125
C. U. ANIZ ET AL.
tion (TPD), ICP-AES, TG/DTG, etc. The results of the
BET surface area and ammonia-TPD data are given in
Tables 1 and 2 respectively. TPD studies were done us-
ing Pulse Chemisorb 2705 from Micromeritics. Samples
were degassed in helium in a quartz reactor, at 200˚C,
and then cooled to ambient temperature. 5% NH3 in he-
lium gas (from Bheruka gases, Bangalore) is admitted to
the sample for 15 min to saturate the sample. Physi-
sorbed ammonia was purged away, keeping the sample at
100˚C. Sample temperature was raised to 700˚C at a rate
of 10˚C/min measuring the outlet desorbed ammonia
using a calibrated TCD.
Nitrogen adsorption-desorption measurements were
done on a volumetric Micromeritics Tristar apparatus at
Liquid N2 temperature, 77.35 K. On an average 33
points were taken for each sample. The average mass of
the sample was 0.3 g. Pore size distributions were calcu-
lated using BJH method and surface areas were taken
from BET isotherms.
The temperature-programmed reduction studies (TPR)
were performed in Micromeritics PulseChemisorb-2705,
which incorporates a thermal conductivity detector
(TCD). Samples were activated/surface-cleaned in he-
lium at 200˚C for 30 minutes, then cooled to ambient
temperatures. The reactive gas composition was 10%
balance N2, its consumption was measured while heating
the sample up to 750˚C at a rate of 10˚C/min.
2.3. Kinetic and Catalytic Studies
Kinetic and catalytic reaction studies of carbon monox-
ide oxidation have been done with all the catalyst sam-
ples, granulated to 16 × 20 mesh sizes after shaping to
cylindrical tablets. 0.5 g of the activated catalyst samples
were supported between glass wool plugs and flanked by
inert porcelain beads in the middle of a specially de-
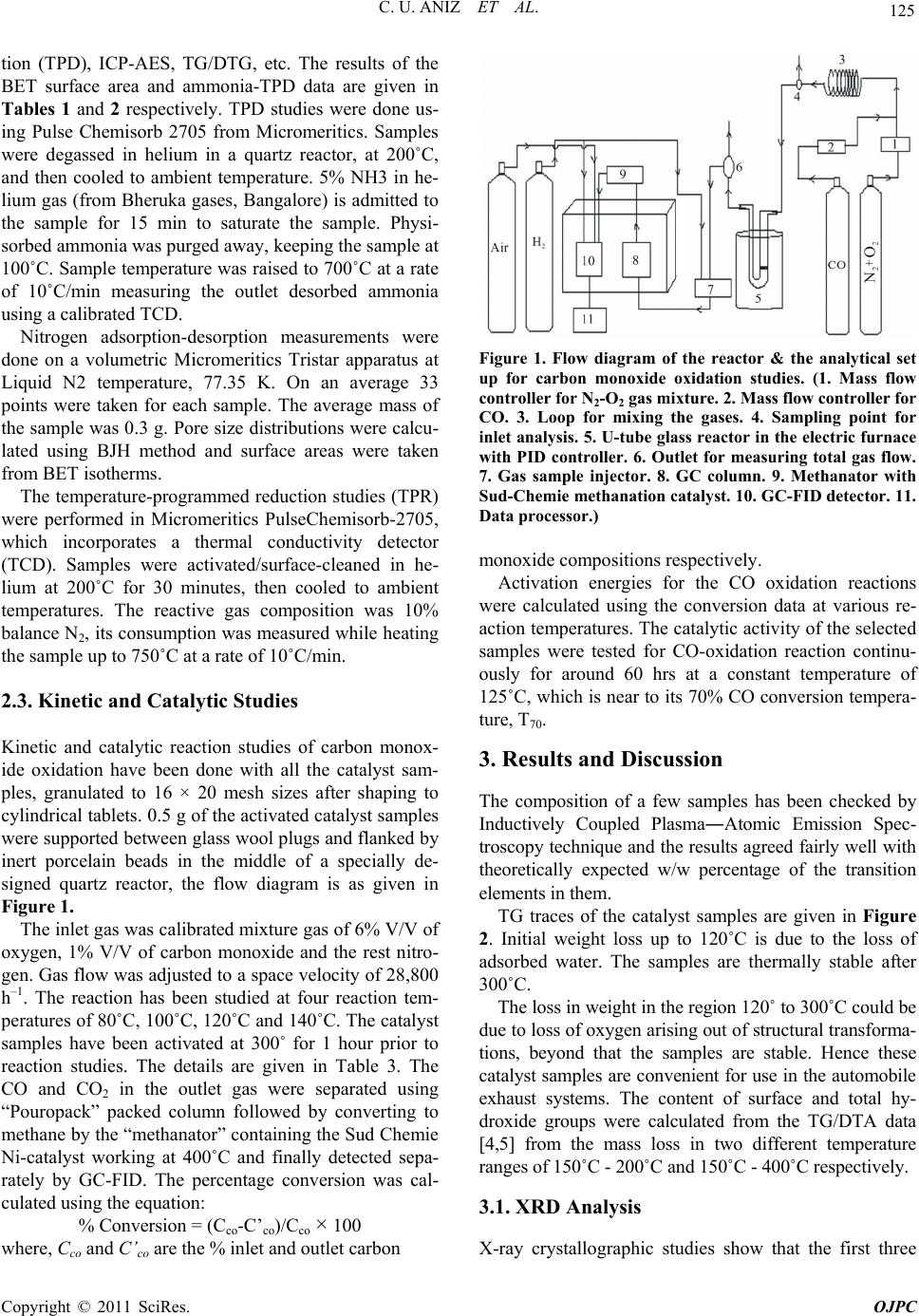
signed quartz reactor, the flow diagram is as given in
Figure 1.
The inlet gas was calibrated mixture gas of 6% V/V of
oxygen, 1% V/V of carbon monoxide and the rest nitro-
gen. Gas flow was adjusted to a space velocity of 28,800
h–1. The reaction has been studied at four reaction tem-
peratures of 80˚C, 100˚C, 120˚C and 140˚C. The catalyst
samples have been activated at 300˚ for 1 hour prior to
reaction studies. The details are given in Table 3. The
CO and CO2 in the outlet gas were separated using
“Pouropack” packed column followed by converting to
methane by the “methanator” containing the Sud Chemie
Ni-catalyst working at 400˚C and finally detected sepa-
rately by GC-FID. The percentage conversion was cal-
culated using the equation:
% Conversion = (Cco-C’co)/Cco × 100
where, Cco and C’co are the % inlet and outlet carbon
Figure 1. Flow diagram of the reactor & the analytical set
up for carbon monoxide oxidation studies. (1. Mass flow
controller for N2-O2 gas mixture. 2. Mass flow controller for
CO. 3. Loop for mixing the gases. 4. Sampling point for
inlet analysis. 5. U-tube glass reactor in the electric furnace
with PID controller. 6. Outlet for measuring total gas flow.
7. Gas sample injector. 8. GC column. 9. Methanator with
Sud-Chemie methanation catalyst. 10. GC-FID detector. 11.
Data processor.)
monoxide compositions respectively.
Activation energies for the CO oxidation reactions
were calculated using the conversion data at various re-
action temperatures. The catalytic activity of the selected
samples were tested for CO-oxidation reaction continu-
ously for around 60 hrs at a constant temperature of
125˚C, which is near to its 70% CO conversion tempera-
ture, T70.
3. Results and Discussion
The composition of a few samples has been checked by
Inductively Coupled Plasma―Atomic Emission Spec-
troscopy technique and the results agreed fairly well with
theoretically expected w/w percentage of the transition
elements in them.
TG traces of the catalyst samples are given in Figure
2. Initial weight loss up to 120˚C is due to the loss of
adsorbed water. The samples are thermally stable after
300˚C.
The loss in weight in the region 120˚ to 300˚C could be
due to loss of oxygen arising out of structural transforma-
tions, beyond that the samples are stable. Hence these
catalyst samples are convenient for use in the automobile
exhaust systems. The content of surface and total hy-
droxide groups were calculated from the TG/DTA data
[4,5] from the mass loss in two different temperature
ranges of 150˚C - 200˚C and 150˚C - 400˚C respectively.
3.1. XRD Analysis
X-ray crystallographic studies show that the first three
Copyright © 2011 SciRes. OJPC