 Open Journal of Polymer Chemistry, 2011, 1, 1-9 doi:10.4236/ojpchem.2011.11001 Published Online November 2011 (http://www.SciRP.org/journal/ojpchem) Copyright © 2011 SciRs. OJPChem Organosoluble and Thermally Stable of Benzazole- Containing Poly(Imide-Urea)s: One-Pot Synthesis and Characterization Hojjat Toiserkani1,2* 1Department of Chemistry, College of Sciences, Hormozgan University, Bandar Abbas, Iran. 2College of Oil Engineering, Hormozgan University, Bandar Abbas, Iran. E-mail: *Toiserkani@yahoo.com Received October 12, 2011; revised November 10, 2011; accepted November 18, 2011 Abstract The preparation of new types of poly(imide-urea)s (PIUs) with high thermal stability and improved solubility was investigated. Three series of aromatic poly(imide-urea)s (PIUOa-c, PIUSa-c, and PIUNa-c) bearing pen- dent benzoxazole, benzothiazole or benzimidazole rings were prepared by one-pot polycondensation reaction of three bis(imide-carboxylic acid)s, 2-[3,5-bis(N-trimellitimidoyl)-phenyl]benzoxazole (1O), 2-[3,5-bis(N- trimellitimidoyl)-phenyl]benzothiazole (1S), or 2-[3,5-bis(N-trimellitimidoyl)-phenyl]benzimidazole (1N) with various kinds of aromatic diamines (a-c). The effects of the benzazole pendent groups on the polymer properties such as solubility and thermal stability were investigated by comparison of the polymers. All of the resulting polymers exhibited excellent solubility in common polar solvents. The glass transition tem- perature of the polymers determined by DSC thermograms were in the range 192˚C - 236˚C. The tempera- tures at 10% weight loss from their TGA curves were found to be in the range 390˚C - 441˚C in nitrogen. Keywords: Poly(Imide-Urea)s, Structure-Property Relation, Thermal Properties, Benzazole Pendent Groups 1. Introduction Aromatic polyimides (PIs) are well known as materials of high performance were developed in the early 1960s and since then have been of great technological impor- tance because of their outstanding thermal stability and chemical resistance, together with their balanced electric and mechanical properties [1,2]. Because many of them are insoluble and infusible, however, their applications in some fields were limited. It is well known that the poor properties of PIs have a close connection with its chemi- cal composition and chain structure, in other words, the chemical composition and chain structure of PIs will be a head ingredient leading them to infusible within proc- essing temperature and insoluble in organic solvents. Thus, incorporating new functionalities to make polyim- ides more tractable without decreasing their many desir- able properties has become one important target of polyimides’ chemistry [3,4]. So far, many efforts on chemical modifications of polyimides structure have been made to enhance their processability and solubility while other advantageous polymer properties are retained either by introducing flexible linkages, bulky groups, or molecular asymmetry into the polymer backbone [5-19]. Among these approaches, the method of incorporating bulky, rigid benzazole hetero rings as pendent groups into the polymer backbone has become very attractive [20-23]. The advantages of this method include two main parts: (1) close chain packing and intermolecular interac- tions of the resulting polyimide are restricted, and this results in relatively high polymer solubility, and (2) the main chain rigidity of the polyimide can be maintained by restricting the segmental mobility, in addition, these benzazole hetero rings are thermally stable, and these allow the polyimide to have a high glass-transition tem- perature (Tg) and excellent thermal properties. As reported in our previous publications [22,23], three bis(imide-carboxylic acid)s, 2-[3,5-bis(N-trimellitimi- doyl)-phenyl]benzoxazole (1O), 2-[3,5-bis(N-trimelliti- midoyl)-phenyl]benzothiazole (1S), and 2-[3,5-bis(N-tri- mellitimidoyl)-phenyl]benzimidazole (1N) were synthe- sized via a two-stage procedure that included the con- densation reaction between 2-aminophenol, 2-amino- thiophenol, or m-phenylenediamine and 3,5-diamino- benzoic acid in the presence of polyphosphoric acid (PPA) in 190˚C, followed by their reaction two mole 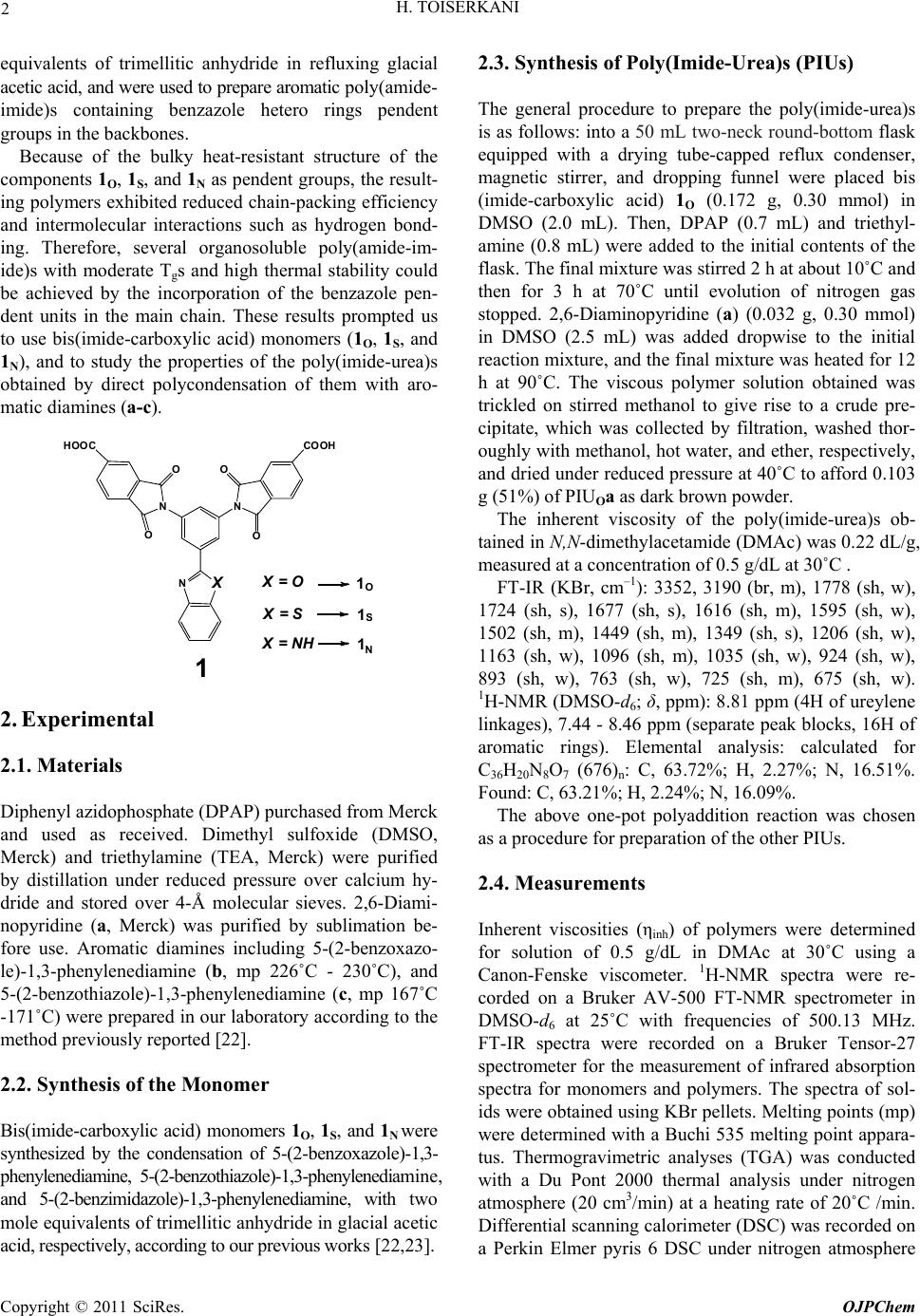 H. TOISERKANI 2 equivalents of trimellitic anhydride in refluxing glacial acetic acid, and were used to prepare aromatic poly(amide- imide)s containing benzazole hetero rings pendent groups in the backbones. Because of the bulky heat-resistant structure of the components 1O, 1S, and 1N as pendent groups, the result- ing polymers exhibited reduced chain-packing efficiency and intermolecular interactions such as hydrogen bond- ing. Therefore, several organosoluble poly(amide-im- ide)s with moderate Tgs and high thermal stability could be achieved by the incorporation of the benzazole pen- dent units in the main chain. These results prompted us to use bis(imide-carboxylic acid) monomers (1O, 1S, and 1N), and to study the properties of the poly(imide-urea)s obtained by direct polycondensation of them with aro- matic diamines (a-c). X=NH X=O X=S 1 1 1 NX NN COOH O O O O HOOC 1N O S 2. Experimental 2.1. Materials Diphenyl azidophosphate (DPAP) purchased from Merck and used as received. Dimethyl sulfoxide (DMSO, Merck) and triethylamine (TEA, Merck) were purified by distillation under reduced pressure over calcium hy- dride and stored over 4-Å molecular sieves. 2,6-Diami- nopyridine (a, Merck) was purified by sublimation be- fore use. Aromatic diamines including 5-(2-benzoxazo- le)-1,3-phenylenediamine (b, mp 226˚C - 230˚C), and 5-(2-benzothiazo le)-1,3-phenylenediamine (c, mp 167˚C -171˚C) were prepared in our laboratory according to the method previously reported [22]. 2.2. Synthesis of the Monomer Bis(imide-carboxylic acid) monomers 1O, 1S, and 1N were synthesized by the condensation of 5-(2-benzoxazole)-1,3- phenylenediamine, 5-(2-benzothiazole)-1,3-phenylenediamine, and 5-(2-benzimidazole)-1,3-phenylenediamine, with two mole equivalents of trimellitic anhydride in glacial acetic acid, respectively, according to our previous works [22,23]. 2.3. Synthesis of Poly(Imide-Urea)s (PIUs) The general procedure to prepare the poly(imide-urea)s is as follows: into a 50 mL two-neck round-bottom flask equipped with a drying tube-capped reflux condenser, magnetic stirrer, and dropping funnel were placed bis (imide-carboxylic acid) 1O (0.172 g, 0.30 mmol) in DMSO (2.0 mL). Then, DPAP (0.7 mL) and triethyl- amine (0.8 mL) were added to the initial contents of the flask. The final mixture was stirred 2 h at about 10˚C and then for 3 h at 70˚C until evolution of nitrogen gas stopped. 2,6-Diaminopyridine (a) (0.032 g, 0.30 mmol) in DMSO (2.5 mL) was added dropwise to the initial reaction mixture, and the final mixture was heated for 12 h at 90˚C. The viscous polymer solution obtained was trickled on stirred methanol to give rise to a crude pre- cipitate, which was collected by filtration, washed thor- oughly with methanol, hot water, and ether, respectively, and dried under reduced pressure at 40˚C to afford 0.103 g (51%) of PIUOa as dark brown powder. The inherent viscosity of the poly(imide-urea)s ob- tained in N,N -dimethylacetamide (DMAc) was 0.22 dL/g, measured at a concentration of 0.5 g/dL at 30˚C . FT-IR (KBr, cm–1): 3352, 3190 (br, m), 1778 (sh, w), 1724 (sh, s), 1677 (sh, s), 1616 (sh, m), 1595 (sh, w), 1502 (sh, m), 1449 (sh, m), 1349 (sh, s), 1206 (sh, w), 1163 (sh, w), 1096 (sh, m), 1035 (sh, w), 924 (sh, w), 893 (sh, w), 763 (sh, w), 725 (sh, m), 675 (sh, w). 1H-NMR (DMSO-d6; δ, ppm): 8.81 ppm (4H of ureylene linkages), 7.44 - 8.46 ppm (separate peak blocks, 16H of aromatic rings). Elemental analysis: calculated for C36H20N8O7 (676)n: C, 63.72%; H, 2.27%; N, 16.51%. Found: C, 63.21%; H, 2.24%; N, 16.09%. The above one-pot polyaddition reaction was chosen as a procedure for preparation of the other PIUs. 2.4. Measurements Inherent viscosities (ηinh) of polymers were determined for solution of 0.5 g/dL in DMAc at 30˚C using a Canon-Fenske viscometer. 1H-NMR spectra were re- corded on a Bruker AV-500 FT-NMR spectrometer in DMSO-d6 at 25˚C with frequencies of 500.13 MHz. FT-IR spectra were recorded on a Bruker Tensor-27 spectrometer for the measurement of infrared absorption spectra for monomers and polymers. The spectra of sol- ids were obtained using KBr pellets. Melting points (mp) were determined with a Buchi 535 melting point appara- tus. Thermogravimetric analyses (TGA) was conducted with a Du Pont 2000 thermal analysis under nitrogen atmosphere (20 cm3/min) at a heating rate of 20˚C /min. Differential scanning calorimeter (DSC) was recorded on a Perkin Elmer pyris 6 DSC under nitrogen atmosphere Copyright © 2011 SciRes. OJPChem 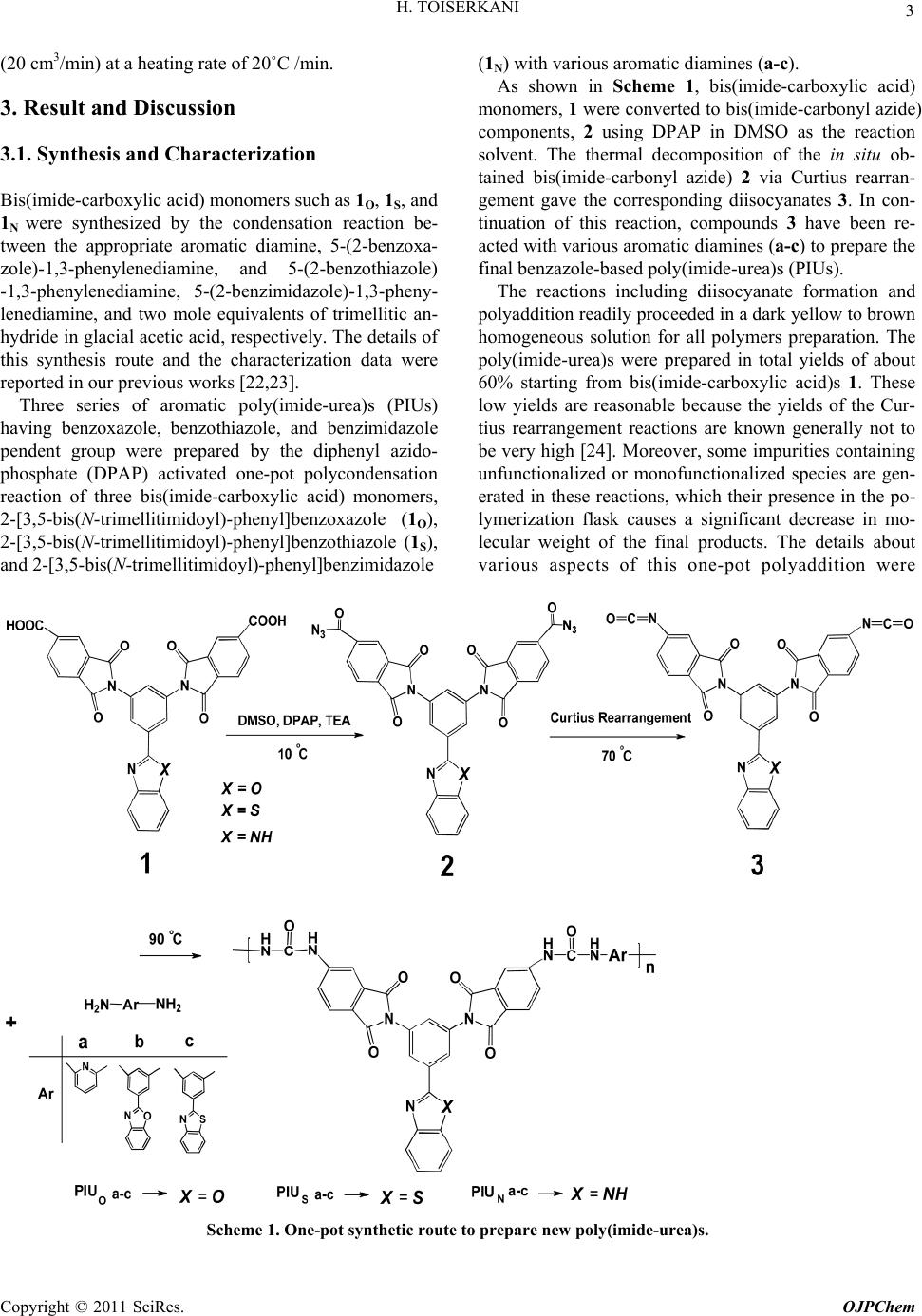 H. TOISERKANI Copyright © 2011 SciRes. OJPChem 3 (20 cm3/min) at a heating rate of 20˚C /min. (1N) with various aromatic diamines (a-c). As shown in Scheme 1, bis(imide-carboxylic acid) monomers, 1 were converted to bis(imide-carbonyl azide) components, 2 using DPAP in DMSO as the reaction solvent. The thermal decomposition of the in situ ob- tained bis(imide-carbonyl azide) 2 via Curtius rearran- gement gave the corresponding diisocyanates 3. In con- tinuation of this reaction, compounds 3 have been re- acted with various aromatic diamines (a-c) to prepare the final benzazole-based poly(imide-urea)s (PIUs). 3. Result and Discussion 3.1. Synthesis and Characterization Bis(imide-carboxylic acid) monomers such as 1O, 1S, and 1N were synthesized by the condensation reaction be- tween the appropriate aromatic diamine, 5-(2-benzoxa- zole)-1,3-phenylenediamine, and 5-(2-benzothiazole) -1,3-phenylenediamine, 5-(2-benzimidazole)-1,3-pheny- lenediamine, and two mole equivalents of trimellitic an- hydride in glacial acetic acid, respectively. The details of this synthesis route and the characterization data were reported in our previous works [22,23]. The reactions including diisocyanate formation and polyaddition readily proceeded in a dark yellow to brown homogeneous solution for all polymers preparation. The poly(imide-urea)s were prepared in total yields of about 60% starting from bis(imide-carboxylic acid)s 1. These low yields are reasonable because the yields of the Cur- tius rearrangement reactions are known generally not to be very high [24]. Moreover, some impurities containing unfunctionalized or monofunctionalized species are gen- erated in these reactions, which their presence in the po- lymerization flask causes a significant decrease in mo- lecular weight of the final products. The details about various aspects of this one-pot polyaddition were Three series of aromatic poly(imide-urea)s (PIUs) having benzoxazole, benzothiazole, and benzimidazole pendent group were prepared by the diphenyl azido- phosphate (DPAP) activated one-pot polycondensation reaction of three bis(imide-carboxylic acid) monomers, 2-[3,5-bis(N-trimellitimidoyl)-phenyl]benzoxazole (1O), 2-[3,5-bis(N-trimellitimidoyl)-phenyl]benzothiazole (1S), and 2-[3,5-bis(N-trimellitimidoyl)-phenyl]benzimidazole Scheme 1. One-pot synthetic route to pre par e new poly(imide-urea)s. 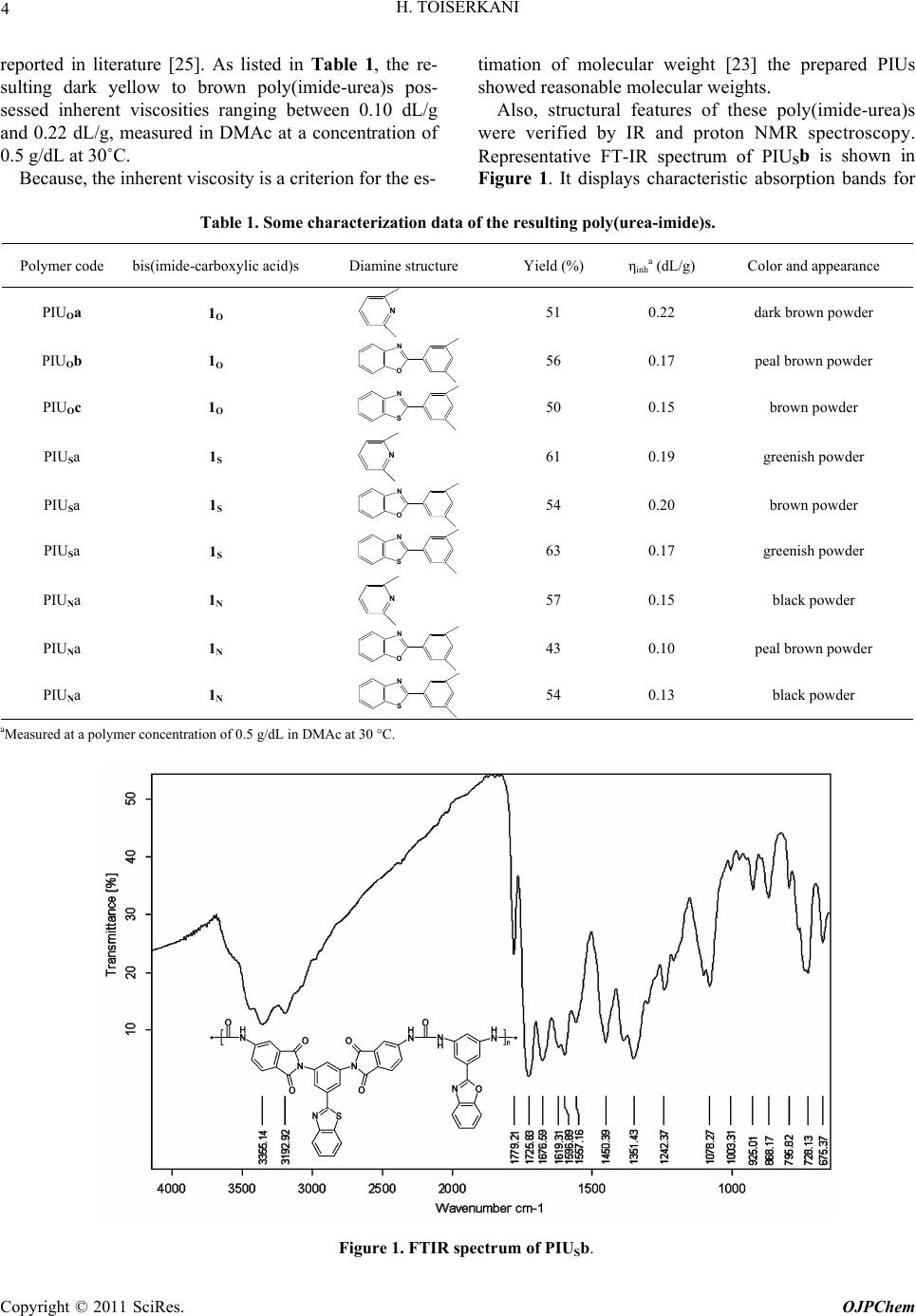 H. TOISERKANI 4 reported in literature [25]. As listed in Table 1, the re- sulting dark yellow to brown poly(imide-urea)s pos- sessed inherent viscosities ranging between 0.10 dL/g and 0.22 dL/g, measured in DMAc at a concentration of 0.5 g/dL at 30˚C. Because, the inherent viscosity is a criterion for the es- timation of molecular weight [23] the prepared PIUs showed reasonable molecular weights. Also, structural features of these poly(imide-urea)s were verified by IR and proton NMR spectroscopy. Representative FT-IR spectrum of PIUSb is shown in Figure 1. It displays characteristic absorption bands for Table 1. Some characterization data of the resulting poly(urea-imide)s. Polymer code bis(imide-carboxylic acid)s Diamine structure Yield (%) ηinha (dL/g) Color and appearance PIUOa 1O N 51 0.22 dark brown powder PIUOb 1O N O 56 0.17 peal brown powder PIUOc 1O N S 50 0.15 brown powder PIUSa 1S N 61 0.19 greenish powder PIUSa 1S N O 54 0.20 brown powder PIUSa 1S N S 63 0.17 greenish powder PIUNa 1N N 57 0.15 black powder PIUNa 1N N O 43 0.10 peal brown powder PIUNa 1N N S 54 0.13 black powder aMeasured at a polymer concentration of 0.5 g/dL in DMAc at 30 °C. Figure 1. FTIR spectrum of PIUSb. Copyright © 2011 SciRes. OJPChem 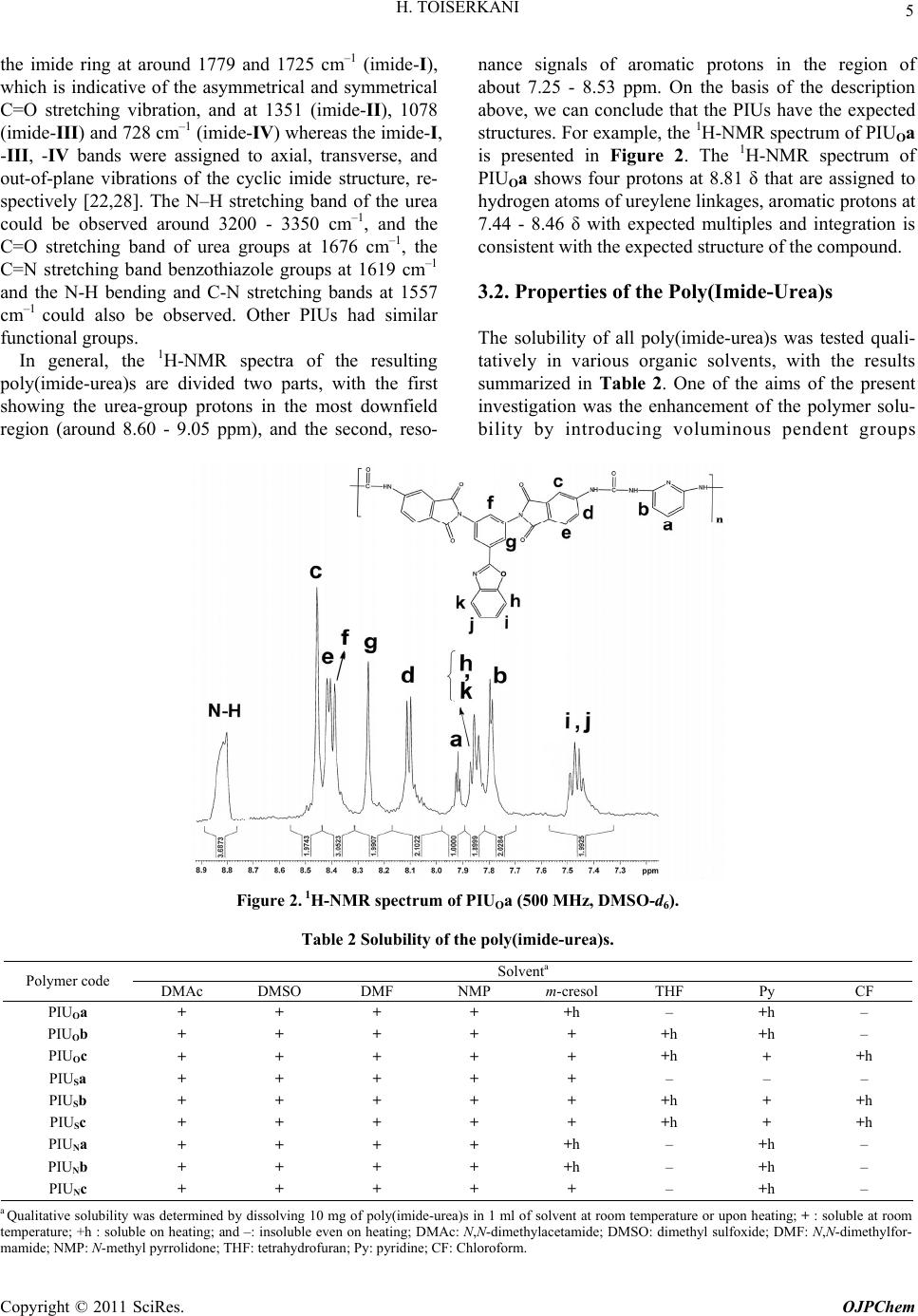 5 H. TOISERKANI the imide ring at around 1779 and 1725 cm–1 (imide-I), which is indicative of the asymmetrical and symmetrical C=O stretching vibration, and at 1351 (imide-II), 1078 (imide-III) and 728 cm–1 (imide-IV) whereas the imide-I, -III, -IV bands were assigned to axial, transverse, and out-of-plane vibrations of the cyclic imide structure, re- spectively [22,28]. The N–H stretching band of the urea could be observed around 3200 - 3350 cm–1, and the C=O stretching band of urea groups at 1676 cm–1, the C=N stretching band benzothiazole groups at 1619 cm–1 and the N-H bending and C-N stretching bands at 1557 cm–1 could also be observed. Other PIUs had similar functional groups. In general, the 1H-NMR spectra of the resulting poly(imide-urea)s are divided two parts, with the first showing the urea-group protons in the most downfield region (around 8.60 - 9.05 ppm), and the second, reso- nance signals of aromatic protons in the region of about 7.25 - 8.53 ppm. On the basis of the description above, we can conclude that the PIUs have the expected structures. For example, the 1H-NMR spectrum of PIUOa is presented in Figure 2. The 1H-NMR spectrum of PIUOa shows four protons at 8.81 δ that are assigned to hydrogen atoms of ureylene linkages, aromatic protons at 7.44 - 8.46 δ with expected multiples and integration is consistent with the expected structure of the compound. 3.2. Properties of the Poly(Imide-Urea)s The solubility of all poly(imide-urea)s was tested quali- tatively in various organic solvents, with the results summarized in Table 2. One of the aims of the present investigation was the enhancement of the polymer solu- bility by introducing voluminous pendent groups Figure 2. 1H-NMR spectrum of PIUOa (500 MHz, DMSO-d6). Table 2 Solubility of the poly(imide-urea)s. Solventa Polymer code DMAc DMSO DMF NMP m-cresol THF Py CF PIUOa + + + + +h – +h – PIUOb + + + + + +h +h – PIUOc + + + + + +h + +h PIUSa + + + + + – – – PIUSb + + + + + +h + +h PIUSc + + + + + +h + +h PIUNa + + + + +h – +h – PIUNb + + + + +h – +h – PIUNc + + + + + – +h – a Qualitative solubility was determined by dissolving 10 mg of poly(imide-urea)s in 1 ml of solvent at room temperature or upon heating; + : soluble at room temperature; +h : soluble on heating; and –: insoluble even on heating; DMAc: N,N-dimethylacetamide; DMSO: dimethyl sulfoxide; DMF: N,N-dimethylfor- mamide; NMP: N-methyl pyrrolidone; THF: tetrahydrofuran; Py: pyridine; CF: Chloroform. Copyright © 2011 SciRes. OJPChem  H. TOISERKANI 6 along the polymer backbone. The poly(imide-urea)s, PIUOa-c were readily soluble in polar aprotic solvents (DMF, NMP, DMAc, DMSO). They also dissolved in m-cresol and pyridine, while they were partially soluble in chloroform and tetrahydrofuran upon heating at about 70˚C. Poly(imide-urea)s, PIUSa-c, compared to PIUOa-c displayed almost the same solubil- ity in these solvents. In general, the flexible amide, ether, and urea linkages affect the solubility of a copolyimide to a great extent due to a salvation effect. Besides the solvation effects related to enthalpy factor, the good solubility of these polymers is also caused, mainly by the entropy advantage which resulted from bulky pendant groups in the polymer structures that lead to expansion of the macromolecular chains in their solution state. Fur- thermore, this block of copolyimides showed somewhat further solubility toward the above solvents compared with that of the other copolyimides with analogous struc- ture [22,23,28,29]. The better solubility of poly(imide-urea)s, should be attributed to the interchain steric repulsion caused by the bulky benzoxazole, benzothiazole or ben- zimidazole side groups. The chain separation effect ac- counts for a weakening of the strong interactions through hydrogen bonding and the steric factor must be dominant because the other effects, such as dipole attraction or chain rigidity enhancement, should work against good solubility. The thermal properties of the resulting poly(im- ide-urea)s were determined by means of differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The thermal behavior data of the poly- mers are summarized in Table 3. The DSC profiles were achieved at a heating rate of 20˚C/min in a nitrogen at- mosphere in the temperature range from 100˚C to 300˚C. The DSC thermograms of the poly(imide-urea)s showed glass-transition temperatures (Tg’s) in the range between 192˚C and 236˚C. The nature of the diamine moiety played a significant role on the Tg values. These went down in the series as follows b > c > a (Table 3). This could be attributed to the incorporation of rigid ben- zoxazole or benzothiazole ring in diamine segments, which restricted the free rotation of the macromolecular chains leading to an enhanced Tg value. Upon comparing the chemical structures of the pendent groups, it is seen that benzoxazole was somewhat more Tg than benzothi- azole [20,22]. Figure 3 shows DSC curves of the poly- mers PIUOb, PIUSb, and PIUNb. In order to compare the thermal properties, some ho- mo and copolymers, including polyimide, polyurea, poly (imide-amide), poly(imide-ether), and poly(imide-amide- ether) with the structures shown in Scheme 2 were con- sidered. Table 3. Thermal properties of the poly(imide-urea)s. Polymer code Tg a (°C ) T10 b (°C ) Char yield c (wt%) PIUOa 209 408 38 PIUOb 236 441 47 PIUOc 216 412 44 PIUSa 194 390 30 PIUSb 223 417 41 PIUSc 219 394 38 PIUNa 192 397 36 PIUNb 201 437 42 PIUNc 197 415 39 a Form the second heating traces of DSC measurements with a heating rate of 20°C.min-1 in nitrogen.b Temperature at which 10% weight loss was recorded by TGA at a heating rate of 20°C.min-1 in nitrogen.c Char yield at 800°C. Figure 3. DSC thermograms of PIUOb, PIUSb, and PIUNb. The thermal properties of these reference polymers are also listed in Table 4. All the poly(imide-urea)s exhib- ited lower Tg’s than the fully aromatic homopolyimide R1, the poly(imide-amide) R4, the poly(imide-ether) R5, and the poly(imide-amide-ether) R6 due to higher flexi- bility of the urea linkages than those of imide, amide and ether bonds. Thermal stability of the polymers was evaluated by TGA in nitrogen at a heating rate of 20˚C/min. The poly(imide-urea)s obtained were stable up to 400˚C and lost 10% of their total weight between 390˚C and 441˚C, which showed a remarkably improvement of decomposi- tion temperature in comparison with common ho- mopolyurea R3. There are similarities in the thermal sta- bilities of all PIUs. In fact, all of them showed two step thermal decomposition pattern in their thermograms. The temperature maximum decomposition of the first stage is Copyright © 2011 SciRes. OJPChem  7 H. TOISERKANI Scheme 2. The chemical structure of some reference polymers. Table 4. Thermal properties of some reference polymers. Polymer code Polymer class Tg (°C ) T10 (°C ) Char yield (wt%)a Ref. R1 Polyimide 412 551c 57 20, 26 R2 Polyimide —b 616c 62 20 R3 Polyurea —b 300 —b 27 R4 Poly(imide-amide) —d 561 60 22 R5 Poly(imide-ether) 279 543 59 28 R6 Poly(imide-amide-ether) 263 513 52 29 a Residual wt% at 800°C in nitrogen. b Was not reported. c Maximum polymer decomposition temperature. d No discernible transition was observed on the DSC traces. at around 360˚C - 385˚C, which corresponds to the de- composition of the urea groups. The second-stage de- decomposition of the imide linkages. Therefore, the 10% weight loss temperatures are mainly caused by the de- composition, started around 540˚C - 570˚C, is due to the composition of ureylene linkages. As shown in Table 4, Copyright © 2011 SciRes. OJPChem  H. TOISERKANI 8 lation reactions of bis(imide-carboxylic cid)s, namely, 1, 1, and 1with a number of aromatic D. Stenzenberger and P. M. Hergenrother, Polyimides. New York: Chapman & Hall, 1990, pp. the poly(imide-urea)s exhibited less thermal stability than those of the homopolyimide R1 and R2, the poly(imide-amide) R4, the poly(imide-ether) R5, and the poly(imide-amide-ether) R6. However, they showed higher T10% values than the homopolyurea R3. These obs- ervations might be attributed to the early degradation of the urea linkages than those of the imide, amide and even ether groups against high temperatures. Furthermore, when the heat-resistant benzoxazole or benzothiazole ring incorporated as pendent group to the diamines c and b, an obvious increase was observed for decomposition profiles aromatic PIU than unsubstituted diamine a in each series. Upon comparing the chemical structures of the pendent groups, it is seen that benzoxazole was some- what more thermally stable than benzothiazole (Table 3 ). 4. Conclusions The one-pot polyurey aO SN diamines (a-c) resulted in preparation of aromatic poly(imide-urea)s (PIUOa-c, PIUSa-c, and PIUNa-c). The main objectives of this study were to improve the solu- bility of homopolyimides as well as the thermal stability of homopolyurea by the introduction of both imide and urea linkages into the macromolecular chains. Most of the poly(imide-urea)s presented an excellent solubility in polar aprotic solvents such as, NMP, DMF, DMAc, and DMSO, while they were partially soluble in m-cresol and pyridine and also they were not soluble in less polar sol- vents such as chloroform and tetrahydrofuran at room temperature. The resulting poly(imide-urea)s showed a better solubility in common organic solvents than the polyimides, poly(imide-amide)s, and even poly(ether-imide)s with the same aromatic structure. Furthermore, the amorphous polymers obtained exhibited a desirable heat resistance in comparison with aromatic polyureas due to the presence of thermally stable imide groups as well as heat-resistant benzoxazole and benzothiazole rings into the fully aromatic structures. 5. References [1] D. Wilson, H. 1-352. [2] M. K. Ghosh and K. L. Mittal, “Polyimides: Fundamen- tals and applications,” New York: Marcel Dekker, 1996, pp. 1-6. [3] P. M. Hergenrother and S. J. Havens, “Polyimides Con- taining Quinoxaline and Benzimidazole Units,” Macro- molecules, Vol. 27, No. 17, 1994, pp. 4659-4664. doi:10.1021/ma00095a004 [4] E. M. Maya, A.E. Lozano, J. G. Campa and J. Abajo, “Soluble Polyamides and Polyimides Functionaliz Benzo-15-Crown-5-Pendant ed with Groups,” Macromolecular Rapid Communications, Vol. 25, No. 4, 2004, pp. 592-597. doi:10.1002/marc.200300092 O. Ozarslan, M. K. Bayazit and E. Catiker, “Preparation and Properties of Flame Retardant Poly(urethane-imide)s Containing Phosphine Oxide Moiety,” Journal o [5] f Applied Polymer Science, Vol. 114, No. 2, 2009, pp. 1329-1338. doi:10.1002/app.30601 [6] S. Shadpour Mallakpour and M. H. Shahmohammadi, “Synthesis and Characterization of Novel Optically Ac- tive Poly(imide-urethane)s Derived from N,N'-(pyromel- litoyl)-bis-(L-leucine) Diisocyanate and Aromatic Diols,” Polymer International, Vol. 53, No. 2, 2004, pp. 184-190. [7] H. Behniafar, B. Akhlaghinia and S. Habibian, “Synthesis and Characterization of New Soluble and Thermally Sta- ble Poly(ester-imide)s Derived from N-[3,5-bis(N-trimel- litoyl)phenyl]phthalimide and Various Bisphenols,” European Polymer Journal, Vol. 41, No. 5, 2005, pp. 1071-1078. doi:10.1016/j.eurpolymj.2004.12.001 [8] N. H. You, Y. Suzuki, D. Yorifuji, S. Ando and M. Ueda, “Synthesis of High Refractive Index Polyimides Derived from 1,6-Bis(p-aminophenylsulfanyl)-3,4,8,9-tetrahydro- 2,5,7,10-tetrathiaanthracene and Aromatic Dianhydrides,” Macromolecules, Vol. 41, No. 17, 2008, pp. 6361-6366. doi:10.1021/ma800982x [9] H. Choi, I. S. Chung, K. Hong, C. E. Park and S. Y. Kim, “Soluble Polyimides from Unsymmetrical Diamine Con taining Benzimidazole Ri - ng and Trifluoromethyl Pendent Group,” Polymer, Vol. 49, No. 11, 2008, pp. 2644-2649. doi:10.1016/j.polymer.2008.04.019 [10] C. Hamciuc, E. Hamciuc and M. Bruma, “New Fluori- nated Poly(1,3,4-oxadiazole-ether-imide)s,” Polymer, Vol. 46, No. 16, 2005, pp. 5851-5859. doi:10.1016/j.polymer.2005.05.074 [11] A. Ghosh and S. Banerjee, “Synthesis, Characterization, and Comparison of Properties Poly(imide siloxane) Copolymers,” of Novel Fluorinated Journal of Applied Polymer Science, Vol. 107, No. 3, 2008, pp. 1831-1841. doi:10.1002/app.27241 [12] C. H. Lin and C. H. Lin, “Synthesis and Properties of Polyimides Derived from 1,4-Bis(4-aminophenoxy)-2- (6-oxido-6H-dibenz[c,e] [1,2]oxaphosphorin-6-yl) pheny- lene,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 45, No. 14, 2007, pp. 2897-2912. doi:10.1002/pola.22046 [13] G. S. Liou, H. W. Chen and H. J. Yen, “Poly(amine- amide-imide)s Bearing Pendent N-Carbazolylph Moieties: Synthesis and enyl Electrochromic Properties,” Ma- cromolecular Chemistry and Physics, Vol. 207, No. 17, 2006, pp. 1589-1598. doi:10.1002/macp.200600130 [14] H. Behniafar and S. Haghighat, “Thermally Stable and Organosoluble Binaphthylene-Based Poly(urea-ether-im- ide)s: One-Pot Preparation and Characterization,” Poly- mers for Advanced Technologies, Vol. 19, No. 8, 2008, pp. 1040-1047. doi:10.1002/pat.1072 [15] Y. C. Kung, G. S. Liou and S. H. Hsiao, “Synthesis and Characterization of Novel Electroactive Polyamides and Polyimides with Bulky 4-(1-Adamantoxy)triphenylamine Moieties,” Journal of Polymer Science Part A: Polymer Copyright © 2011 SciRes. OJPChem  H. TOISERKANI Copyright © 2011 SciRes. OJPChem 9 Chemistry, Vol. 47, No. 7, 2009, pp. 1740-1755. doi:10.1002/pola.23264 [16] W. Li, S. Li, Q. Zhang and S. Zhang, “Synthesis of Bis (amine anhydride)s for Novel High Tgs and Organosolu- ble Poly(amine imide)s by Palladium-Catalyzed tion of 4-Chlorophthalid Am e Anhydride,” Macromolecules, ina- Vol. 40, No. 23, 2007, pp. 8205-8211. doi:10.1021/ma071213c [17] M. Ghaemy and R. Alizadeh, “Synthesis of Soluble and Thermally Stable Polyimides from Unsymmetrical Dia- mine Containing 2,4,5-triaryl Imidazol European Polymer Journ e Pendent Group,” al, Vol. 45, No. 6, 2009, pp. 1681-1688. doi:10.1016/j.eurpolymj.2009.03.006 [18] Y. Shao, Y. Li, X. Zhao, T. Ma, C. Gong and F. Yang, “Synthesis and Characterization of Soluble Polyimides Derived from a Novel Unsymmetrical Diamine Monomer: 1,4-(2',4''-diaminodiphenoxy) Benzene,” European Poly- mer Journal, Vol. 43, No. 10, 2007, pp. 4389-4397. doi:10.1016/j.eurpolymj.2007.07.002 [19] Y. T. Chern, J. Y. Tsai and J. J. Wang, “High T g and High Organosolubility of Novel Unsymmetric Polyim- ides,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 47, No. 10, 2009, pp. 2443-2452. doi:10.1002/pola.23337 [20] J. A. Mikroyannidis, “Aromatic Polyamides and Polyim- ides with Benzoxazole or Benzothiazole Pendent Groups Prepared from 5-(2-Benzoxazole) or 5-(2-Benzothia- zole)-1,3-phenylenediamine,” Macromolecules, Vol. 28, No. 15, 1995, pp. 5177-5183. doi:10.1021/ma00119a003 [21] J. A. Mikroyannidis, “Aromatic Polyamides and Polyim- ides with Benzimidazole or Benzoxazinone Pendent Groups Prepared from 5-(2-benzimidazole)-or 5-(2-ben- zoxazinone)-l,3-phenylenediamine,” Polymer, Vol. 37, No. 13, 1996, pp. 2715-2721. doi:10.1016/0032-3861(96)87633-6 [22] H. Toiserkani, H. Sheibani and K. Saidi, “New Organo- soluble and Thermally Stable Poly(amide-imide)s with Benzoxazole or Benzothiazole Pendent Groups: Synthesis and Characterization,” Polymers for Advanced Technolo- gies, Vol. 22, No. 10, 2011, pp. 1494-1501. doi:10.1002/pat.1633 [23] H. Toiserkani, K. Saidi and H. Sheibani, “S Characterization of New ynthesis and Soluble and Thermally Stable Poly(Amide-Imide)s Containing Pendent Benzimidazole Moieties,” Journal of Applied Polymer Science, Vol. 114, No. 1, 2009, pp. 185-192. doi:10.1002/app.30454 [24] H. S. Dong, W. Jia, J. D. Cui and Q. L. Wang, “The Syn- thesis of Some New N-[1-(2,5-Dichlorophenyl)-5-methyl ction with Diphenylphosphoryl -1,2,3-triazol-4-yl]-carbamic Acid Ester Derivatives,” Journal of the Chinese Chemical Society, Vol. 50, No. 6, 2003, pp. 1209-1213. [25] N. Nishi, M. Tsunemi, K. Nakamura and H. Tokura, “Polymerization Rea Azide, Preparation of Polyamides, Polyureas and Poly- urethanes,” Die Makromolekulare Chemie, Vol. 192, No. 8, 1991, pp. 1811-1820. doi:10.1002/macp.1991.021920814 [26] J. A. Mikroyannidis, “Co Polyimides, Polyamides and Poly mparison of the Properties of the ureas Derived from 1-[(dialkoxyphosphinyl)-methyl]-2,4- and -2,6-diamino- benzenes and m-phenylenediamine,” European Polymer Journal, Vol. 22, No. 4, 1986, pp. 277-284. doi:10.1016/0014-3057(86)90192-8 [27] Y. Oishi, M. Padmanaban, M. A. Kakimoto “Synthesis and Properties of Aromati and Y. Imai, c Polyureas from N, N'-Bis ( trimethylsilyl )-Substituted Aromatic Diamines and Aromatic Diisocyanates,” Journal of Polymer Sci- ence Part A: Polymer Chemistry, Vol. 30, No. 7, 1992, pp. 1363-1368. doi:10.1002/pola.1992.080300714 [28] H. Toiserkani, “Synthesis and Evaluation of Properties of Novel Aromatic Poly(ether-imide) with Benzazole Pen- dent Groups and Flexible Ether Linkages,” High Per- formance Polymers, 2011 in press. doi:10.1177/0954008311421988 [29] H. Toiserkani, “Modified Poly (ethe Pendent Benzazole Structures: Sy r-imide-amide)s with nthesis and Characteri- zation,” Journal of Applied Polymer Science, 2011 in press. doi:10.1002/app.35634
|