 World Journal of AIDS, 2011, 1, 155-164 doi:10.4236/wja.2011.14023 Published Online December 2011 (http://www.SciRP.org/journal/wja) Copyright © 2011 SciRes. WJA 155 Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection Based on the Expert Consensus* Hee Jung Choi1, Kyoung Ae Kong2, Jung Won Min3, Hun-Jae Lee4, Hae-Sook Park3 1Department of Internal Medicine, School of Medicine, Ewha Womans University, Seoul, South Korea; 2Clinical Trial Center, Ewha Womans University Medical Center, Seoul, South Korea; 3Department of Preventive Medicine, School of Medicine, Ewha Woma ns Uni- versity, Seoul, South Korea; 4Department of Social and Preventive Medicine, College of Medicine, Inha University, Incheon, South Korea. E-mail: heechoi@ewha.ac.kr Received August 4th, 2011; revised October 27th, 2011; accepted N ovember 14th, 2011. ABSTRACT Purpose: This study was conducted to develop a guideline for HIV testing in South Korea where the HIV prevalence is very low. It is necessary to make the recommendations presented here based on the epidemiology of opportunistic infec- tion and cancer in the community. Methods: The development of a guideline for HIV testing was conducted using lit- erature reviews and the agreement of Korean experts for HIV patients using a two-round Delphi consensus technique. Each expert was asked to independently answer to each item related to HIV risk. Ten experts participated in the two- round survey. Results: The individual items were determined based on the following categories: high risk behavior, symptom and diseases, colo-rectal diseases, uro-genital diseases and gynecology, skin and oral diseases. The final guideline consisted of 4-scale agreement rating (strongly recommended, recommended, not routin ely recommended, no recommendation for routine provision). Discussion: We have developed the guideline for HIV testing in a country where the HIV prevalence is low based on a systematic investigation and expert consensus. Keywords: HIV, Testing, Guideline, Consensus, Delphi Studies 1. Introduction The HIV prevalence of South Korea is still far less than 0.01% of its population, which is considered to be much lower than that of other countries [1]. Recommendations for HIV testing are already available from the Centers for Disease Control and Prevention (CDC), which was de- veloped by the United States, where there is greater than 1% HIV prevalence. According to a recent survey in South Korea [2], compliance with HIV testing by Korean physicians has been relatively poor due to the lo w preva- lence of HIV infection and insufficient experience with HIV patients in clinics in South Korea. Furthermore, tuberculosis (TB) or viral hepatitis B of general popula- tion is still more common, which are considered to be indicative diseases for HIV testing, in Korea compared to the United States statistics [3,4]. Therefore, previous recommendations by the United States may be not appli- cable to the country whose HIV prevalence is very low, and a new guideline for HIV testing is required for the use by primary physicians in the co mmun ity. Although some individual guidelines for HIV testing are currently available in Korea, these guidelines have been developed by individual medical societies associ- ated with HIV through the Kor ean NIH and CDC, which had been recognized by only a few physicians in Korean clinics. In addition, these are not new guidelines that were systematically developed. However, It is required to establish the recommendations that were made based on agreements by experts treating HIV patients with consid- eration of the Korean environment in terms of epidemi- ology. Accordingly, they need to be appropriately revised to reflect the current prevalence of HIV and other dis- eases in Korea. Due to the increasing demand and as a preparative measure, we have developed the guideline for HIV testing in South Korea based on a systematic investigation and expert consensus. 2. Methods *This study was presented at the 57th Conference of the Korean Asso- ciation of Internal Medicine, Seoul, Korea, October 28th, 2006. 1) Selection of items for the questionnaire for HIV test-  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 156 Based on the Expert Consensus ing. The statements for the HIV testing survey were ex- tracted from the CDC recommendations and individual guidelines by Korean medical societies associated with HIV [5-7]. The references that were recently developed in Korea included the HIV/AIDS clinical guide that was published by the Korean Society of Infectious Diseases and the Korean Alliance to Defeat AIDS that focused on Korean methods of HIV testin g, diagnosis and co nfirma- tion, reporting, and therapy [7]. In the questionnaires, each item was categorized into the following areas. The HIV testing requirements in the questionnaires based on high-risk behaviors were made from the CDC guideline for HIV counseling. Other items for HIV testing re- quested to special clinics were selected from each guide- line developed by Korean Dermatological Association, Korean society of Obstetrics and Gynecology, Korean Society of Coloproctology, Korean Surgical Society, Korean Urological Association, Korean Academy of Dental Sciences, and Korean Society of Emergency Medicine [7]. 2) Expert panel selection and guideline development using the two-round Delphi process This study was conducted from January to February, 2006. Experts for the panel were composed of ten infec- tious disease specialists in Korea who had AIDS research experience at university hospitals and were also AIDS advisory council in the Korean CDC. To request their participation into the panel, experts were contacted by electronic mail. All ten experts agreed to participate in the survey. The questionnaires were sent to an expert panel via an attached file in an electronic mail and re- turned to the participants. In the first Delphi round, each panelist was asked to independently respond on a scale (Yes or No). In addition, they were asked to give com- ments and to suggest new items. After responses were received from the panel, their answers were collected, and the revised questionnaires containing additional items for HIV testing suggested by the panel were re- turned to them in the second round of th e Delphi survey. The newly added items for questionnaires were as fol- lows: homosexual man; oral candidiasis and oral hairy leukoplakia in dental clinic; syphilis; UTI due to rare organisms; prostatitis or prostatic abscess; and any STD in the uro-genital field. After receiving the responses to the second survey from the expert panel, the final items were categorized into a 4-scale for agreement rating (strongly recom- mended, seven or more of the ten experts agreed on the need for an HIV screening test; recommended, four to six out of the ten experts agreed; not routinely recommended, one to three of the ten experts agreed; no recommenda- tion for routine provi s ion, no one ag r e ed). 3. Results 1) Results of the survey of HIV testing requirement based on hi gh-risk beha viors. Out of the ten experts surveyed for this study, nine have commonly conducted HIV tests for patients who had unprotected intercourse with prostitutes. Addition- ally, six have conducted HIV tests on patients who had unprotected intercou rse with those who had unknown sex partners. Although only two experts had experienced HIV testing of IV drug abuser s who had shared injecting needles, all of the panel members agreed to recommend HIV testing to these individuals. In addition, all experts agreed to recommend HIV testing to patients who had unprotected intercourse with those who had multiple sex partners and to any homosexual men. Although preg- nancy is not compatible with HIV-high risk behavior, all of the experts recommended HIV testing of pregnant woman. 2) Survey results of the requirements of HIV testing according to the AIDS case definition by the CDC. These items are consistent with the opportunistic dis- eases of HIV and most experts agreed with the necessity of HIV testing of those items listed in Table 2. All ten experts have performed HIV testing of patients with can- didiasis of the esophagus, bron chus, and lung. Moreover, nine of the ten experts agreed to recommend the HIV testing to patients with extra-pulmonary cryptococcosis, chronic fatigue, fever for more than 30 days, nonsponta- neous weight loss, chronic diarrhea for more than 30 days, and pneumocystis carinii pneumonia. Although the Korean panel has limited experience with chronic cry- ptosporidiosis, extrapulmonary histoplasmosis, isosporo- sis, Kaposi sarcoma in individuals younger than 60 years old, progressive multifocal leukoencephalopathy, recur- rent nontyphoid salmonellosis, and toxoplasmosis of viscera, the experts accepted the recommendation of HIV testing of patients with these d iseases. Only one member of the panel had experience recommending HIV testing to a cervical cancer patient, but six panelists agreed on the need for HIV testing of individuals with invasive cervical cancer. For TB, eight panelists had experience with patients with military TB and four had experience with pulmonary TB patients. However, only four experts recommended HIV testing of patients with pulmonary TB. 3) Survey results of HIV testing of the items in colo- rectal clinics. The panel did not have experience with HIV testing of colorectal diseases except for syphilis. However, all of the panel members agreed to recommend HIV testing for Copyright © 2011 SciRes. WJA 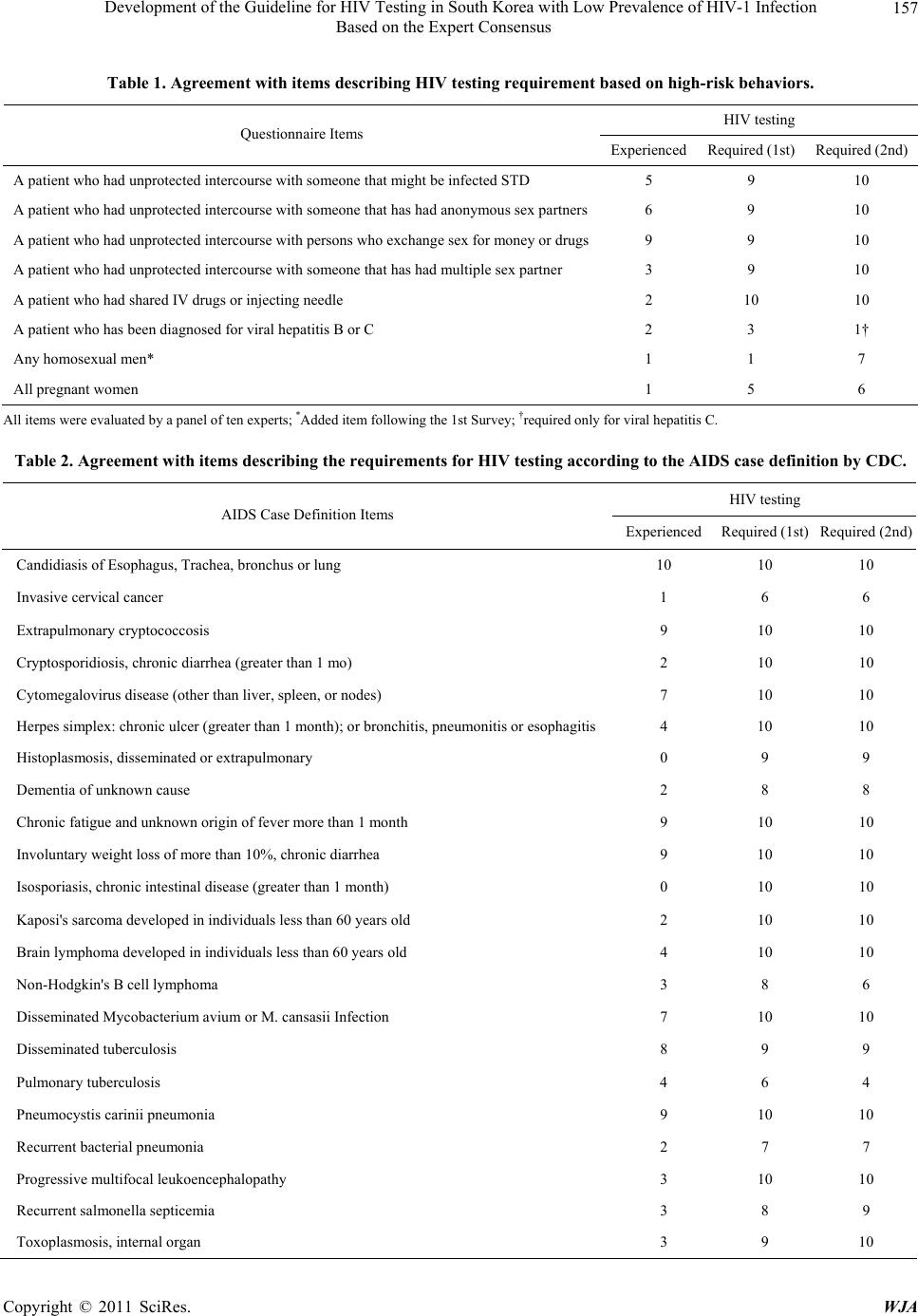 Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection Based on the Expert Consensus Copyright © 2011 SciRes. WJA 157 Table 1. Agreement with items describing HIV testing requirement based on high-risk behaviors. HIV testing Questionnaire Items ExperiencedRequired (1st) Required (2nd) A patient who had unprotected intercourse with someone that might be infected STD 5 9 10 A patient who had unprotected intercourse with someone that has had anonymous sex partners6 9 10 A patient who had unprotected intercourse with persons who exchange sex for money or drugs9 9 10 A patient who had unprotected intercourse with someone that has had multiple sex partner 3 9 10 A patient who had shared IV drugs or injecting needle 2 10 10 A patient who has been diagnosed for viral hepatitis B or C 2 3 1† Any homosexual men* 1 1 7 All pregnant women 1 5 6 All items w ere evaluated by a panel of te n experts; *Added item following the 1st Survey; †required only for viral hepatitis C. Table 2. Agreement with items describing the requirements for HIV testing according to the AIDS case definition by CDC. HIV testing AIDS Case Definition Items Experienced Required (1st) Requir ed (2nd) Candidiasis of Eso phagus, Trachea, bronchus or lung 10 10 10 Invasive cervical cancer 1 6 6 Extrapulmonary cryptococcosis 9 10 10 Cryptosporidiosis, chronic diarrhea (greater than 1 mo) 2 10 10 Cytomegalovirus disease (other t han liver, spleen, or nodes) 7 10 10 Herpes simplex: chronic ulcer (greater than 1 month); or bronchitis, pneumonitis or esophagitis 4 10 10 Histoplasmosis, disseminated or extrapulmonary 0 9 9 Dementia of unknown cause 2 8 8 Chronic fatigue and unknown origin of fever more than 1 month 9 10 10 Involuntary weight loss of more than 10%, chronic diar rhea 9 10 10 Isosporiasis, chronic intestinal disease (greater than 1 month) 0 10 10 Kaposi's sarcoma developed in individuals less than 6 0 years old 2 10 10 Brain lymphoma developed in indivi duals less than 60 year s old 4 10 10 Non-Hodgkin's B cell lymphoma 3 8 6 Disseminated Mycobacterium avium or M. cansasii Infection 7 10 10 Disseminated tuberculosis 8 9 9 Pulmonary tuberculosis 4 6 4 Pneumocystis carinii pneumonia 9 10 10 Recurrent bacterial pneumonia 2 7 7 Progressive multifocal leukoencephalopathy 3 10 10 Recurrent salmonella septicemia 3 8 9 Toxoplasmosis, internal organ 3 9 10  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 158 Based on the Expert Consensus gonococcal infection of the lower rectum and anus, Chlamydia, CMV colitis, Lymphogranuloma venereum, Herpes simplex viral (HSV) infection, syphilis (Chancre, condyloma lata), anal papilloma, anal dysplasia, squa- mous carcinoma of anorectum, Non-Hodgkin’s B cell lymphoma, and Kaposi’s sarcoma (Table 3). 4) Survey results of HIV testing of the items in uro- genital fields. All ten experts agreed on recommending HIV testing to any patients with syphilis seen in urological or gyne- cological exams, and of patients with recurrent genital HSV, recurrent Herpes simplex genitalis, severe genital Herpes or candidiasis, other genital ulcers, and refractory or multiple condyloma accuminata. Eight of the ten ex- perts agreed with HIV testing of patients with persistent or recurrent candida vaginitis. In the first survey, HIV testing of patients with pelvic inflammatory disease or abnormal findings upon Pap smear was required by four experts, but in the second survey testing of these indi- viduals was not required by any of the experts. Urinary tract infection and prostatitis or prostatic abscess by rare organisms were newly posted after the first survey, but only two experts agreed with HIV testing of individuals with these items in the second survey. 5) Survey results regarding HIV testing requirements for the items in dermatology and dental clinics Herpes zoster was found to be the disease for which HIV testing was most often recommended among der- matology diseases, with all ten exp erts reportin g th ey had performed testing on such individuals. This was followed by recurrent oral candidiasis patients, who have been tested for HIV by seven experts, and patients with anal or genital ulcers, who were tested by six experts. It was also suggested that HIV testing of herpes zoster patients be limited to more specific subjects, such as those who are younger than 50 years old. Some of the items in the den- tal field overlapped with those in other fields that were removed in the first survey. However, some experts pro- posed that patients with oral hairy leukoplakia and oral candidiasis be tested for HIV; therefore, they were in- cluded in the second survey. All ten experts agreed to test for HIV in herpes zoster patients that were younger than 50 years old, as well as in patients with anal or genital ulcer, Kaposi’s sarcoma, genital warts, bacillary angiomatosis, and oral hairy leu- koplakia. It was recommended that patients with mol- luscum contagiosum of the face or genitalia be tested for HIV by nine of the experts. Moreover, patients with per- sistent generalized lymphadenopathy, which are lymph nodes more than 1 cm in diameter that are located at more than two sites excluding inguinal areas without other distinct diseases and patients with recurrent oral candidiasis were recommended for HIV testing by eight experts. Six of the experts recommended that patients with bartonellosis and severe eosinophilic purulent fol- liculitis undergo HIV testing, while five experts recom- mended that individuals with non-Hodgkin’s lymphoma and four of the experts recommended that those with skin T cell lymphoma and recurrent aphthous ulcer undergo HIV testing. Five experts responded that severe gingivitis Table 3. The requirements for HIV testing in colorectal clinics. HIV testing Diseases Items Experienced Required (1st) Required (2nd) Gonorrhea 2 10 10 Chlamydial infection in recto-anal area 1 10 10 Nonspecific proctitis 2 7 6 CMV proctitis/colitis 4 10 10 Lymphogranuloma venereum 2 10 10 Herpes simplex infection 4 10 10 Syphilis 7 10 10 Idiopathic rectal-anal ulcer 3 8 9 Conlyloma 3 10 10 Anal dyspla s ia, squamous c ancer 1 10 10 Non-Hodgkin’s B cell lymphoma, Kaposi’ s s arcoma 0 10 10 Rare anal cancer; adenocarcinoma, basal cell carcinoma 0 8 7 Copyright © 2011 SciRes. WJA  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 159 Based on the Expert Consensus should lead to HIV testing in the first survey, but only three experts agreed with this recommendation for the second survey. Other items including squamous cell car- cinoma of the skin, melanoma, refractory seborrheic dermatitis, maculopapular rash, recurrent bacterial fol- liculitis, and generalized Tinea corporis were considered to necessitate HIV testing by four or five experts, but only one or two experts recommended HIV testing of individuals with those diseases in the second survey. Moreover, none of the panel recommended HIV testing of individuals with scabies, atopic dermatitis, drug erup- tion, pyoderma, skin abscess, cellulitis, and necrotizing fasciitis in the second survey. One expert pointed out that HIV testing was required for patients older than 20 years who developed atopic dermatitis for the first time and for young males who developed maculo-papular rash. 6) Guideline endorsed by the expert panel for HIV testing recommendation in Korea. All of the questionnaire items considered by the ten experts in Korea are shown in Tables 1-5. These items were integrated and developed into a guideline (Table 6). The recommendations were categorized into the follow- ing four different levels as described in the methods: Strongly-recommended, Recommended, Not routinely recommended, and Not recomme n de d. 4. Discussion The number of HIV-infected patients has been increasing in South Korea since the first HIV-infected person was confirmed in 1985. Indeed, the number of reported cases was more than 6000 in 2009. However, the prevalence of HIV in Korea is still only 0.01% of the national popula- tion, which is lower than the average prevalence of the world, whic h was 0. 8% i n 2 00 8 [ 1] . In 2001, the United States CDC recommended routine HIV screening of persons aged 13 - 64 years in medical settings in which the HIV sero-prevalence exceeds 1% [5]. This recommendation was broadened in 2006 to in- clude routine HIV testing for all persons aged 13 - 64 years in all healthcare settings [6]. However, the UK guideline recommends that HIV testing should only be conducted for high-risk group s and in medical settings in local areas in which the prevalence exceeds 0.2% [8]. The European guideline for HIV testing was updated to provide advice for sexu ally transmitted infection serv ices in 2008 [9]. The WHO recommends HIV testing in countries in which HIV is prevalent, but they clarified that health care providers should not recommend HIV testing to all persons attend ing health facilities in settin gs with low-level epidemics [10]. Therefore, it is necessary Table 4. Agreement with items describing HIV testing requirements in uro-genital field. HIV testing Diseases items Experienced Required (1st) Required (2nd) Syphilis 2 2 10 Any STD 1 1 9 UTI due to rare organisms 0 1 2 Prostatitis or prostatic abscesses 0 1 2 Recurrent Herpes simplex genitalis* 3 8 10 Severe genital Herpes or candidiasis 1 9 10 Other genital ulcer 3 9 10 Refractory or multiple Condyloma accuminata 2 9 10 Abnormal findings in Pap smear 0 4 0 Any PID 1 4 0 Any STD 3 8 10 Persistent or re current candida vaginitis** 1 7 8 *Twice during six months or more than four times per year, the cases occurring two times in frequency though one year; **Persistent diseases after two rounds of treatment, twice during six months or more than four times per year, the cases occurring two times in frequency through one year; †All items in the Urology department were suggeste d by experts following the first survey. Copyright © 2011 SciRes. WJA  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 160 Based on the Expert Consensus Table 5. Agreement with items describing HIV testing requirements in dermatology and dental clinics. HIV testing Items for D ermatology and Dental Clinic s Experienced Required (1st) Required (2nd) Herpes zoster, age <50 years old 10 9 10 Ulcer of anal or genital area 6 10 10 Molluscum contagiosum in face, genitalia 2 8 9 Scabies 0 4 0 Bartonellosis 0 7 6 Kaposi’s sarcoma 4 10 10 Non-Hodgkins’s l ymphoma 1 6 5 Skin T cell lymphoma 0 6 4 Squamous cell ca r ci n oma 0 5 2 Melanoma 0 5 2 Severe eosinophilic purulent folliculitis 1 6 6 Psoriasis 1 2 0 Refractory seborrheic dermatitis 1 5 2 Atopic dermatitis 0 2 0 Drug eruption 1 1 0 Maculopapular rash 2 4 2 Persistent generalized lymphadenopathy* 2 9 8 Generalized Tinea corporis 2 4 1 Genital condyloma 2 7 10 Recurrent bacterial folliculitis 1 4 2 Pyoderma, abscess, cellulitis, necrotizing fasciitis 1 2 0 Bacillary angiomatosis 1 7 10 Recurrent oral candidiasis 7 10 8 Oral hairy leukoplakia 2 9 10 Recurrent aphthous ulcer 2 4 4 Severe gingivitis, periodontitis 3 5 3 *Lymph nodes more than 1 cm in diameter located in more than two sites except inguinal areas without other distinct diseases. to establish a Korean national recommendation for HIV testing that reflects the prevalence of infectious diseases in Korea. This is the first study to integrate evidence available in the literature, several recommendations for other countries, and Korean expert consensus using a Delphi survey [11]. The experts agreed on an HIV testing requirement for all high-risk behaviors, except for viral hepatitis B or C. The United States guideline recommends HIV testing of hepatitis B positive patients, but the Korean guidelines do not consistently adopt this recommendation. This is likely due to the difference in the prevalence of viral he- patitis B. The sero-prevalence of chronic hepatitis B was about 3.7% in South Korea in 2005 [3]. In contrast, the prevalence of hepatitis B in the United States was about 0.3% - 0.5% in 2006 [12]. The physicians who partici- pated in the present survey con sidered viral hepatitis B to be too common to recommend HIV testing in Copyright © 2011 SciRes. WJA  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 161 Based on the Expert Consensus Table 6. The guideline endorsed by the expert panel for HIV testing recommendation in Korea. Strongly recommended A. High risk behaviors A patient who had unprotected intercourse with someone that might be infected with an STD A patient who had unprotected intercourse with som eone that has had an anonymous sex partner A patient who had unprotected intercourse with someone that has exchanged sex for money A patient who had unprotected intercourse with someone that has had multiple sex partners A patient who has shared IV drug or injecting needle Any homosexual men B. Symptom and Diseases Candidiasis in esophagus, trachea, bronchus or lung Extrapulmonary cryptococcosis Cryptosporidiosis accompanying chronic diarrhea for more than 1 month Cytomegalovirus disease (other than liver, spleen, or nodes) Herpes simplex viral infections: chronic ulcer (s) (duration greater than 1 month) HSV bronchitis, pneumonitis, or esophagitis Extrapulmonary Histoplasmosis Dementia, unkno wn origin Involuntary weight loss greater than 10% and unknown fever for greater than 30 d ays Isosporiasis, chronic intestinal (duration greater than 1 month) Kaposi's sarcoma in individual less than 60 years old Brain lymphoma in individual less than 60 years old Disseminated Mycobacterium avium or M. cansasii Disseminated tuberculosis Pneumocystis carinii pneumonia Recurrent bacte rial pneumonia Progressive multifocal leukoencephalopathy Recurrent salmonella bacteremia Toxoplasmosis of internal organ C. Colo-rectal Diseases Gonorrheal diseases in rectum and anal canal Chlamydial infection, CMV colitis in rectum and anal canal Lymphogranuloma venereum Herpes simplex infection Syphilis Idiopathic rectal ulcer Condyloma Dysplasia Non-Hodgkin's B cell lymphoma, Kaposi samcoma Rare anal cancer; adenocarcinoma, small cell cancer, basal cell carcinoma D. Uro-genital Diseases/Gynecology Syphilis Sexually Transmitted Diseases Recurrent Herpes simplex genitalis (Twice during six mo n th s o r more than four times per year, Severe genital Herpes or candidiasis Copyright © 2011 SciRes. WJA 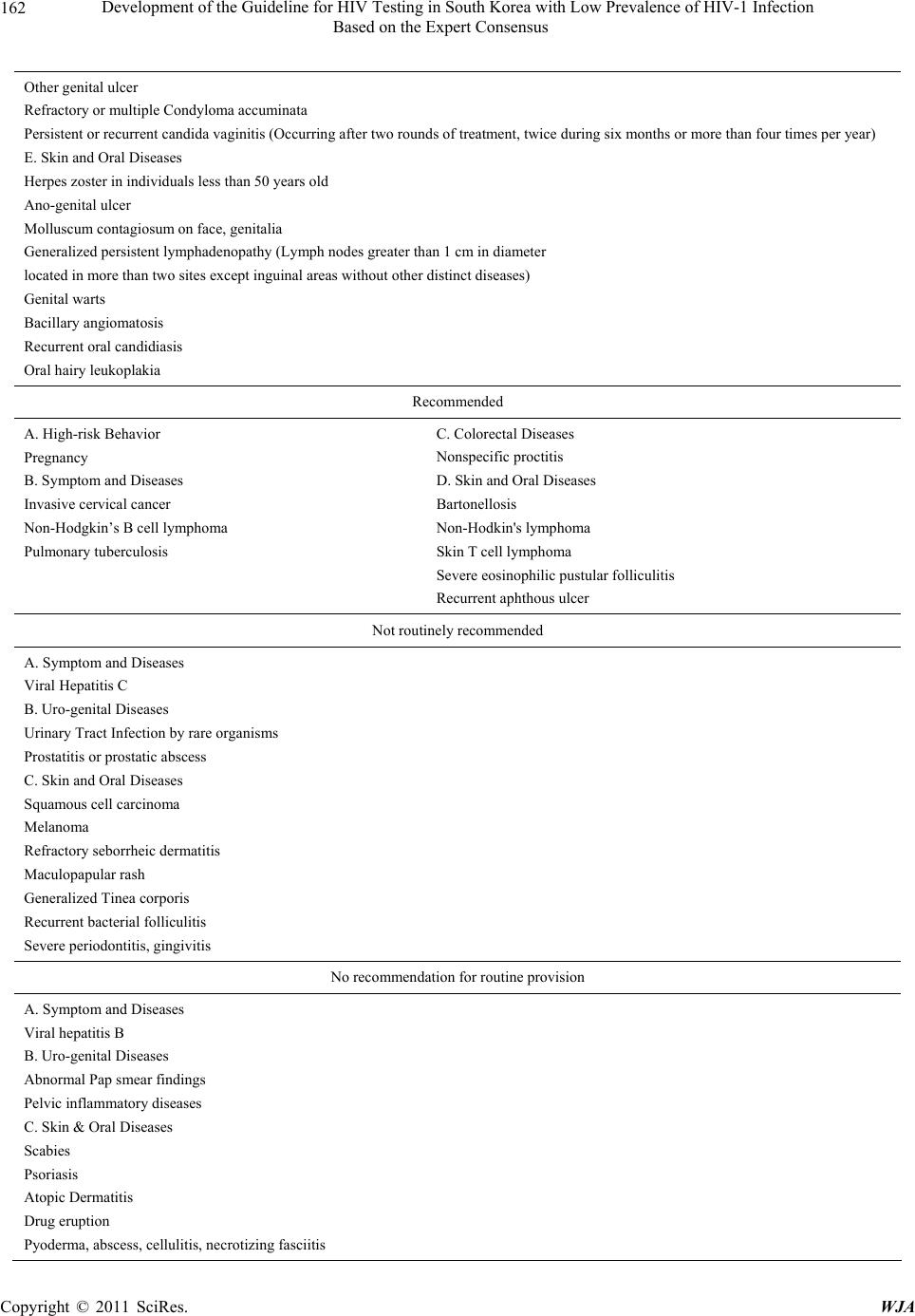 Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 162 Based on the Expert Consensus Other genital ulcer Refractory or multiple Condyloma accuminata Persistent or recurrent candida vaginitis (Occurring after two rounds of treatment, twice during six months or more than four times per year) E. Skin and Oral Diseases Herpes zoster in individuals less than 50 years old Ano-genital ulcer Molluscum contagiosum on face, genitalia Generalized persistent lymphadenopathy (Lymph nodes greater than 1 cm in diameter located in more than two sites except inguinal areas without other distinct diseases) Genital warts Bacillary angiomatosis Recurrent oral candidiasis Oral hairy leuko plakia Recommended A. High-risk Behavior Pregnancy B. Symptom and Diseases Invasive cervical cancer Non-Hodgkin’s B cell lymphoma Pulmonary tuberculosis C. Colorectal Diseases Nonspecific proctitis D. Skin and Oral Diseases Bartonellosis Non-Hodkin's lymphoma Skin T cell lymphoma Severe eosinophilic pustular folliculitis Recurrent aphthous ulcer Not routinely recommended A. Symptom and Diseases Viral Hepatitis C B. Uro-genital Diseases Urinary Tract Infection by rare organisms Prostatitis or prostatic abscess C. Skin and Oral Diseases Squamous cell carcinoma Melanoma Refractory seborrheic dermatitis Maculopapular rash Generalized Tinea corporis Recurrent bacterial folliculitis Severe periodontitis, gingivitis No recommendation for routine provision A. Symptom and Diseases Viral hepatitis B B. Uro-genital Diseases Abnormal Pap smear findings Pelvic inflammatory diseases C. Skin & Oral Diseases Scabies Psoriasis Atopic Dermatitis Drug eruption Pyoderma, abscess, cellulitis, necrotizing fasciitis Copyright © 2011 SciRes. WJA  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection Based on the Expert Consensus Copyright © 2011 SciRes. WJA 163 Korea. In 2001, the CDC recommended all pregnant women undergo an HIV test as part of routine screening for pre- natal care [13]. The universal HIV screening for the pre- natal period and HAART therapy demonstrated that 20% of the perinatal transmission cases decreased to less than 2% due to the recommendation for HIV testing [14,15]. Therefore, despite the low HIV prevalence among Ko- rean women, experts recommended routine screening of pregnant women for HIV based on evidence for the re- duction of perinatal HIV transmission when it was de- tected early. As shown in Table 2, most of the panel members agreed to the recommendation for screening of individu- als with the opportunistic diseases mentioned in the AIDS-surveillance case definition [16]. The prevalence of TB is relatively high in Korea, with 18,000 (0.039%) smear positive and 224,000 (0.32%) radiologically activ e TB patients being present in 2006 [4]. In contrast, the United States reported only 4.2 cases of TB per 100,000 in 2008, which was 10-f old lower than that of the Kor ean smear-positive cases [17]. TB prevalence is also de- creasing in Korea, but it is an important opportunistic infection of HIV patients. Therefore, although TB is still prevalent in Korea, our expert panel recommended HIV testing of individuals diagnosed with TB. Meanwhile, in the uogenital and anorectal field, most experts in this study agreed to recommend HIV testing of individuals with well-known AIDS defining opportunistic infections or cancer. However, it was not recommended that indi- viduals with nonspecific diseases of the skin such as sca- bies, atopic dermatitis, drug eruption, and psoriasis un- dergo HIV testing. All the recommendations were inte- grated, scaled and completed to a guideline. Since 2006, the CDC has recommended universal HIV testing at hospitals, but it was reported that many physi- cians did not routinely provide HIV tests [18], and only 35.8% of residents of internal medicine in New York administered HIV tests [19]. The CDC recommends HIV testing of newly diagnosed TB patients. In the United States, 63% of TB patients were tested for HIV in 1993, but only 37% of such patients were tested in Singapore in 2006 [20,21]. This discrepancy suggests that the guide- line should be publicized to practicing doctors. This guideline could help Korean physicians properly apply HIV testing to their patients. In summary, this literature review and Korean expert consensus condu cted through the Delphi process re sulted in the development of guideline for HIV testing reflect- ing national epidemiology of HIV-indicating diseases, which were different points compared with both CDC in US recommending universal HIV testing at ho spital. The guideline presented here should be revised again accord- ing to the change of HIV-prevalence. 5. Acknowledgments This study was funded by Korean CDC grant 2005- E31003-00. We thank the ten experts who participated in the Del- phi survey: Nam Joong Kim, Seoul National University School of Medicine; Yang-Ri Kim, Catholic University School of Medicine, Department of Internal Medicine, Division of Infectious Diseases; Woo Joo Kim, Korea University School of Medicine; June Myung Kim, Yon- sei University College of Medicine; Sun-Hee Lee, Busan National University School of Medicine; Hong-Bin Kim; Seoul National University College of Medicine; Hyo- Yeul Kim, Yousei University College of Medicine; Hyunjoo Pai; Hanyang University College of Medicine; Myoung-don Oh, Seoul National University College of Medicine; Jun Yong Choi, Yousei University College of Medicine. 6. Author Disclosure Statement Hee Jung Choi, Kyoung Ae Kong, Jung Won Min, Hun-Jae Lee and Hae-Sook Park have no potential con- flicts of interest to disclose. REFERENCES [1] UNAIDS, “AIDS Epidemic Update,” 2009. http://www.unaids.org [2] Korean CDC, “Development of Strategies for Activation in HIV Diagnostic Test in Korean Healthcare Settings,” 2006. [3] A. Jeong, H. W. Yim, S. H. Bae and W. C. Lee, “Changes of Hepatitis B Surface Antigen Seroprevalence in Korea, 1998-2005,” Korean Journal of Epidemiology, Vol. 30, No. 1, 2008, pp. 119-127. doi:10.4178/kje.2008.30.1.119 [4] H. J. Kim, “Current Situation of Tuberculosis and Its Control in Korea,” Journal of the Korean Medical Asso- ciation, Vol. 49, No. 9, 2006, pp. 762-772. doi:10.5124/jkma.2006.49.9.762 [5] Centers for Disease Control and Prevention, “Revised Guidelines for HIV Counseling, Testing, and Referral,” MMWR Recommendations and Reports, Vol. 50, 2001, pp. 1-57. [6] B. M. Branson, H. H. Handsfield, M. A. Lampe, R. S. Janssen, A. W. Taylor, S. B. Lyss, et al., “Revised Rec- ommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings,” MMWR Recommendations and Reports, Vol. 55, 2006, pp. 1-17. [7] Korean CDC, “Revised Guidelines for HIV Counseling, Testing, and Referral,” 1999. [8] British HIV Association, British Association of Sexual Health and HIV, British Infection Society, “UK National  Development of the Guideline for HIV Testing in South Korea with Low Prevalence of HIV-1 Infection 164 Based on the Expert Consensus Guidelines for HIV Testing,” 2008. http://www.bhiva.org/documents/Guidelines/Testing/Glin esHIVTest08.pdf [9] M. Poljak, E. Smit and J. Ross, “2008 European Guide- line on HIV Testing,” International Journal of STD & AIDS, Vol. 20, No. 2, 2009, pp. 77-83. doi:10.1258/ijsa.2008.008438 [10] World Health Organization and UNAIDS, “Guidance on Provider-Initiated Testing and Counseling in Health Fa- cilities,” 2007. http://www.whqlibdoc.who.int/publications/2007/978924 1595568_eng.pdf [11] H. A. Linstone, M. Turoff and O. Helmer, “The Delphi Method: Techniques and Applications,” 2002. http://is.njit.edu/pubs/delphibook/delphibook.pdf [12] Centers for Disease Control and Prevention, “Recom- mendations for Identification and Public Health Man- agement of Persons with Chronic Hepatitis B Virus Infec- tion,” MMWR Recommendations and Reports, Vol. 57, No. RR-8, 2008, pp. 1-20. [13] Centers for Disease Control and Prevention, “Revised Recommendations for HIV Screening of Pregnant Wom- en,” MMWR Recommendations and Reports, Vol. 50, No. RR-19, 2001, pp. 63-85. [14] E. R. Cooper, M. Charurat, L. Mofenson, I. C. Hanson, J. Pitt and C. Diaz, “Combination Antiretroviral Strategies for the Treatment of Pregnant HIV-1—Infected Women and Prevention of Perinatal HIV-1 Transmission,” Jour- nal of Acquired Immune Deficiency Syndromes, Vol. 29, No. 5, 2002, pp. 484-494. [15] L. M. Mofenson, “U.S. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States,” MMWR Recommendations and Re- ports, Vol. 51, No, RR-18, 2002, pp. 1-38. [16] Centers for Disease Control and Prevention, “1993 Re- vised Classification System for HIV Infection and Ex- panded Surveillance Case Definition for AIDS among Adolescents and Adults,” MMWR Recommendations and Reports, Vol. 41, No. RR-7, 1992, pp. 1-19. [17] Centers for Disease Control and Prevention, “Trends in Tuberculosis—United States, 2008,” MMWR Recom- mendations and Reports, Vol. 58, No. 10, 2009, pp. 249- 253. [18] R. C. Burke, K. A. Sepkowitz, K. T. Bernstein, A. M. Karpati, J. E. Myers, B. W. Tsoi, et al. “Why Don’t Phy - sicians Test for HIV? A Review of the United States Lit- erature,” AIDS, Vol. 21, No. 12, 2007, pp. 1617-1624. doi:10.1097/QAD.0b013e32823f91ff [19] C. L. Jain, C. M. Wyatt, R. Burke, K. Sepkowitz and E. M. Begier, “Knowledge of the Centers for Disease Con- trol and Prevention’s 2006 Routine HIV Testing Recom- mendations among New York City Internal Medicine Residents,” AIDS Patient Care and STDs, Vol. 23, No. 3, 2009, pp. 167-176. doi:10.1089/apc.2008.0130 [20] S. M. Asch, A. S. London, P. F. Barnes and L. Gelberg, “Testing for Human Immunodeficiency Virus Infection among Tuberculosis Patients in Los Angeles,” American Journal of Respiratory and Critical Care Medicine, Vol. 155, No. 1, 1997, pp. 378-381. [21] S. Y. Low and P. Eng, “Human Immunodeficiency Virus Testing in Patients with Newly-Diagnosed Tuberculosis in Singapore,” Singapore Medical Journal, Vol. 50, 2009, pp. 479-481. Copyright © 2011 SciRes. WJA
|