mays Grown in Solu- tion Culture,” Annal of Botany, Vol. 61, No. 1, 1988, pp. 41-53. [45] S. H. Shah, “Effects of Salt Stress on Mustard as Affected by Gibberellic Acid Application,” General Appied Plant Physiology, Vol. 33, No. 1-2, 2007, pp. 97-106. [46] E. A. Dorgham, “Effect of Water Stress, Irradiation and Nitrogen Fertilization on Grain Filling, Yield and Quality of Certain Wheat Cultivars,” Ph. D. Thesis, Ain Shams University of Cairo, Cairo, 1991. [47] L. Bernstein, A. Laüchli and W. K. Silk, “Kinematics and Dynamics of Sorghum (Sorghum bicolor L.) Leaf De- velopment at Various Na+/Ca++ Salinities,” Plant Physi- ology, Vol. 103, No. 2, 1993, pp. 1107-1114. [48] R. Munns, “Physiological Process Limiting Plant Growth in Saline Soils: Some Damage and Hypotheses,” Plant Cell Environmental, Vol. 16, No. 1, 1993, pp. 15-24. doi:10.1111/j.1365-3040.1993.tb00840.x [49] M. Nabil and A. Coudret, “Effects of Sodium Chloride on Growth, Tissue Elasticity and Solute Adjustment in Two Acacia Nilotica Subspecies,” Physiologia Plantarum, Vol. 93, No. 2, 1995, pp. 217-224. doi:10.1111/j.1399-3054.1995.tb02220.x [50] R. Munns and S. Termaut, “Whole-Plant Response to Sa- linity,” Austrian Journal Plant Physiology, Vol. 13, No. 1, 1986, pp.143-160. doi:10.1071/PP9860143 [51] S. K. Yadav, N. J. Lakshmi, M. Maheswari, M. Vanaja and B. Venkateswarlu, “Influence of Water Deficit at Vegetative, Anthesis and Grain Filling Stages on Water Relation and Grain Yield in Sorghum,” Indian Journal of Plant Physiology, Vol. 10, No. 1, 2005, pp. 20-24. [52] A. R. Reddy, K. V. Chaitanya and M. Vivekanandan, “Drought Induced Responses of Photosynthesis and An- tioxidant Metabolism in Higher Plants,” Journal Plant Physiology, Vol. 161, No. 11, 2004, pp. 1189-1202. doi:10.1016/j.jplph.2004.01.013 [53] M. A. Shaddad and M. A. El-Tayeb, “Interactive Effects of Soil Moisture Content and Hormonal Treatment on Dry Matter and Pigment Contents of Some Crop Plants,” Acta. Agronomica, Vol. 39, No. 1-2, 1989, pp. 49-57. [54] M. A. K. Shaddad, H. M. Abd-ElSamad and M. M. Ra- gaey, “Drought Tolerance of Wheat Genotypes at the Early Vegetative Stage,” Assiut University Journal of Bo- tany, Vol. 37, No. 2, 2008, pp. 15-32 [55] A. H. Ibrahim and H. S. Aldesuquy, “Glycine Betaine and Shikimic Acid-Induced Modification in Growth Cri- teria, Water Relation and Productivity of Droughted Sor- ghum Bicolor Plants,” Phyton (Horn, Austria), Vol. 43, No. 2, 2003, pp. 351-363. [56] U. A. A. Radwan, “Plant Water Relations, Stomatal Be- havior, Photosynthetic Pigments and Anatomical Charac- teristics of Solenostemma arghel (Del.) Hayne under Hy- perarid Environmental Conditions,” American-Eurasian Journal of Scientific Research, Vol. 2, No. 2, 2000, pp. 80-92. [57] S. Majumdar, S. Ghosh, B. R. Glick and E. B. Dumbroff, “Activities of Chlorophyllase, Phosphoenolpyruvate Car- boxylase and Ribulose 1,5-biphosphate Carboxylase in Pri- mary Leaves of Soybean during Senescence and Drought,” Physiologia Plantarum, Vol. 81, No. 4, 1991, pp. 473-480. doi:10.1111/j.1399-3054.1991.tb05087.x [58] S. Kulshreshtha, D. P. Mishra and R. K. Gupta, “Changes in Contents of Chlorophyll, Proteins and Lipids in Whole Chloroplast Membrane Fractions at Different Leaf Water Potentials in Drought Resistant and Sensitive Genotypes of Wheat,” Photothenthetica, Vol. 21, 1987, pp. 65-70. [59] M. M. Chaves, “Effects of Water Deficits on Carbon Assimilation,” Journal of Experimentsl Botany, Vol. 42, No. 1, 1991, pp. 1-16. [60] M. Drazkiewicz, “Chlorophyllase: Occurrence, Functions, Mechanism of Action, Effects of External and Internal Factors,” Photosynthetica, Vol. 30, No. 3, 1994, pp. 321- 331. doi:10.1093/jxb/42.1.1 [61] G. R. Sun, Y. Z. Peng, H. B. Shao and L. Y. Chu, “Does Puccinelia tenuiflora Have the Ability of Salt Exuda- tion?” Colloids and Surfaces B: Biointerfaces, Vol. 46, No. 4, 2005, pp. 197-203. do i:1 0. 10 16 / j. co l sur fb. 20 05 . 11 .0 03 [62] H. B. Shao, M. A. Shao and Z. S. Liang, “Osmotic Ad- justment Comparison of 10 Wheat (Triticum aestivum L.) Genotypes at Soil Water Deficits,” Colloids and Surfaces  Interactive Effects of Drought Stress and Phytohormones or Polyamines on Growth and Yield of Two M (Zea maize L.) Genotypes Copyright © 2011 SciRes. AJPS 807 B: Biointerfaces, Vol. 47, No. 2, 2006, pp. 132-139. do i:1 0. 10 16 / j. co l sur fb. 20 05 . 11 .0 28 [63] Y. Tan, Z. S. Liang and H. B. Shao, “Effect of Water Deficits on the Activity of Anti-Oxidative Enzymes and Osmoregulation among 3 Different Genotypes of Radix Astragali at Seeding Stage,” Colloids and Surfaces B: Biointerfa ces, Vol. 49, No. 1, 2006, pp. 60-65. do i:1 0. 10 16 / j. co l sur fb. 20 06 . 02 .0 14 [64] X. A. Liu and D. R. Bush, “Expression and Transcrip- tional Regulation of Amino Acid Transporters in Plants,” Amino Acids, Vol. 30, No. 2, 2006, pp. 113-120. do i:1 0. 10 07 / s00 72 6 -0 05 -02 48 - z [65] P. D. R. Van Heerden and R. Laurie, “Effects of Pro- longed Restriction in Water Supply on Photosynthesis, Shoot Development and Storage Root Yield in Sweet Po- tato,” Physiologia Plantarum, Vol. 134, No. 1, 2008, pp. 99-109. doi:10.1111/j.1399-3054.2008.01111.x [66] K. Liu, Y. Ye, C. Tang, Z. Wang and J. Yang, “Re- sponses of Ethylene and ACC in Rice Grains to Soil Moisture and Their Relations to Grain Filling,” Frontiers of Agriculture in China, Vol. 2, No. 2, 2008, pp. 172-180. do i:1 0. 10 07 / s11 70 3 -0 08 -00 08 - 4 [67] Y. Katoa, A. Kamoshitab and J. Yamagishia, “Preflow- ering Abortion Reduces Spikelet Number in Upland Rice (Oryza sativa L.) under Water Stress,” Crop Science So- ciety of A m erica, Vol. 48, No. 6, 2008, pp. 2389-2395. [68] E. S. Ober, J. T. Setter and J. F. Madison and P. S. Sha- piro, “Influence of Water Deficit on Maize Endosperm Development. Enzyme Activities and RNA Transcripts of Starch and Zein Synthesis, Abscisic Acid and Cell Divi- sion,” Plant Physiology, Vol. 95, No. 1, 1991, pp. 154- 164. doi:10.1104/pp.97.1.154 [69] J. Yang, J. Zhang, Z. Wang, K. Liu and P. Wang, “Post- anthesis Development of Inferior and Superior Spikelets in Rice in Relation to Abscisic Acid and Ethylene,” Jour- nal of Experimental Botany, Vol. 57, No. 1, 2006, pp. 149- 160. doi:10.1093/jxb/erj018 [70] N. Sunderland, “Cell Division and Expansion in Growth of the Leaf,” Journal of Experiment Botany, Vol. 11, No. 1, 1960, pp. 68-80. doi:1 0. 10 93 / jx b /11 . 1. 68 [71] Z. Z. Xu and G. S. Zhou, “Photosynthetic Recovery of a Perennial Grass Leymus chinesis After Different Periods of Soil Drought,” Plant Production Science, Vol. 10, No. 3, 2007, pp. 277-285. doi:10.1626/pps.10.277 [72] S. Asseng and A. F. van Herwaarden, “Analysis of the Benefits to Wheat Yield from Assimilates Stored Prior to Grain Filling in a Range of Environments,” Plant and Soil, Vol. 256, No. 1, 2000, pp. 3217-3219. [73] Z. Plaut, B. J. Butow and C. S. Blumenthal, “Transport of Dry Matter into Developing Wheat Kernels and Its Con- tribution to Grain Yield under Post-Anthesis Water Defi- cit and Elevated Temperature,” Field Crops Research, Vol. 86, No. 2-3, 2004, pp. 185-198. doi:10.1016/j.fcr.2003.08.005 [74] A. Mostajeran and V. Rahimi-Eichi, “Drought Stress Effects on Root Anatomical Characteristics of Rice Cul- tivars (Oryza sativa L.),” Pakistan Journal of Biological Science, Vol. 11, No. 18, 2008, pp. 2173-2183. do i:1 0. 39 23 / p jb s. 20 08 .2 17 3 .2 18 3
|