 Open Journal of Physical Chemistry, 2011, 1, 118-123 doi:10.4236/ojpc.2011.13016 Published Online November 2011 (http://www.SciRP.org/journal/ojpc) Copyright © 2011 SciRes. OJPC Buffer Standards for Physiological pH of the Buffer N-(2-Acetamido)-2-aminoethanesulfonic Acid from 5˚C to 55˚C Lakshmi N. Roy, Rabindra N. Roy*, Zachary M. Downs, Blake M. Bodendorfer, Jaime A. Veliz, Jessica M. Stegner, Isaac B. Henson, Joshua T. Wollen Hoffman Department of Chemistry, Drury University, Springfield, USA E-mail: rroy@drury.edu Received August 23, 2011; revised September 27, 2011; accepted November 1, 2011 Abstract Electromotive force (emf) measurements of the Cell Pt(s), H2(g)|ACES(m1) + NaACES(m2) + NaCl (m3)| AgCl, Ag(s) have been carried out from 5˚C to 55˚C. The agreement of pH values between two calculated (extended Debye-Hückel and liquid junction correction) is very good. Two buffer solutions without the chlo- ride ion and seven buffer solutions with NaCl, at an ionic strength (I = 0.16 mol·kg–1) similar to that of physiological fluids, have been studied. The pH values for these buffer solutions have been evaluated in the temperature range of 5˚C to 55˚C using the extended Debye-Hückel equation of the Bates-Guggenheim con- vention. Values of the residual liquid junction potential (δEj) between the ACES solutions and the saturated KCl calomel electrode solution have been estimated at 25˚C and 37˚C from the previously determined Ej val- ues using the flowing junction cell to determine the operational pH values at 25˚C and 37˚C. These ACES buffer solutions are recommended as secondary standard reference solutions for pH measurements in the range of physiological application at I = 0.16 mol·kg–1. Keywords: Buffer, Emf, pH, Zwitterionic 1. Introduction The zwitterionic amino acid buffer solutions recom- mended by Good et al. [1,2] have been studied in the authors’ laboratory for the purpose of pH measurement control in the physiological range of pH. Concerning the current investigation, the authors’ goal is to provide pH values for the ACES, which is depicted by the following structure: N O H2N S O OOH H N-(2-acetamido)-2-aminoethanesulfonic acid ACES This phosphate buffer [3,4] is comprised of KH2PO4 (0.008695 mol·kg–1) and Na2HPO4 (0.03043 mol·kg–1). This buffer solution is widely used as the primary refer- ence standard for routine laboratory measurements. Some of the limitations have been previously reported [5-8]. The zwitterionic buffer compound ACES may form complexes with cations such as Mg+2 and Ca+2. At a high NaCl concentration, the complex formation is mini- mized. Wu et al. [7] have studied MOPSO using two point pH calibration measurements. Roy et al. [9] studied pK2 and pH values of 3-(N-morpholino)propanesulfonic acid (MOPS) from 5˚C to 55˚C. The following two solutions of ACES were studied without the presence of the chloride ion: (a) ACES (0.02 mol·k g–1) + NaACES (0.06 mol·kg–1); (b) ACES (0.04 mol·k g–1) + NaACES (0.08 mol·kg–1). The following seven solutions were also studied but contain the chloride ion yielding an ionic strength close to that of blood plasma (I = 0.16 mol·kg–1): (c) ACES (0.08 mol·kg–1) + NaACES (0.08 mol·kg–1) + NaCl (0.08 mol·k g–1); (d) ACES (0.06 mol·kg–1) + NaACES (0.06 mol·k g–1) + NaCl (0.10 mol·kg–1); (e) ACES (0.04 mol·k g–1) + NaACES (0.04 mol·kg–1) + NaCl (0.12 mol·k g–1); (f) ACES (0.03 mol·kg–1) + NaACES (0.03 mol·k g–1) + NaCl (0.13 mol·kg–1); (g) ACES (0.03  119 L. N. ROY ET AL. mol·k g–1) + NaACES (0.09 mol·kg–1) + NaCl (0.07 mol·k g–1); (h) ACES (0.02 mol·kg–1) + NaACES (0.08 mol·k g–1) + NaCl (0.08 mol·kg–1); (i) ACES (0.02 mol·k g–1) + NaACES (0.10 mol·kg–1) + NaCl (0.06 mol·k g–1). 2. Experimental Section The ACES was obtained from Research Organics (Cle- veland, OH) and was crystallized further. The method and the assay have been reported elsewhere [8,9]. The analyses averaged 99.97% with a standard deviation of 0.05%. Buffer were prepared from the weighed amount of the ACES solid, ACS reagent grade NaCl, a standard NaOH solution (for preparation of NaACES), and CO2- free doubly distilled water. Buoyancy corrections were made for all masses. The cell design, method of preparation of hydrogen electrodes, hydrogen gas purification, silver-silver chlo- ride electrodes, solution preparation, temperature meas- urements, and emf techniques have been described [8,10, 11]. 3. Methods and Results The emf values required for the pH(s) calculations are given in Table 1 for cell A. Cell A contains two solutions without the chloride ion and seven solutions with Cl–. The cell voltage values have been corrected to a hydro- gen pressure of 1 atm. At 25˚C, the emf values are aver- aged from readings taken twice at the beginning and the middle. The uncertainty lies within 0.02 mV on the av- erage in. pH of the ACES Buffer. The Bates et al. [7,8,11-13] method has been used to evaluate the conventional stan- dard pH values for solutions (a) to (i). For accurate cal- culations of pH for nine buffer solutions, the following cell A is used: Pt(s), H2(g), 101.325 kPa|ACES (m1) + NaACES (m2) + NaCl(m3)|AgCl(s), Ag(s) (A) where m1, m2 and m3 denote the molalities of the respec- tive species at 1 atm = 101.325 kPa. Cell A is known as the Harned-type cell. Cell B, the flowing junction cell, was used for the evaluation of the liquid junction potential: Pt(s), H2(g), 101.325 kPa|ACES(m1) + NaACES(m2) + NaCl(m3)||KCl(satd), Hg2Cl2(s), Hg(l) (B) where the abbreviations “s” “l” and “g” imply the solid, liquid, and gaseous states, respectively. For cell C, a primary standard phosphate buffer solu- tion, NBS/NIST certified, was used. The diagram of cell C is shown below: Pt(s), H2(g), 101.325 kPa|KH2PO4 + Na2HPO4||KCl(satd), Hg2Cl2(s), Hg(l) (C) The SCE values of the saturated calomel electrode were taken as –0.2415 and –0.2335 at 25˚C and 37˚C, respectively [14]. The residual liquid junction potential δEj values of the buffer solution of ACES were calcu- lated from cells B and C using the following equation: E δEj = E + – k˚pH (1) SCE E where k = 0.059156 and pH = 7.415 at 25˚C and k = 0.061538 and pH = 7.395 at 37˚C. The pH values are that of the standard phosphate buffer solution and buffer so- lution of ACES obtained using the extended De- bye-Hückel equation. The operational definition of pH, denoted as pH(x), can be calculated by use of the subse- quent equation pH(x) = pH(s) + (Ex – Es + δEj)/k (2) where “x” refers to the unknown buffer, “s” is the NBS/NIST standard phosphate buffer solution of known pH, and Ej = Ej(s) – Ej(x). To calculate the pH(s) values for the buffer solutions under investigation, calculations were made to determine acidity function, p(aH Cl), in the temperature range of 25˚C and 37˚C. These calculations were made using emf values listed in Table 1, the molality of the chloride ion, and the standard electrode potential of the silver-silver chloride electrode (E˚). The values of p(aH Cl) were ob- tained from the equation below: () log Cl Cl EE pa m k (3) where “k” is the Nernst slope. The values of p(aH Cl) were plotted against the mo- lality of the chloride ion. A linear line was obtained. The intercept is the p(aH Cl)˚ value at mCl¯ = 0. The p(aH Cl) values for the seven buffer solutions containing Cl¯ are entered in Tables 2 and 3 from 5˚C to 55˚C. Conventional pH(s) values for solutions without liquid junction and absence a chloride ion were determined using the equation: o ()() log Cl Cl pHsp a (4) where the single-ion activity coefficient, o Cl , can be estimated based on corrections. A previous publication outlines the method used for obtaining this figure [9]. The pH values obtained from the liquid junction cell are indicated by pH whereas the “conventional” pH calcu- lated from Equations (5) and (6) are designated as pH(s). The Bates-Guggenheim convention [15,16], is expressed y the equation: b Copyright © 2011 SciRes. OJPC  L. N. ROY ET AL. Copyright © 2011 SciRes. OJPC 120 Table 1. Cell Potential of Cell A (in Volts): Pt(s); H2(g), 101.35 kPa|ACES(m1), NaACES(m2), NaCl(m3)|AgCl(s), Ag (s). m1 m2 m3 T/˚C mol· kg–1 5 10 15 20 25 30 35 37 40 45 50 55 0.02 0.06 0.005 0.02 0.06 0.010 0.02 0.06 0.015 0.02 0.06 0.020 0.04 0.08 0.005 0.04 0.08 0.010 0.04 0.08 0.015 0.04 0.08 0.020 0.08 0.08 0.080 0.06 0.06 0.100 0.04 0.04 0.120 0.03 0.03 0.130 0.03 0.09 0.070 0.02 0.08 0.080 0.02 0.10 0.060 0.79035 0.7736 0.76380 0.75659 0.77395 0.75930 0.75154 0.74645 0.69577 0.69083 0.68684 0.68510 0.72884 0.73082 0.75064 0.79189 0.77487 0.76467 0.75741 0.77491 0.75990 0.75176 0.74651 0.69532 0.69030 0.69629 0.68453 0.72906 0.73107 0.75128 0.79332 0.77591 0.76539 0.75803 0.77581 0.76029 0.75188 0.74629 0.69475 0.68972 0.68565 0.68391 0.72911 0.73119 0.75170 0.79440 0.77671 0.76607 0.75848 0.77641 0.76038 0.75143 0.74557 0.69403 0.68869 0.68498 0.68322 0.72905 0.73119 0.75202 0.79541 0.77739 0.76655 0.75880 0.77666 0.76004 0.75064 0.74427 0.69331 0.68805 0.68379 0.68195 0.72980 0.73109 0.75210 0.79654 0.77819 0.76717 0.75921 0.77766 0.76140 0.75253 0.74672 0.69244 0.68710 0.68281 0.68093 0.72878 0.73086 0.75213 0.79731 0.77868 0.76745 0.75943 0.77828 0.76166 0.75276 0.74680 0.69146 0.68600 0.68160 0.67968 0.72846 0.73068 0.75174 0.79779 0.77898 0.76763 0.75949 0.77844 0.76190 0.75301 0.74714 0.69104 0.68558 0.68126 0.67935 0.72827 0.73047 0.75196 0.79807 0.77914 0.76773 0.75957 0.77855 0.76266 0.75411 0.74884 0.69035 0.68487 0.68052 0.67860 0.72792 0.73020 0.75184 0.79866 0.77941 0.76794 0.75956 0.77884 0.76262 0.75418 0.74891 0.68924 0.68373 0.67922 0.67726 0.72743 0.72970 0.75169 0.79921 0.77968 0.76790 0.75948 0.77917 0.76292 0.75449 0.74955 0.68815 0.68257 0.67805 0.67601 0.72674 0.72912 0.75128 0.79961 0.77980 0.76781 0.75941 0.77911 0.76266 0.75428 0.74911 0.68797 0.68193 0.67702 0.67486 0.72590 0.72814 0.75082 am = 1 mol·kg–1. Table 2. p(aHγCl)˚ of ACES + NaACES buffer solutions from 5˚C to 55˚C obtained by extrapolation for chloride-free solutions, p(aHγCl) of ACES + NaACES buffer solutions from 5˚C to 55˚C, computed using Equations (4)-(6)a. T (˚C) 0.02 m ACES + 0.06 m NaACES + 0.00 m NaCl I = 0.06 m 0.04 m ACES + 0.08 m NaACES + 0.00 m NaCl I = 0.08 m 0.08 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m 0.06 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m 0.04 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m 0.03 m ACES + 0.03 m NaACES + 0.13 m NaCl I = 0.16 m 5 10 15 20 25 30 35 37 40 45 50 55 7.781 7.679 7.582 7.484 7.391 7.306 7.218 7.189 7.136 7.056 6.980 6.904 7.446 7.341 7.241 7.145 7.050 6.956 6.871 6.836 6.780 6.694 6.145 6.537 7.267 7.159 7.056 6.956 6.863 6.772 6.684 6.650 6.600 6.520 6.446 6.388 7.275 7.167 7.065 6.966 6.871 6.780 6.692 6.659 6.608 6.530 6.455 6.392 7.281 7.175 7.073 6.977 6.878 6.788 6.699 6.668 6.617 6.538 6.464 6.396 7.285 7.178 7.077 6.981 6.882 6.791 6.702 6.671 6.621 6.541 6.467 6.397 Table 3. p(aHγCl) of ACES + NaACES buffer solutions from 5˚C to 55˚C, computed using Equations (4)-(6)a. T (˚C) 0.03 m ACES + 0.09 m NaACES + 0.07 m NaCl I = 0.16 m 0.02 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m 0.02 m ACES + 0.10 m NaACES + 0.06 m NaCl I = 0.16 m 5 10 15 20 25 30 35 37 40 45 50 55 7.809 7.702 7.599 7.500 7.406 7.318 7.231 7.197 7.146 7.067 6.989 6.912 7.902 7.796 7.693 7.595 7.501 7.411 7.325 7.291 7.241 7.161 7.084 7.005 8.136 8.030 7.926 7.828 7.731 7.639 7.544 7.515 7.434 7.384 7.305 7.228 am = 1 mol·kg–1. 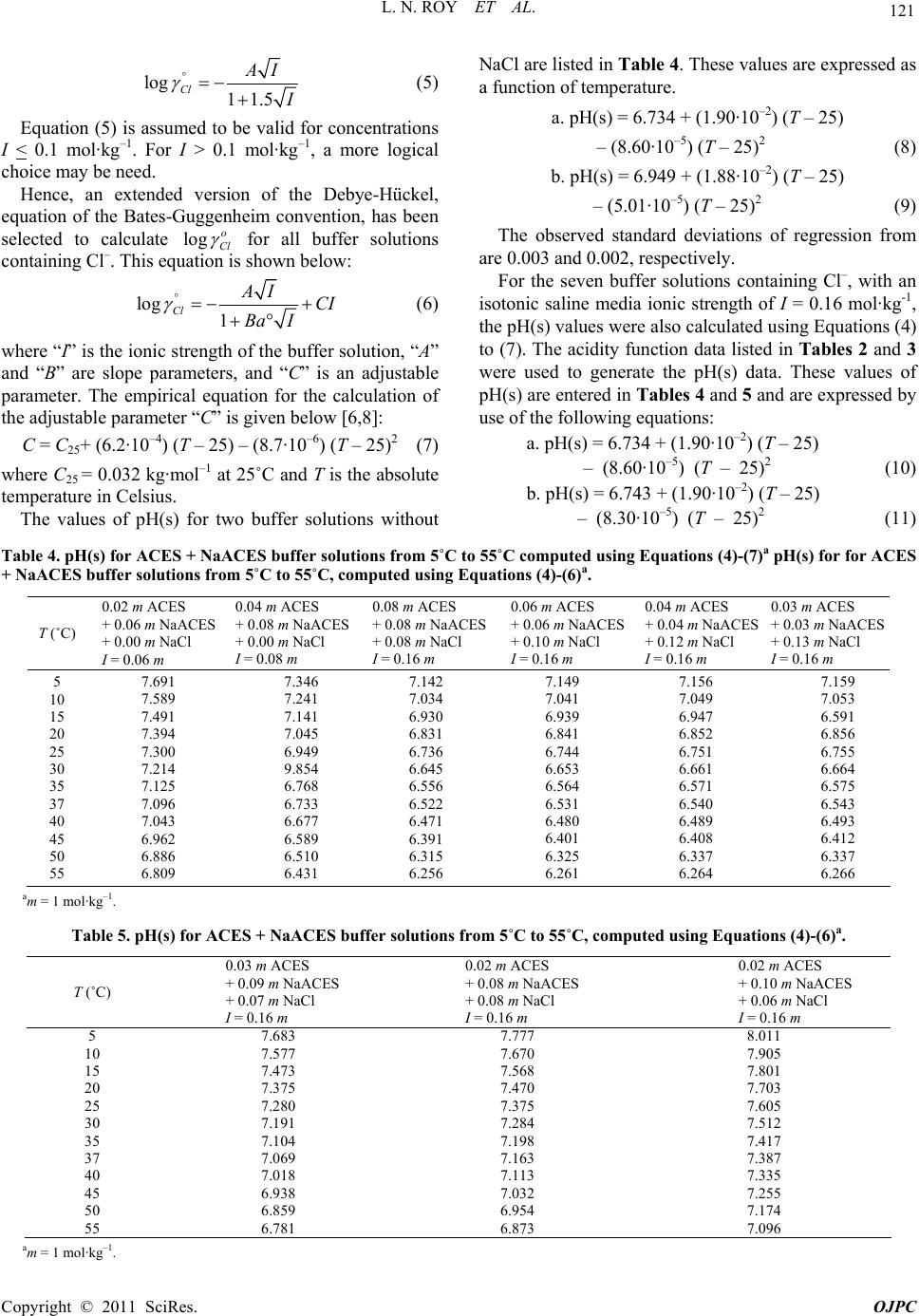 121 L. N. ROY ET AL. log 11.5 Cl I (5) Equation (5) is assumed to be valid for concentrations I < 0.1 mol·kg–1. For I > 0.1 mol·kg–1, a more logical choice may be need. Hence, an extended version of the Debye-Hückel, equation of the Bates-Guggenheim convention, has been selected to calculate log o Cl for all buffer solutions containing Cl–. This equation is shown below: log 1 Cl AI CI Ba I (6) where “I” is the ionic strength of the buffer solution, “A” and “B” are slope parameters, and “C” is an adjustable parameter. The empirical equation for the calculation of the adjustable parameter “C” is given below [6,8]: C = C25+ (6.2·10–4) (T – 25) – (8.7·10–6) (T – 25)2 (7) where C25 = 0.032 kg·mol–1 at 25˚C and T is the absolute temperature in Celsius. The values of pH(s) for two buffer solutions without NaCl are listed in Table 4. These values are expressed as a function of temperature. a. pH(s) = 6.734 + (1.90·10–2) (T – 25) – (8.60·10–5) (T – 25)2 (8) b. pH(s) = 6.949 + (1.88·10–2) (T – 25) – (5.01·10–5) (T – 25)2 (9) The observed standard deviations of regression from are 0.003 and 0.002, respectively. For the seven buffer solutions containing Cl–, with an isotonic saline media ionic strength of I = 0.16 mol·kg-1, the pH(s) values were also calculated using Equations (4) to (7). The acidity function data listed in Tabl es 2 and 3 were used to generate the pH(s) data. These values of pH(s) are entered in Tab l es 4 and 5 and are expressed by use of the following equations: a. pH(s) = 6.734 + (1.90·10–2) (T – 25) – (8.60·10–5) (T – 25)2 (10) b. pH(s) = 6.743 + (1.90·10–2) (T – 25) – (8.30·10–5) (T – 25)2 (11) Table 4. pH(s) for ACES + NaACES buffer solutions from 5˚C to 55˚C computed using Equations (4)-(7)a pH(s) for for ACES + NaACES buffer solutions from 5˚C to 55˚C, computed using Equations (4)-(6)a. T (˚C) 0.02 m ACES + 0.06 m NaACES + 0.00 m NaCl I = 0.06 m 0.04 m ACES + 0.08 m NaACES + 0.00 m NaCl I = 0.08 m 0.08 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m 0.06 m ACES + 0.06 m NaACES + 0.10 m NaCl I = 0.16 m 0.04 m ACES + 0.04 m NaACES + 0.12 m NaCl I = 0.16 m 0.03 m ACES + 0.03 m NaACES + 0.13 m NaCl I = 0.16 m 5 10 15 20 25 30 35 37 40 45 50 55 7.691 7.589 7.491 7.394 7.300 7.214 7.125 7.096 7.043 6.962 6.886 6.809 7.346 7.241 7.141 7.045 6.949 9.854 6.768 6.733 6.677 6.589 6.510 6.431 7.142 7.034 6.930 6.831 6.736 6.645 6.556 6.522 6.471 6.391 6.315 6.256 7.149 7.041 6.939 6.841 6.744 6.653 6.564 6.531 6.480 6.401 6.325 6.261 7.156 7.049 6.947 6.852 6.751 6.661 6.571 6.540 6.489 6.408 6.337 6.264 7.159 7.053 6.591 6.856 6.755 6.664 6.575 6.543 6.493 6.412 6.337 6.266 am = 1 mol·kg–1. Table 5. pH(s) for ACES + NaACES buffer solutions from 5˚C to 55˚C, computed using Equations (4)-(6)a. T (˚C) 0.03 m ACES + 0.09 m NaACES + 0.07 m NaCl I = 0.16 m 0.02 m ACES + 0.08 m NaACES + 0.08 m NaCl I = 0.16 m 0.02 m ACES + 0.10 m NaACES + 0.06 m NaCl I = 0.16 m 5 10 15 20 25 30 35 37 40 45 50 55 7.683 7.577 7.473 7.375 7.280 7.191 7.104 7.069 7.018 6.938 6.859 6.781 7.777 7.670 7.568 7.470 7.375 7.284 7.198 7.163 7.113 7.032 6.954 6.873 8.011 7.905 7.801 7.703 7.605 7.512 7.417 7.387 7.335 7.255 7.174 7.096 am = 1 mol·kg–1. Copyright © 2011 SciRes. OJPC  L. N. ROY ET AL. 122 Table 6. Emf of Cell B and pH values with δEj correction at 25˚C and 37˚C for ACES buffer. E/V δEjb/mV Withoutc δEj corr Withd δEj corr Extendede D-H eqn. Withoutc δEj corr Withd δEj corr Extendede D-H eqn. m1 m2 m3 I 25˚C 37˚C 25˚C 37˚C25˚C 37˚C 0.02 0.06 0.00 0.06 0.67559 0.67263 0.3 0.4 7.294 7.299 7.300 7.089 7.095 7.096 0.02 0.08 0.08 0.16 0.67825 0.67498 2.1 2.2 7.338 7.374 7.375 7.127 7.162 7.163 0.03 0.09 0.07 0.16 0.67263 0.66917 2.1 2.2 7.244 7.279 7.280 7.033 7.068 7.069 0.02 0.01 0.06 0.16 0.69186 0.68874 2.1 2.2 7.569 7.604 7.605 7.351 7.386 7.387 Emf of Cell Ca 0.008695 m KH2PO4 + 0.03043 m Na2HPO4 0.68275 0.69147 2.6 2.9 aCorrected to a hydrogen pressure of 101.325 kPa for physiological phosphate buffer solutions (primary reference standard buffer) at 25˚C and 37˚C; bδEj = E + – k˚ pH from Equation (1); the pH of the primary standard phosphate buffer solution is 7.415 and 7.395 at 25˚C and 37˚C, respectively; = elec- trode potential of the saturated calomel electrode = –0.2415 and –0.2335 at 25˚C and 37˚C [14], respectively; units of m, mol·kg–1; cValues obtained from Equa- tion (2) where δEj = 0 and Table 6; dObtained from Equation (2) and cell potential data from Table 6; eObtained from extended Debye-Hückel (DH) equation of the Bates-Guggenheim convention. SCE E SCE E c. pH(s) = 6.752 + (1.90·10–2) (T – 25) – (7.80·10–5) (T – 25)2 (12) d. pH(s) = 6.756 + (1.90·10–2) (T – 25) – (7.66·10–5) (T – 25)2 (13) e. pH(s) = 7.282 + (1.86·10–2) (T – 25) – (6.68·10–5) (T – 25)2 (14) f. pH(s) = 7.376 + (1.86·10–2) (T – 25) – (6.57·10–5) (T – 25)2 (15) g. pH(s) = 7.604 + (1.90·10–2) (T – 25) – (6.90·10–5) (T – 25)2 (16) The observed standard deviations of regression from Equations (10)-(17) are 0.003, 0.002, 0.002, 0.002, 0.002, 0.002 and 0.002, respectively. 4. Discussion and Conclusions The operational pH values at 25˚C and 37˚C were evalu- ated from cells with a liquid junction (cells B and C) by means of the flowing junction cell [6,8]. The emf values of cells B and C at 25˚C and 37˚C are given in Table 6 for four buffer solutions of ACES. The values of δEj are also listed in Table 6. The estimated uncertainties are due to 1) the calculation of logγCl, 2) extrapolation of the acidity function, 3) emf measurements, and 4) the esti- mation of δEj. From Table 6, the pH values of four buffer solutions lie in the range of 7.1 to 7.6. Thus, these buffer solutions are recommended as useful standards for pH measurements in the clinical laboratory. The overall uncertainty of pH is within 0.01 in the experimental tem- perature range. Work is in progress for the calculation of pH of the buffer solution using the specific ionic interac- tion theory of Pitzer [17,18] for the calculation of logγCl. The main application of these pH data is to establish a unified pH scale applicable to a wide range of ionic str- engths for practical pH measurements. 5. Acknowledgements The authors are grateful for the funding from the Na- tional Institutes of Health (AREA), under the grant 2- R15 GM 066866-03 and the diversity supplemental grant 3-R15 GM 066866-03 S1. The authors would also like to thank Levi Lowther for his dedicated and hard work. The content of this paper is the sole responsibility of the au- thors and does not necessarily represent the official views of the National Institutes of Health or the National Institutes of General Medical Sciences. 6. References [1] N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa and R. M. M. Singh, “Hydrogen Ion Buffers for Biological Research,” Biochemistry, Vol. 5, No. 2, 1966, pp. 467-477. doi:10.1021/bi00866a011 [2] W. J. Ferguson, K. L. Braunschweiger, W. R. Braun- schweiger, J. R. Smith, J. J. McCormick, C. C. Wasmann, N. P. Jarvis, D. H. Bell and N. E. Good, “Hydrogen Ion Buffers for Biological Research,” Analytical Biochemis- try, Vol. 104, 1980, pp. 300-310. doi:10.1016/0003-2697(80)90079-2 [3] R. N. Roy, J. Bice, J. Greer, J. A. Carlsten, J. Smithson, W. S. Good, L. N. Roy and K. M. Kuhler, “Buffers for the Physiological pH Range: Acidic Dissociation Con- stants of Zwitterionic Compounds (ACES and CHES) in Water from 5˚C to 55˚C,” Journal of Chemical & Engi- neering Data, Vol. 42, No. 1, 1997, pp. 41-44. doi:10.1021/je960279s [4] V. E. Bower, M. Paabo and R. G. Bates, “A Standard for the Measurement of the pH of Blood and Other Physio- logical Media,” Journal of Research of the National Bu- reau of Standards, Vol. 65A, 1961, pp. 267-270. [5] R. A. Durst and B. R. Staples, “Tris/Tris HCl: Standard Buffer for Use in the Physiological pH Range,” Clinical Chemistry, Vol. 18, 1972, pp. 206-208. Copyright © 2011 SciRes. OJPC  123 L. N. ROY ET AL. [6] D. Feng, W. F. Koch and Y. C. Wu, “Second Dissocia- tion Constant and pH of N-(2-Hydroxyethyl) piperazine- N’-2-ethanesulfonic Acid from 0˚C to 50˚C,” Analytical Chemistry, Vol. 61, No. 13, 1989, pp. 1400-1405. doi:10.1021/ac00188a019 [7] Y. C. Wu, P. A. Berezansky, D. Feng and W. F. Koch, “Second Dissociation Constant of 3-(N-Morpholino)-2- hydroxypropanesulfonic Acid and pH of Its Buffer Solu- tions,” Analytical Chemistry, Vol. 65, No. 8, 1993, pp. 1084-1087. doi:10.1021/ac00056a023 [8] R. N. Roy, D. R. Mrad, P. A. Lord, J. A. Carlsten, W. S. Good, P. Allsup, L. N. Roy, K. M. Kuhler, W. F. Koch and Y. C. Wu, “Thermodynamics of the Second Disso- ciation Constant and Standards for pH of 3-(N-Morpho- lino)propanesulfonic Acid (MOPS) from 5˚C to 55˚C,” Journal of Solution Chemistry, Vol. 27, No. 1, 1998, pp. 73-87. doi:10.1023/A:1022692629289 [9] R. G. Bates, C. A. Vega and D. R. White Jr., “Standards for pH Measurements in Isotonic Saline Media of Ionic Strength I = 0.16,” Analytical Chemistry, Vol. 50, No. 9, 1978, pp. 1295-1300. doi:10.1021/ac50031a026 [10] R. G. Bates, “Determination of pH,” 2nd Edition, Wiley & Sons, New York, 1973. [11] R. N. Roy, L. N. Roy, C. E. Denton, S. R. LeNoue, S. Ashkenazi, M. S. Fuge, C. D. Dunseth, J. L. Durden, C. N. Roy, A. Bwashi, J. T. Wollen and S. J. DeArmon, “Buffer Standards for the Physiological pH of 3-[(1,1-Di methyl-2-hydroxymethyl)-amino]-2-hydroxypropanesulf onic Acid from 278.15 to 328.15 K,” Journal of Chemi- cal & Engineering Data, Vol. 54, 2009, pp. 428-435. [12] L. N. Roy, R. N. Roy, C. E. Denton, S. R. LeNoue, C. N. Roy, S. Ashkenazi, T. B. Williams, D. R. Church, M. S. Fuge and K. N. Sreepada, “Second Dissociation Constant of Bis-[(2-hydroxyethyl)amino]acetic Acid (BICINE) and pH of Its Buffer Solutions from 5˚C - 55˚C,” Journal of Solution Chemistry, Vol. 35, No. 4, 2006, pp. 605-624. doi:10.1007/s10953-005-9009-6 [13] R. N. Roy, L. N. Roy, S. Ashkenazi, J. T. Wollen, C. D. Dunseth, M. S. Fuge, C. N. Roy, H. M. Hughes, B. T. Morris and K. L. Cline, “Buffer Standards for pH Meas- urement of N-(2-Hydroxyethyl)piperazine-N’-2-ethane- sulfonic Acid (HEPES) for I = 0.16 mol·kg–1 from 5˚C to 55˚C,” Journal of Solution Chemistry, Vol. 38, No. 4, 2009, pp. 449-458. doi:10.1007/s10953-009-9378-3 [14] W. M. Latimer, “Oxidation Potentials,” 2nd Edition, Prentice-Hall, New York, 1952. [15] C. A. Vega and R. G. Bates, “Buffers for the Physiologi- cal pH Range: Thermodynamic Constants of Four Sub- stituted Aminoethanesulfonic Acids from 5˚C to 50˚C,” Analytical Chemistry, Vol. 48, 1976, pp. 1293-1296. [16] R. G. Bates and E. A. Guggenheim, “Report on the Stan- dardization of pH and Related Terminology,” Pure and Applied Chemistry, Vol. 1, 1960, pp. 163-168. doi:10.1351/pac196001010163 [17] K. S. Pitzer and G. J. Mayorga, “Thermodynamics of Electrolytes. II. Activity and Osmotic Coefficients for Strong Electrolytes with One or Both Ions Univalent,” Journal of Physical Chemistry, Vol. 77, No. 19, 1973, pp. 2300-2307. doi:10.1021/j100638a009 [18] M. I. A. Ferra, “A Pitzer Theory Approach to Assignment of pH to Standard Buffer Solutions,” Electrochimica Acta, Vol. 16, 1998, pp. 133-142. Copyright © 2011 SciRes. OJPC
|