 Modern Mechanical Engineering, 2011, 1, 77-83 doi:10.4236/mme.2011.12010 Published Online November 2011 (http://www.SciRP.org/journal/mme) Copyright © 2011 SciRes. MME Bio-Diesel from Mustard Oil: A Renewable Alternative Fuel for Small Diesel Engines Zannatul Moiet Hasib1, Jomir Hossain2, Saikat Biswas2, Asif Islam3 1Ryerson University, Toronto, Canada 2Department of Mechani cal Engineering, Bangladesh Uni versi t y of Engi neeri ng an d Technology, Dhaka, Bangladesh 3Energypac Engineeri ng Lt d. C / A , Dhaka, Bangladesh E-mail: zannatulmoiet.hasib@ ryerson.ca, jomir_h@yahoo.com, {saikat.buet, asif038}@gmail.com Received October 8, 2011; revised November 8, 2011; accepted November 15, 2011 Abstract This paper represents the prospect of mustard oil as a renewable and alternative fuel. To cope up with present load-shedding situation and to reduce the dependency on imported fuel, Bangladesh government is encour- aging the use of renewable energy sources. Since diesel engines have versatile uses including small irrigation pumping systems, and standby small electricity generators, use of diesel fuel is much higher than any other gasoline fuels. In Bangladesh mustard oil has been in use as edible oil throughout the country. Mustard is a widely growing plant in Bangladesh and every year the production of mustard seed exceeds the demand. So the endeavor was to use the surplus mustard oil as an alternative to diesel fuel. Fuel properties are deter- mined in the fuel testing laboratory with standard procedure. An experimental set-up is then made to study the performance of a small diesel engine in the heat engine laboratory using different blends of bio-diesel converted from mustard oil. It is found that bio-diesel has slightly different properties than diesel fuel. It is also observed that with bio-diesel, the engine is capable of running without difficulty but with a deviation from its optimum performance. Initially different blends of bio-diesel (i.e. B20, B30, B50 etc.) have been used to avoid complicated modification of the engine or the fuel supply system. Finally, a comparison of en- gine performance for different blends of bio-diesel has been carried out to determine the optimum blend for different operating conditions. Keywords: Transesterification, Mustard Oil, Bio-Diesel, Heating Value, Pyrolysis, Viscosity 1. Introduction Modern civilization is much dependent on fossil energy. Energy obtained from fossil resources is much higher than any other resources. Majority of the world’s energy needs are supplied thorough petrochemical resources, coal, oil and natural gas. The consumption of fossil fuels is on in- crease from year to year. As the fossil resource is non- renewable, so fuel price is gouging as a consequence of spiraling demand and diminishing supply. Diesel fuel has higher energy density than other gaso- line fuel. Therefore, diesel engines are widely used in hea- vy-duty transportation, power generation and also in ag- ricultural sectors. As a result, the depletion rate of diesel fuel is much higher than other gasoline fuels, which sub- sequently causes higher price of diesel fuel than other gasoline. In Bangladesh, resource of petrochemical fuels is very limited. So, for energy demand, Bangladesh is fully dependent on crude oil import from Middle Eastern coun- tries. Moreover, as Bangladesh imports Arabian Light Crude oil (ALC), so the cost associated with oil refining is also huge. Moreover, the growing concern about envi- ronmental issues in the 90’s (i.e. clean air act) has in- creased the interest in alternative fuels paving the way to greater funding and effort for research studies. The in- creaseing amount of Green-House Gases (GHG) such as CO2 which is causing global warming and climate change, as well as the declining reserve of fossil fuels, and more importantly, the high fuel prices have strongly increased the interest in the use of bio-oils and biodiesel for land, transport and power generation. The sources of bio-fuels are renewable, and the use of bio-fuels ensures reduced amount of particulate matter, HC and NOX emission to the environment. Thus bio-fuels can emerge as an excel- lent alternative to fossil fuels. The use of vegetable oils as an alternative fuel for 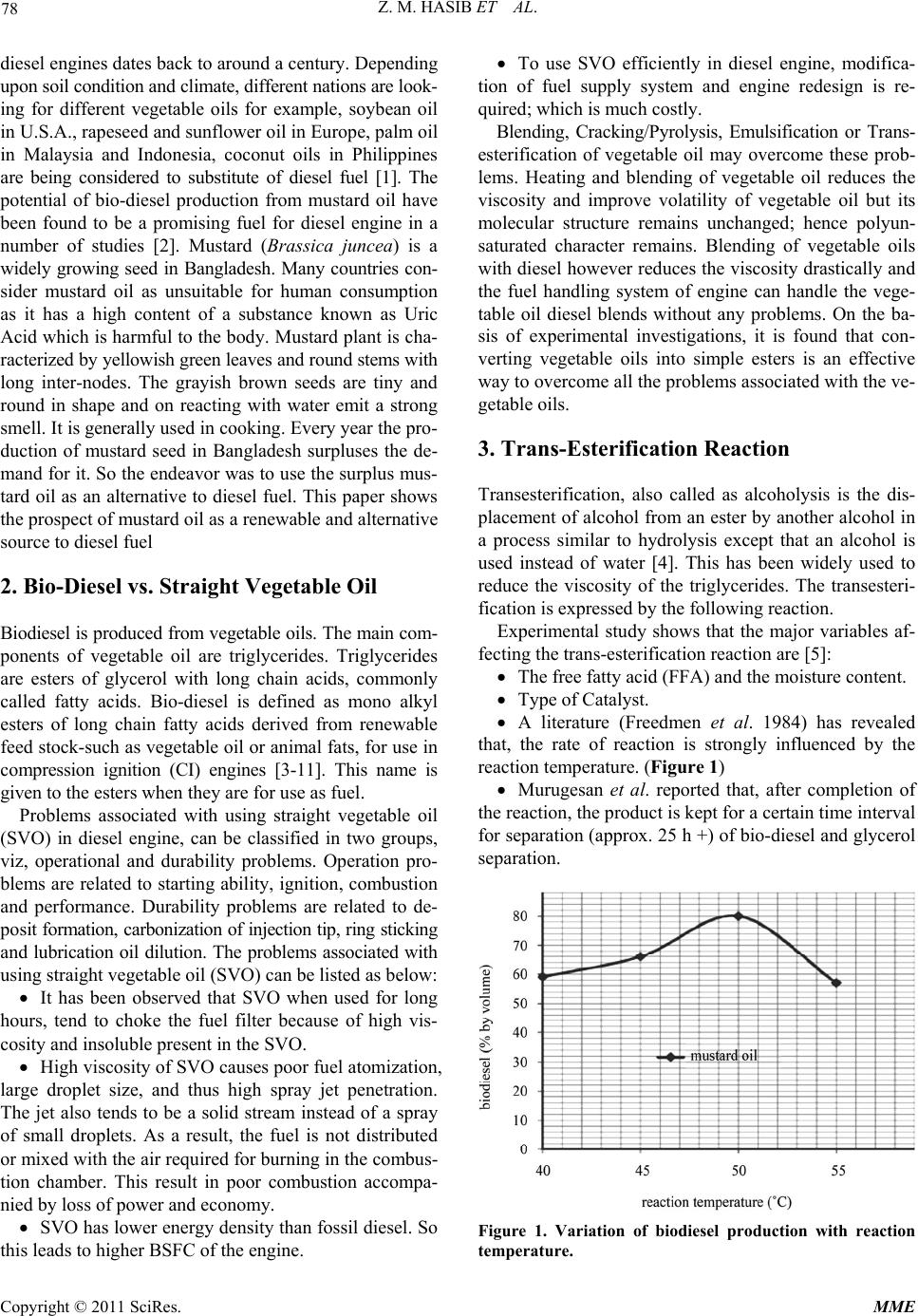 Z. M. HASIB ET AL. 78 diesel engines dates back to around a century. Depending upon soil condition and climate, different nations are look- ing for different vegetable oils for example, soybean oil in U.S.A., rapeseed and sunflower oil in Europe, palm oil in Malaysia and Indonesia, coconut oils in Philippines are being considered to substitute of diesel fuel [1]. The potential of bio-diesel production from mustard oil have been found to be a promising fuel for diesel engine in a number of studies [2]. Mustard (Brassica juncea) is a widely growing seed in Bangladesh. Many countries con- sider mustard oil as unsuitable for human consumption as it has a high content of a substance known as Uric Acid which is harmful to the body. Mustard plant is cha- racterized by yellowish green leaves and round stems with long inter-nodes. The grayish brown seeds are tiny and round in shape and on reacting with water emit a strong smell. It is generally used in cooking. Every year the pro- duction of mustard seed in Bangladesh surpluses the de- mand for it. So the endeavor was to use the surplus mus- tard oil as an alternative to diesel fuel. This paper shows the prospect of mustard oil as a renewable and alternative source to diesel fuel 2. Bio-Diesel vs. Straight Vegetable Oil Biodiesel is produced from vegetable oils. The main com- ponents of vegetable oil are triglycerides. Triglycerides are esters of glycerol with long chain acids, commonly called fatty acids. Bio-diesel is defined as mono alkyl esters of long chain fatty acids derived from renewable feed stock-such as vegetable oil or animal fats, for use in compression ignition (CI) engines [3-11]. This name is given to the esters when they are for use as fuel. Problems associated with using straight vegetable oil (SVO) in diesel engine, can be classified in two groups, viz, operational and durability problems. Operation pro- blems are related to starting ability, ignition, combustion and performance. Durability problems are related to de- posit formation, carbonization of injection tip, ring sticking and lubrication oil dilution. The problems associated with using straight vegetable oil (SVO) can be listed as below: It has been observed that SVO when used for long hours, tend to choke the fuel filter because of high vis- cosity and insoluble present in the SVO. High viscosity of SVO causes poor fuel atomization, large droplet size, and thus high spray jet penetration. The jet also tends to be a solid stream instead of a spray of small droplets. As a result, the fuel is not distributed or mixed with the air required for burning in the combus- tion chamber. This result in poor combustion accompa- nied by loss of power and economy. SVO has lower energy density than fossil diesel. So this leads to higher BSFC of the engine. To use SVO efficiently in diesel engine, modifica- tion of fuel supply system and engine redesign is re- quired; which is much costly. Blending, Cracking/Pyrolysis, Emulsification or Trans- esterification of vegetable oil may overcome these prob- lems. Heating and blending of vegetable oil reduces the viscosity and improve volatility of vegetable oil but its molecular structure remains unchanged; hence polyun- saturated character remains. Blending of vegetable oils with diesel however reduces the viscosity drastically and the fuel handling system of engine can handle the vege- table oil diesel blends without any problems. On the ba- sis of experimental investigations, it is found that con- verting vegetable oils into simple esters is an effective way to overcome all the problems associated with the ve- getable oils. 3. Trans-Esterification Reaction Transesterification, also called as alcoholysis is the dis- placement of alcohol from an ester by another alcohol in a process similar to hydrolysis except that an alcohol is used instead of water [4]. This has been widely used to reduce the viscosity of the triglycerides. The transesteri- fication is expressed by the following reaction. Experimental study shows that the major variables af- fecting the trans-esterification reaction are [5]: The free fatty acid (FFA) and the moisture content. Type of Catalyst. A literature (Freedmen et al. 1984) has revealed that, the rate of reaction is strongly influenced by the reaction temperature. (Figure 1) Murugesan et al. reported that, after completion of the reaction, the product is kept for a certain time interval for separation (approx. 25 h +) of bio-diesel and glycerol separation. Figure 1. Variation of biodiesel production with reaction temperature. Copyright © 2011 SciRes. MME  79 Z. M. HASIB ET AL Murugesan et al. reported that, washing is a proc- ess to remove catalyst, soap and excess methanol. 4. Synthesis of Bio-Diesel from Mustard Oil For the transesterification of mustard oil, Dr. Peepers style has been followed in our work [6,7]. First 250 ml (90% pure) methanol was mixed with 150 ml (1 N) NaOH. This mixture was swirled in a glass container until NaOH is fully dissolved in methanol. As this is an exothermic reaction, so the mixture would get hot. This solution is known as methoxide, which is a powerful corrosive base and is harmful for human skin. So, safety precautions should be taken to avoid skin contamination during meth- oxide producing [10-15]. Next, methoxide was added with 1 liter of mustard oil, which was preheated about 55 degree Celsius. Then the mixture was jerked for 5 minutes in a glass container. After that, the mixture was left for 24 hours (the longer is better) (Figures 2(a) and (b)) for the separation of glyc- erol and ester. This mixture then gradually settles down (a) (b) (c) Figure 2. (a) Biodiesel production after 3 hours of separa- tion. (b) Biodiesel production after 24 hours of separation. (c) Produced biodiesel is separated and then heated to re- move methanol and water. in two distinctive layers. The upper more transparent la- yer is 100% bio-diesel and the lower concentrated layer is glycerol. The heavier layer is then removed either by gravity separation or with a centrifuge. In some cases if the mustard oil contains impurities, then a thin white layer is formed in between the two layers. This thin layer composes soap and other impurities. Bio-diesel produced in the above process contains mois- ture (vaporization temperature 100 degree Celsius) and methanol (vaporization temperature 60 degree celsius) and usually some soap. If the soap level is low enough (300 ppm - 500 ppm), the methanol can be removed by vaporization and the methanol will usually be dry enough to directly recycle back to the reaction. Methanol tends to act as a co-solvent for soap in biodiesel; so at higher soap levels the soap will precipitate as a viscous sludge when the methanol is removed. Anyway, heating the biodiesel at temperature above 100 degree Celsius would cause the removal of both the moisture and methanol as well. In our study, food grade quality mustard oil was used, other than raw mustard oil to ensure that the vegetable oil contains lesser impurities. 5. Fuel Properties of Biodiesel and Their Blends Biodiesel produced from mustard oil has comparable fuel properties with the conventional fossil diesel. A com- parative study of fuel properties for fossil diesel, neat biodiesel and their blends have been carried out in this work to find out the suitable blending of biodiesel. In our study, we have prepared B20, B30, B40, B50 and B100 blend to compare the fuel properties for different blends. 5.1. Heating Value Heating value indicates the energy density of the fuel. In our study, ASTM 2382 method has been applied to mea- sure the heating value of biodiesel and their blends. Table 1 shows the heating value of diesel, neat biodiesel and their blends in MJ/Kg. Table 1. Comparison of heating value of different fuels. Heating value (MJ/Kg) Fossil Diesel 44.00 Neat biodiesel B100 39.51 B50 41.97 B40 42.18 B30 42.21 B20 42.65 Copyright © 2011 SciRes. MME 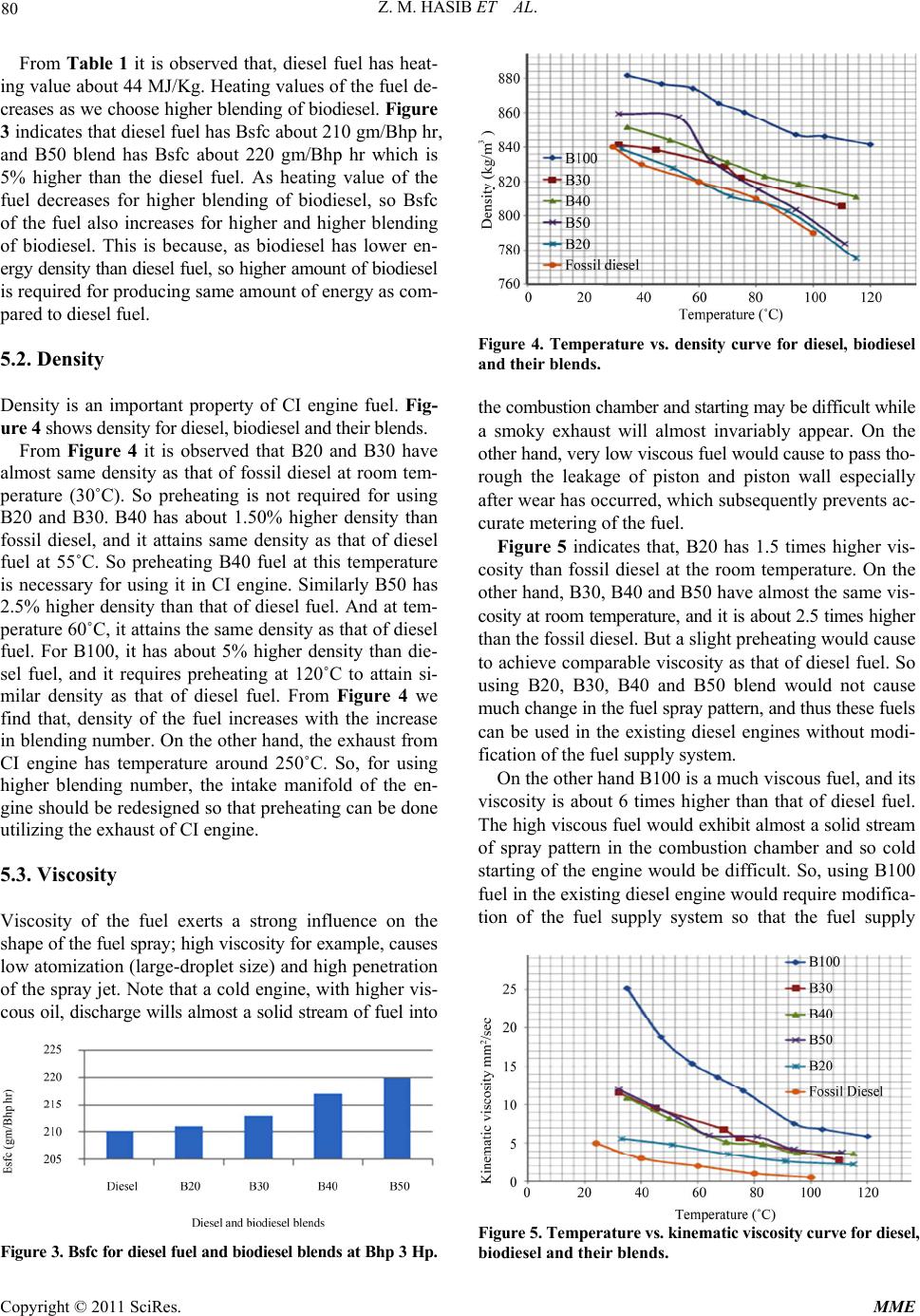 Z. M. HASIB ET AL. 80 From Table 1 it is observed that, diesel fuel has heat- ing value about 44 MJ/Kg. Heating values of the fuel de- creases as we choose higher blending of biodiesel. Figure 3 indicates that diesel fuel has Bsfc about 210 gm/Bhp hr, and B50 blend has Bsfc about 220 gm/Bhp hr which is 5% higher than the diesel fuel. As heating value of the fuel decreases for higher blending of biodiesel, so Bsfc of the fuel also increases for higher and higher blending of biodiesel. This is because, as biodiesel has lower en- ergy density than diesel fuel, so higher amount of biodiesel is required for producing same amount of energy as com- pared to diesel fuel. 5.2. Density Density is an important property of CI engine fuel. Fig- ure 4 shows density for diesel, biodiesel and their blends. From Figure 4 it is observed that B20 and B30 have almost same density as that of fossil diesel at room tem- perature (30˚C). So preheating is not required for using B20 and B30. B40 has about 1.50% higher density than fossil diesel, and it attains same density as that of diesel fuel at 55˚C. So preheating B40 fuel at this temperature is necessary for using it in CI engine. Similarly B50 has 2.5% higher density than that of diesel fuel. And at tem- perature 60˚C, it attains the same density as that of diesel fuel. For B100, it has about 5% higher density than die- sel fuel, and it requires preheating at 120˚C to attain si- milar density as that of diesel fuel. From Figure 4 we find that, density of the fuel increases with the increase in blending number. On the other hand, the exhaust from CI engine has temperature around 250˚C. So, for using higher blending number, the intake manifold of the en- gine should be redesigned so that preheating can be done utilizing the exhaust of CI engine. 5.3. Viscosity Viscosity of the fuel exerts a strong influence on the shape of the fuel spray; high viscosity for example, causes low atomization (large-droplet size) and high penetration of the spray jet. Note that a cold engine, with higher vis- cous oil, discharge wills almost a solid stream of fuel into Figure 3. Bsfc for diesel fuel and biodiesel blends at Bhp 3 Hp. Figure 4. Temperature vs. density curve for diesel, biodiesel and their blends. the combustion chamber and starting may be difficult while a smoky exhaust will almost invariably appear. On the other hand, very low viscous fuel would cause to pass tho- rough the leakage of piston and piston wall especially after wear has occurred, which subsequently prevents ac- curate metering of the fuel. Figure 5 indicates that, B20 has 1.5 times higher vis- cosity than fossil diesel at the room temperature. On the other hand, B30, B40 and B50 have almost the same vis- cosity at room temperature, and it is about 2.5 times higher than the fossil diesel. But a slight preheating would cause to achieve comparable viscosity as that of diesel fuel. So using B20, B30, B40 and B50 blend would not cause much change in the fuel spray pattern, and thus these fuels can be used in the existing diesel engines without modi- fication of the fuel supply system. On the other hand B100 is a much viscous fuel, and its viscosity is about 6 times higher than that of diesel fuel. The high viscous fuel would exhibit almost a solid stream of spray pattern in the combustion chamber and so cold starting of the engine would be difficult. So, using B100 fuel in the existing diesel engine would require modifica- tion of the fuel supply system so that the fuel supply Figure 5. Temperature vs. kinematic viscosity curve for diesel, biodiesel and their blends. Copyright © 2011 SciRes. MME  81 Z. M. HASIB ET AL system exerts high spray pressure to achieve the desired spray pattern inside the engine cylinder. 6. Engine Performance Testing and Analysis The final product of biodiesel from mustard oil was used as an alternative fuel to operate a diesel engine and the performance data were recorded. All data was derated as per BS5514 standard. The specification of the engine is given in Table 2. 6.1. Experimental Setup The experimental setup (Figure 6) consisted of engine test bed with fuel supply system and different metering and measuring devices with the engine. A water brake dynamometer was coupled with the engine. Load was va- ried by means of flow control of the dynamometer. Fuel was supplied from an external source. Preheating of fuel was done manually by gas burner. B40 blend was pre- heated at 55˚C and B50 blend was preheated at 60˚C. However B100 was not possible to use directly in the en- gine as it causes excessive vibration. Engine speed was measured by digital tachometer. Lube oil temperature and exhaust gas temperature was measured by K-type ther- mocouple. Operating condition of the engine is given in Table 3. Table 2. Engine specifications. Model ZS 1110 Method of starting Hand starting type Horizontal, 4-stroke, 1 cylinder Cylinder dia 70 mm Piston stroke 75 mm Nominal speed 2600 rpm Nominal power 3 Hp Cooling system Air cooled rotation Anti-clockwise Fuel filter Present Lube oil filter present Figure 6. Experimental setup. Table 3. Engine operating conditions. Engine speed 2200 rpm Engine load 1 kg to 3.5 kg Fuels tested 100% diesel, B20, B30, B40 and B50. Lube oil used SAE-40 6.2. Performance Analysis Figure 7 shows the variation of Bsfc with Bhp for dif- ferent fuels. The curve shows that, Bsfc for biodiesel blends is higher at low % load. And it decreases with the increase in % load. It is also observed from the curve that, specific fuel consumption increases with the increase in biodiesel blend. This is mainly due to the relationship among volumetric fuel injection system, fuel specific grav- ity, viscosity and heating value. As a result, more biodie- sel blend is needed to produce the same amount of en- ergy due to its higher density and lower heating value in comparison to conventional diesel fuel. Again as bio- diesel blends have different viscosity than diesel fuel, so biodiesel causes poor atomization and mixture formation and thus increases the fuel consumption rate to maintain the power. Figure 8 shows the relation in between Bhp and brake thermal efficiency ηb for different fuels. Bsfc is a meas- ure of overall efficiency of the engine. Bsfc is inversely related with efficiency. So, lower the value of Bsfc, higher is the overall efficiency of the engine. However, for dif- ferent fuels with different heating values, the Bsfc values are misleading and hence brake thermal efficiency is em- ployed when the engines are fueled with different types of fuels. From the figure, it is evident that Bsfc for bio- diesel blends is always higher and ηb is always lower than that of diesel fuel. This is because biodiesel has lower heating value than conventional diesel fuel. One other cause for lower ηb for biodiesel blends is the poor at- omization which is attributed to higher density and ki- nematic viscosity of biodiesel blends. Figure 9 depicts about variation in exhaust gas tem- perature with Bhp for different fuels. From the curve it is observed that except B30, all other biodiesel blends have higher exhaust gas temperature than diesel fuel. At start- ing condition, higher exhaust gas temperature but low power output for biodiesel blends indicate late burning to the high proportion of biodiesel. This would increase the heat loss, making the combustion a less efficient. At higher load condition, B30 and B40 have lower exhaust temperature as compared to diesel fuel. Figure 10 shows the relation in between lube oil tem- perature and Bhp for different fuels. At lower Bhp, diesel fuel and biodiesel blends have similar lube oil tempera- ture. At higher % load condition, B50 shows higher lube Copyright © 2011 SciRes. MME  Z. M. HASIB ET AL. 82 Figure 7. Variation of Bsfc with Bhp for different fuels. Figure 8. Variation of thermal efficiency ηb with Bhp for different fuels. Figure 9. Variation of exhaust gas temperature with Bhp for different fuels. Figure 10. Variation of lube oil temperature with Bhp for different fuels. Table 4. Cost of running engines with different fuels. Fuel Cost (tk/lr) Diesel 40 B20 58 B30 67 B40 76 B50 85 oil temperature than other fuels. This phenomenon can be attributed to the preheating of the B50 fuel at 60˚C. However, there is not wide variance in the lube oil tem- perature for diesel fuel and biodiesel blends; which indi- cates that SAE-40 lube oil is suitable for biodiesel run engines. 7. Cost Analysis The present costing of running a diesel engine with bio- diesel blends derived from mustard oil are given in Table 4. From Table 4 it is clear that, running diesel engine with biodiesel blends is costly as compared to diesel fuel. How- ever, cost can be drastically reduced, if methanol can be recycled after the transesterification reaction. Moreover, in our experiment we have used food grade mustard oil. And using raw or unprocessed oil would also cause to decrease the biodiesel production cost. In Bangladesh, government grants a huge subsidy on diesel fuel, which causes the lower price for diesel fuel. So a thorough study is required for the feasibility analy- sis of biodiesel by comparing it production cost with in- ternational market price of diesel. 8. Conclusions Experiment was conducted on a small four stroke diesel engine to determine the feasibility of mustard oil as an alternative to diesel engine. The following conclusions may be drawn from the experiment. Biodiesel can be produced from mustard oil using transesterification reaction. It is possible to run diesel engine with biodiesel blends. Bsfc for biodiesel increases for higher blending of biodiesel, because of the lower heating value of biodiesel as compared to diesel fuel. For using higher blending of biodiesel, the fuel must be preheated in order to reduce the density and vis- cosity of the fuel. Compared to diesel fuel, a little amount of power loss occurs for biodiesel blends. Copyright © 2011 SciRes. MME  Z. M. HASIB ET AL Copyright © 2011 SciRes. MME 83 9. Acknowledgements This research work was funded by Department of Me- chanical Engineering, Bangladesh University of Engi- neering & Technology (BUET). Laboratory support: Fuel testing lab, Heat engine lab, ME dept. BUET. 10. References [1] A. Srivasata and R. Prasad, “Triglyceride based diesel Fuels,” Renewable and sustainable Energy Reviews, Vol. 4, No. 2, 2000, pp. 111-133. doi:10.1016/S1364-0321(99)00013-1 [2] A. Forhad, A. R. Rowshan, M. A. Habib and M. A. Islam, “Production and Performance of Biodiesel as an Alterna- tive to Diesel,” International Conference on Mechanical Engineering, London, 1-3 July 2009. [3] Y. Yosimoto, M. Onodera and H. Tamaki, “Production and Emission Characteristics of Diesel Engine Fuelled by Vegetable Oils,” The Society of Automotive Engineers, Vol. 2, No. 2, 2001. [4] J. Otera, “Transestrification,” Chemical Reviews, Vol. 93, No. 4, 1993, pp. 1449-1470. doi:10.1021/cr00020a004 [5] B. Freedom, E. H. Pyre and T. L. Mounts, “Variable Af- fecting the Yield of Fatty Asters from Transesterification Vegetable Oils,” Journal of the American oil Chemists’ Society, Vol. 61, No. 10, 1984, pp. 1638-1643 [6] R. Da Tech, “Single StageBase Recipes”, 2010. http://www.make-biodiesel.org/index.php?option=com_ct ent&view=article&id=74:single-stage-base-recipes&catid =51:recipes&Itemid=96 [7] Z. M. Hasib, K. A. Rahman and S. Alam, “Prospect of Bio Diesel Production from Mustard Oil,” Bachelor of Science in Engineering Project and Thesis, Bangladesh University of Engineering and Technology, Dhaka, 2010 [8] E. Ahn, M. Koncar, M. Mittelbach and R. Marr, “A Low Waste Process for the Production of Biodiesel,” Separa- tion Science and Technology, Vol. 30, No. 9, 1995, pp. 2021-2033. doi:10.1080/01496399508010391 [9] C. C. Akoh and B.G. Swanson, “Base Catalyzed Trans- esterification of Vegetable Oils,” Journal of Food Proc- essing & Preservation, Vol. 12, 1988, pp. 139-149. doi:10.1111/j.1745-4549.1988.tb00073.x [10] J. Van Gerpen, “Cetane Number Testing of Biodiesel,” Liquid Fuels and Industrial Products from Renewable Resources—Proceedings of the Third Liquid Fuels Con- ference, Nashville, 15-17 September 1996, pp. 166-176. [11] M. Stumborg, A. Wong and E. Hogan, “Hydroprocessed Vegetable Oils for Diesel Fuel Improvement,” Biore- source Technology, Vol. 56, 1996, pp. 13-18. doi:10.1016/0960-8524(95)00181-6 [12] M. Stumborg, D. Soveran and W. Craig, “Conversion of Vegetable Oils to Renewable Diesel Additives,” Pre- sented at the 1991 International Winter Meeting of the ASAE Paper No. 911567, Chicago, 17-20 December 1991. [13] N. M. Strete, “Evaluation of Detergency Effects of Bio- diesel Using Cummins L10 Injector Depositing Test— Cell 19,” Final report from ETS to NBB, 30 September 1996. [14] L. L. Stavinoha and S. Howell, “Potential Analytical Methods for Stability Testing of Biodiesel and Biodiesel Blends,” Society of Automotive Engineering (Special Edi- tion), USA Paper No. 1999-01-3520, 1999, pp. 73-79. [15] H. Stage, “Principle of the New ATT-Process for Con- verting Vegetable Oils to Diesel Fuels,” Zeitschrift für Wissenschaft und Technologie der Fette, Öle und Wachse, Vol. 90, No. 1, 1988, pp. 28-32. Definitions/Abbreviations Bsfc: Brake specific fuel consumption, gm/Bhp-hr Bhp: Brake horse power, hp LV: Lower heating value of fuel, Mj/kg T: Temperature, ˚C Ηb: Brake thermal efficiency (%)
|