 Open Journal of Physical Chemistry, 2011, 1, 85-93 doi:10.4236/ojpc.2011.13012 Published Online November 2011 (http://www.SciRP.org/journal/ojpc) Copyright © 2011 SciRes. OJPC 85 Organocatalyzed Decarboxylation of Naturally Occurring Cinnamic Acids: Po tential Role in Flavoring Chemicals Production Virginia Aldabalde1#, Mariela Risso1#, María Lucía Derrudi1, Federico Geymonat1, Gustavo Seoane1, Daniela Gamenara1, Patricia Saenz-Méndez1,2* 1Grupo de Fisicoquímica Orgánica y Bioprocesos, Facultad de Química, UdelaR, Montevideo, Uruguay 2Computational Chemistry and Biology Group, Facultad de Química, UdelaR, Montevideo, Uruguay E-mail: *psaenz@fq.edu.uy Received June 26, 2011; revised August 15, 2011; accepted September 17, 2011 Abstract The mechanism and the final outcome of the Knoevenagel-Doebner reaction are discussed. The condensation reaction between different hydroxy-substituted aromatic aldehydes and malonic acid is performed using piperidine as organocatalyst. The key role of the catalyst is clearly pointed out during the decarboxylation of ferulic acid, without the use of a strong decarboxylating agent, leading to a 4-vinylphenol derivative. Based on the results obtained, the studied pathway may be important in the understanding of vinylphenol produc- tion during malting and brewing of wheat and barley grains. Finally, changing the solvent of the reaction from pyridine to water in the Knoevenagel-Doebner reaction of 4-hydroxybenzaldehydes, dimerization of resulting styrene derivatives is observed. These results can be of interest also in the field of food chemistry, since cinnamic acids are frequently found in fruits and vegetables used for human consumption. Keywords: ,β-Unsaturated Carboxylic Acids, Decarboxylation, Vinylphenols, Organocatalysis, Knoevenagel 1. Introduction ,β-Unsaturated carboxylic acids are versatile compo- unds which allow the access to a variety of synthetic intermediates as well as natural products, such as lignin related structures (Figure 1) [1]. Lignin is a component of the cell wall in vascular plants. Its structure involves a variety of different cova- lent bonds result of the oxidative coupling of aromatic alcohols derived from hydroxycinnamic acids [2]. There is an increasing interest in its structural study due to its potential utilization as renewable raw material in the chemical industry. Within a research program devoted to the use of ,β- unsaturated carboxylic acids to prepare lignans as well as high added value building blocks, the synthesis of cin- namic acid derivatives through a Knoevenagel-Doebner reaction was performed [3,4]. The Knoevenagel conden- sation of aldehydes and malonic acid is generally pro- moted by bases, such as pyridine and piperidine, yielding the correspondin g ,β-unsaturated carboxylic acid, under organocatalytic conditions [5]. Organocatalysis has be- come a field of great importance within organic synthesis, allowing the development of widely applicable reactions while avoiding the use of metal catalysts [6-9]. In this context, we condensed different aromatic alde- hydes with malonic acid, using piperidine as organo- catalyst and pyridine as solvent [10,11]. In most cases, the reaction lead to the corresponding ,β-unsaturated carboxylic acid in very good yields (results not shown). However, when vanillin (4-hydroxy-3-methoxybenzald- ehyde) was employed as electrophile, the formation of 2-methoxy-4-vinylphenol 1a, instead of ferulic acid ((E)- 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid) 2a, was observed (Figure 2). This useful transformation was reported in 2005 and since then, some aspects of the outcome of the reaction were described [12,13 ]. 4-Vinylphenols are natural products widely distributed in plants [14-17]. Moreover, such intermediates are also employed as building blocks for the preparation of # Both authors contributed equally.  V. ALDABALDE ET AL. 86 Figure 1. ,β-Unsaturated carboxylic acids as precursors of lignin models. Figure 2. Organocatalyzed conversion of vanillin into 2-methoxy-4-vinylphenol 1a. polymers and bioactive compounds [18,19]. Conse- quently, the design of synthetic strategies to prepare this type of compounds and gaining knowledge regarding their occurrence in nature, are of increasing importance. Recently, a detailed computational study of the mech- anism of the Knoevenagel-Doebner reaction was re- ported [20], answering questions regarding the mecha- nism of the reaction and opening the door to new ones. Hydroxycinnamic acids are ubiquitous in plants, and it has been proposed that enzymatic decarboxylation may be responsible for vinylphenol production [21]. Besides the biocatalytic transformation, is it possible to perform the same reaction employing an organocatalyst? As an answer to this question, herein we describe a decarboxy- lation reaction of hydroxycinnamic acids under mild conditions. In addition, we report new experimental evi- dence for the previously proposed mechanism as well as the final outcome of the organocatalytic Knoevena- gel-Do ebner condensation. 2. Experimental 2.1. General Chemical reagents were purchased from commercial sources and were used without further purification. Sol- vents were purified and dried by standard procedures. Reactions were monitored using thin layer chromatogra- phy (TLC) on silica gel (Kieselgel HF254) and visual- ized with UV light (254 nm) and p-anisaldehyde in acidic ethanolic solution. Flash column chromatography was performed using silica gel 60 (230 - 240 mesh). NMR (1H and 13C) spectra were carried out in a Bruker Advance DPX 400 MHz equipment, at 30˚C in the pres- ence of TMS as internal stan dard. All produ cts were pre- viously reported and the spectroscopic data of all com- Copyright © 2011 SciRes. OJPC  87 V. ALDABALDE ET AL. pounds were found to correspond with literature data. 2.2. General Procedure for the Knoevenagel Condensation in Pyridine Malonic acid (1 g, 9.6 mmol) was dissolved in pyridine (10 mL, 124 mmol). Then, the substituted benzaldehyde (6.4 mmol) and piperidine (0.24 mL, 2.4 mmol) were added. The mixture was refluxed for 2 h. After cooling, the solution was poured into ice water and concentrated HCl was added until pH 3. The aqueous solution was extracted with ethyl acetate (3 × 20 mL), dried over Na2SO4 and the solvent removed in vacuo. Purification by column chromatography over silica gel (7:3 hex- ane/ethyl acetate) yielded the corresponding 4-vinylph- enol (for aldehydes 3a - 3c) or coumarin (for aldehydes 3d and 3e). 2.3. General Procedure for the Reaction in Water Malonic acid (1 g, 9.6 mmol) was dissolved in water (10 mL). Pyridine (0.56 mL, 7.0 mmol), the substituted ben- zaldehyde (6.4 mmol) and piperidine (0.24 mL, 2.4 mmol) were added. The mixture was refluxed for 2 h. After cooling, the solution was poured into ice water and concentrated HCl was added until pH 3. The aqueous solution was extracted with ethyl acetate (3 × 20 mL), dried over Na2SO4 and the solvent removed in vacuo. Purification by column chromatography over silica gel (7:3 hexane/ethyl acetate) yielded the ,β-unsaturated carboxylic acid, 4-vinylphenol and styrene dimer. 2.4. Products Spectroscopic data of all compounds were found to match those in previous reports: 1a [12,13], 1b [21], 1c [13], 2a [12], 8d [22], 8e [23], 9a [24] . 2-Metho xy -4-viny lphenol (1a) 1H RMN (400 MHz, CDCl3) δH (ppm) 3.85 (s, 3H, OCH3), 5.11 (dd, 1H, J1 = 10.8 Hz, J2 = 0.9 Hz, CH = CH2), 5.57 (dd, 1H, J1 = 17.6 Hz, J2 = 0.9 Hz, CH = CH2), 5.77 (bs, 1H, OH), 6.62 (dd, 1H, J1 = 17.6 Hz, J2 = 10.8 Hz, CH = CH2), 6.89 (m, 3H, ArH). 13C RMN (100 MHz, CDCl3) δC (ppm) 55.9, 108.0, 111.5, 114.5, 120.1, 130.3, 136.7, 145.6, 146.7. 4-Vinylp henol (1b) 1H RMN (400 MHz, acetone-d6) δH (ppm) 5.04 (dd, 1H, J1 = 10.9 Hz, J2 = 1.1 Hz, CH = CH2), 5.59 (dd, 1H, J1 = 17.6 Hz, J2 = 1.1 Hz, CH = CH2), 6.65 (dd, 1H, J1 = 17.6 Hz, J2 = 10.9 Hz, CH = CH2), 6.81 (d, 2H, J = 6.7 Hz, ArH3, ArH5), 7.31 (d, 2H, J = 6.6 Hz, ArH2, ArH6), 8.43 (bs, 1H, OH). 13C RMN (100 MHz, CDCl3) δC (ppm) 110.0, 115.3, 127.4, 129.3, 136.6, 157.4. 4-Vinylbenzene-1,2-diol (1c) 1H RMN (400 MHz, acetone-d6) δH (ppm) 5.02 (dd, 1H, J1 = 10.9 Hz, J2 = 1.1 Hz, CH = CH2), 5.55 (dd, 1H, J1 = 17.6 Hz, J2 = 1.1 Hz, CH = CH2), 6.60 (dd, 1H, J1 = 17.6 Hz, J2 = 10.9 Hz, CH = CH2), 6.80 (dd, 2H, J1 = 1.4 Hz, J2 = 0.9 Hz, ArH2, ArH6), 6.99 (dd, 1H, J1 = 1.4 Hz, J2 = 0.9 Hz, Ar H5), 7.99 (bs, 1H, OH). 3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid (ferulic acid) (2a) 1H RMN (400 MHz, DMSO-d6) δH (ppm): 3.82 (s, 3H, OCH3), 6.37 (d , 1H, J = 15 .9 Hz, = CH-COOH) , 6.80 (d, 1H, J = 8.1 Hz, ArH5), 7.09 (dd, 1H , J1 = 8.2 Hz, J2 = 1.9 Hz, ArH6), 7.28 (d, 1H, J = 1.9 Hz, ArH2), 7.50 (d, 1H, J = 15.9 Hz, CH = CH-COOH), 9.57 (bs, 1H, OH). 13C RMN (100 MHz, CDCl3) δC (ppm) 56.1, 111.6, 116.0, 123.3, 126.2, 145.0, 148.4, 149.5, 168.5. 2H-Chromen-2-o n e ( 8d) 1H RMN (400 MHz, acetone-d6) δH (ppm) 6.44 (d, 1H, J = 9.6 Hz, CH = CH-C(O)O), 7.36 (m, 2H, ArH6, ArH7), 7.62 (m, 1H, ArH5), 7.71 (m, 1H, ArH8), 8.01 (d, 1H, J = 9.6 Hz, CH = CH-C(O)O). 8-Methoxy-2H-c hromen-2-one (8e) 1H RMN (400 MHz, CDCl3) δH (ppm) 3.97 (s, 3H, OCH3), 6.45 (d, 1H, J = 9.6 Hz, CH = CH-C( O)O), 7.08 (m, 2H, ArH5, ArH7), 7.22 (m, 1H, ArH6), 7.71 (d, 1H, J = 9.6 Hz, CH = CH-C(O)O). 13C RMN (100 MHz, CDCl3) δC (ppm) 56.2, 113.7, 116.9, 119.3, 124.3, 143.7, 147.2, 160.3, 191.0. (E)-4,5’-(but-1-ene-1,3-diyl)bis(2-methoxyphenol) (9a) 1H RMN (400 MHz, CDCl3) δH (ppm) 1.43 (d, 3H, J = 7.0 Hz, CH3), 3.55 (td, 1H, J1 = 7.0 Hz, J2 = 6.6 Hz, CH-CH3), 3.88 (s, 6H, 2xCH3), 5.52 (s, 1H, OH), 5.59 (s, 1H, OH), 6.19 (dd, 1H, J1 = 15.8 Hz, J2 = 6.5 Hz, CH = CH-CH(CH3)), 6.31 (dd, 1H, J1 = 15.9 Hz, J2 = 1.0 Hz, CH = CH-CH(CH3)), 6.76 (m, 2H, ArH); 6.92 (m, 4H, ArH). 13C RMN (100 MHz, CDCl3) δC (ppm) 21.4, 42.2, 55.9, 108.0, 109.9, 114.2, 114.3, 119.8, 128.0, 130.2, 133.3, 137.8, 143.9, 145.0, 146.4, 146.6. 3. Results and Discussion 3.1. Knoevenagel Condensation: Mechanistics Insights A detailed proposed mechanism that agrees with experi- mental information is shown in Figure 3. This mecha- nism, based on previously suggested ones [12,13], in- cludes new information, such as the role of the organo- catalyst in the entire process. In particular, the contribut- ing effect of the catalyst to the key decarboxylation step. Even though the Knoevenagel reaction has been em- Copyright © 2011 SciRes. OJPC  V. ALDABALDE ET AL. Copyright © 2011 SciRes. OJPC 88 Figure 3. Proposed mechanism for the preparation of ferulic acid and 2-methoxy-4-vinylphenol. ployed to obtain cinnamic acid and derivatives, both the reaction and work-up conditions previously reported are different than those described in this work. Simpson and coworkers stated that the styrene formation took place only when a proper substrate (2- or 4-hydroxybenzalde- hyde) was used jointly with toluene for removing the pyridine by distillation in vacuo [12]. In this work, we found that quenching the reaction with acid works as well. Therefore, contrary to previously described mecha- nism, we demonstrate that styrene formation does not depend on the work-up conditions, and took place before the work-up. We analyzed the outcome of the Knoevenagel-Doeb- ner condensation of 2-hydroxy- and 4-hydroxy substi- tuted benzaldehydes (Figure 4 and Table 1), employing a lower load of catalyst (ten times) than previously de- scribed one and quenching the reaction with HCl. The load of catalyst was evaluated, and we found that 1:0.4 corresponds to the optimum vanillin:piperidine ratio (results not shown). From these results it is evident that only 4-hydroxy- substituted aldehydes lead to the correspon ding vinylph- enol. Presumably, a quinone methide intermediate can be obtained for both 2-hydroxy and 4-hydroxy substituted benzaldehydes (Figure 5). In spite of that, the stability and reactivity of ortho- and para-quinone methides are not exactly the same. Quinone methides are common reactive intermediates extensively employed by nature, particularly in the mechanism of action displayed by a variety of bioactive compounds [25,26]. The stability of such intermediates is essential to evaluate the outcome of the reaction. Or- tho-quinone methides are less stable (ca. 2 times) than the corresponding para- isomers [27], and thus the for- mation of the latter can be favored. Taking this into ac- count, subsequent decarboxylation reaction is also fa- vored, and the 4-vinylphenol is then afforded. When the reactant aldehyde is 2-hydroxy substituted, a lower en- ergy pathway than the formation of the quinone methide intermediate is available, and a different ou tcome for the reaction is observed. 2-Hydroxybenzaldehyde (entry 4, Table 1) and 2-hydroxy-3-methoxybenzaldehyde (entry 5, Table 1) afforded a coumarin derivative. Coumarin (1,2-benzopyrone) is a secondary plant me- tabolite with a pleasant flavor, which use as food flavoring Table 1. Products obtained for the Knoevenagel-Doebner condensation of aromatic aldehy de s and malonic acid. Product distributiona EntryAldehydeCinnamic acid (2) (%) Styrene (1) (%) 1 3a 0 80 2 3b Traces 60 3 3c 0 59 4 3d 0 1d (0%) + 8d (15%) 5 3e Traces 1e (Traces) + 8e (20%) aRemaining percentage corresponds to starting material. 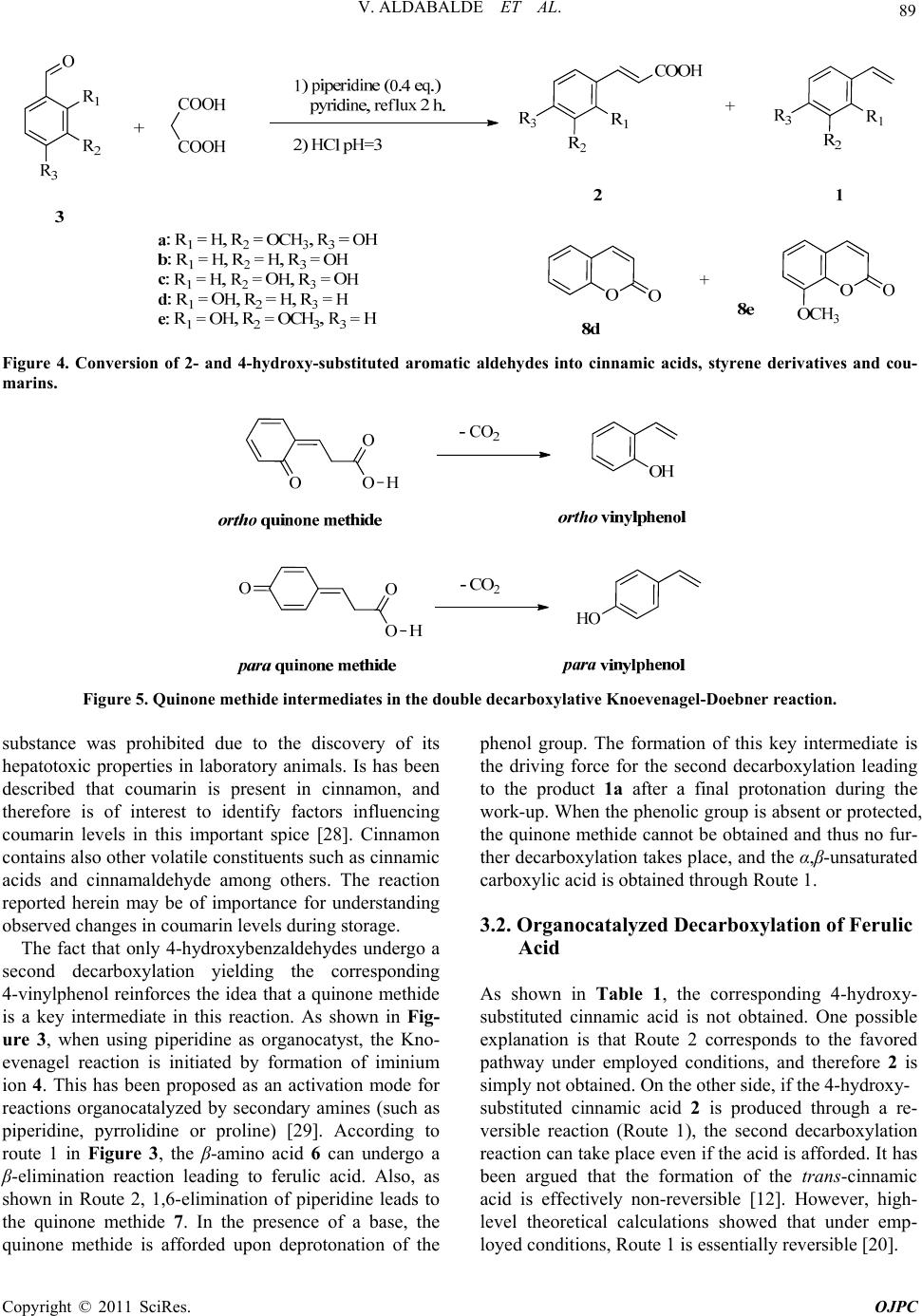 89 V. ALDABALDE ET AL. Figure 4. Conversion of 2- and 4-hydroxy-substituted aromatic aldehydes into cinnamic acids, styrene derivatives and cou- marins. Figure 5. Quinone methide intermediates in the double decarboxylative Knoevenagel-Doebner reaction. substance was prohibited due to the discovery of its hepatotoxic properties in laboratory animals. Is has been described that coumarin is present in cinnamon, and therefore is of interest to identify factors influencing coumarin levels in this important spice [28]. Cinnamon contains also other volatile constitu ents such as cinnamic acids and cinnamaldehyde among others. The reaction reported herein may be of importance for understanding observed changes in coumarin levels during storage. The fact that only 4-hydroxybenzaldehydes undergo a second decarboxylation yielding the corresponding 4-vinylphenol reinforces the idea that a quinone methide is a key intermediate in this reaction. As shown in Fig- ure 3, when using piperidine as organocatyst, the Kno- evenagel reaction is initiated by formation of iminium ion 4. This has been proposed as an activation mode for reactions organocatalyzed by secondary amines (such as piperidine, pyrrolidine or proline) [29]. According to route 1 in Figure 3, the β-amino acid 6 can undergo a β-elimination reaction leading to ferulic acid. Also, as shown in Route 2, 1,6-elimination of piperidine leads to the quinone methide 7. In the presence of a base, the quinone methide is afforded upon deprotonation of the phenol group. The formation of this key intermediate is the driving force for the second decarboxylation leading to the product 1a after a final protonation during the work-up. When the phenolic group is absent or protected, the quinone methide cannot be ob tained and thus no fur- ther decarboxylation takes place, and the α,β-unsaturated carboxylic acid is obtained through Route 1. 3.2. Organocatalyzed Decarboxylation of Ferulic Acid As shown in Table 1, the corresponding 4-hydroxy- substituted cinnamic acid is not obtained. One possible explanation is that Route 2 corresponds to the favored pathway under employed conditions, and therefore 2 is simply not obtained. On the other side, if the 4-hydroxy- substituted cinnamic acid 2 is produced through a re- versible reaction (Route 1), the second decarboxylation reaction can take place even if the acid is afforded. It has been argued that the formation of the trans-cinnamic acid is effectively non-reversible [12]. However, high- level theoretical calculations showed that under emp- loyed condition s , Route 1 is essen tially reversible [20]. Copyright © 2011 SciRes. OJPC  V. ALDABALDE ET AL. 90 To this end, we envisioned an experiment that can demonstrate the role of a secondary amine in the decar- boxylation leading to 4-vinylphenol. When using the Knoevenagel conditions mentioned before, with ferulic acid as reactant, 2-methoxy-4-vinylphenol 1a was ob- tained as a single product. Moreover, if this reaction is performed under the same conditions but without piperi- dine, unchanged ferulic acid is recovered. Herein we report the decarboxylation of ferulic acid 2a to vinyl- phenol 1a under mild conditions, involving a 1,4-addi- tion of piperidine yielding intermediate 6. Most of avail- able protocols for decarboxylation of cinnamic acid de- rivatives involve the use of strong conditions, such as toxic quinoline-metal salts [30]. Intermediate 6 suffer a 1,6-elimination of piperidine affording the key quinone methide 8, which undergoes a decarboxylation reaction leading to the styrene derivative 1a (Figure 6). This result agrees with the mechanistic role proposed for piperidine, suggesting that intermediate 6 facilitates the final decarboxylation reaction leading to the 4-vi- nylphenol 1a. We also propose that this reaction is relevant in food chemistry. Phenolic styrenes have strong flavours and because of that, their presence sometimes is a limitation for the use of the product in foods or beverages. Barley and wheat malts are the most important raw materials for beer production. In those grains, ferulic acid is present and it is known that during brewing, 2-methoxy-4- vinylphenol 1a is produced [31-33]. Until now, thermal or enzymatic decarboxylation of ferulic acid, were proposed as the responsible mecha- nisms for the production of 4-vinylphenol derivatives. We suggest that 4-vinylphenol is produced also through the pathway showed in Figure 6, using a readily avail- able secondary amine, such as proline, which is present in wheat and barley [34]. Thus, under malting and brewing condition, proline (organocatalyst) adds to ferulic acid affording the corre- sponding β-amino acid. Further amine and CO2 elimina- tion leads to 2-methoxy-4-vinylphenol production. In fact, when attempting the decarboxylation of ferulic acid employing proline as organocatalyst, the reaction was complete, leading to 2-methoxy-4-vinylphenol as a sin- gle product. Such processes in natural environments are actually performed in aqueous media. Therefore, the choice of solvent may be critical. The Knoevenagel-Doebner reac- tion of aromatic aldehydes has been performed employ- ing different solvents or even under solvent-free condi- tions [3,1 2]. 3.3. Vinylphenol Dimerization in Water Both the elimination step to get ferulic acid and the de- carboxylation leading to 4-vinylphenol, are expected to be affected by solvent properties, such as polarity. Thus, we performed the reaction in water. One can expect that increasing the dielectic constant will raise both the global conversion and th e 2-methoxy-4-vinylphenol/f erulic acid ratio. Contrary to these assumptions, when performing the reaction in water (dielectric constant of water = 80.1, dielectric constant of pyridine = 13.26) [35] a totally different result was obtained. Ferulic acid and 2-methoxy-4-vinylphenol were ob- tained, but in different amounts than those obtained in pyridine (18% and 8% of yield, respectively). In addition, during the reaction we observed the formation of a new compound 9a (6 7% of yield), w ith a 1H NMR showing a clear ABX system and a trans double bond (Figure 7). Spectroscopic data is in agreement with the proposed structure. The structure of 9a immediately suggests that this product can be obtained through a dimerization of 2-methoxy-4-vinylphenol. Dimerization of styrene de- rivatives has been reported by sever a l authors [36-40]. In order to confirm this hypothesis, we performed the reaction in water under the same conditions as before, but employing 2-methoxy-4-vinylphenol 1a as substrate. After 2 hours of refluxing, the product 9a was obtained as a single product. If the reaction time is extend ed, polar polymeric products are formed, while 9a is consumed. When considering the relative amounts of acid (malo- nic acid) and bases (pyridine/piperidine) employed, one can see that malonic acid is present in a small excess. H3CO OH N H H3CO HO N COOH 6 COOH ferulic acid, 2a H3CO OH N OHHO HO OCH3 1a -CO 2 +H + Figure 6. Organocatalyzed decarboxylation of ferulic acid. Copyright © 2011 SciRes. OJPC 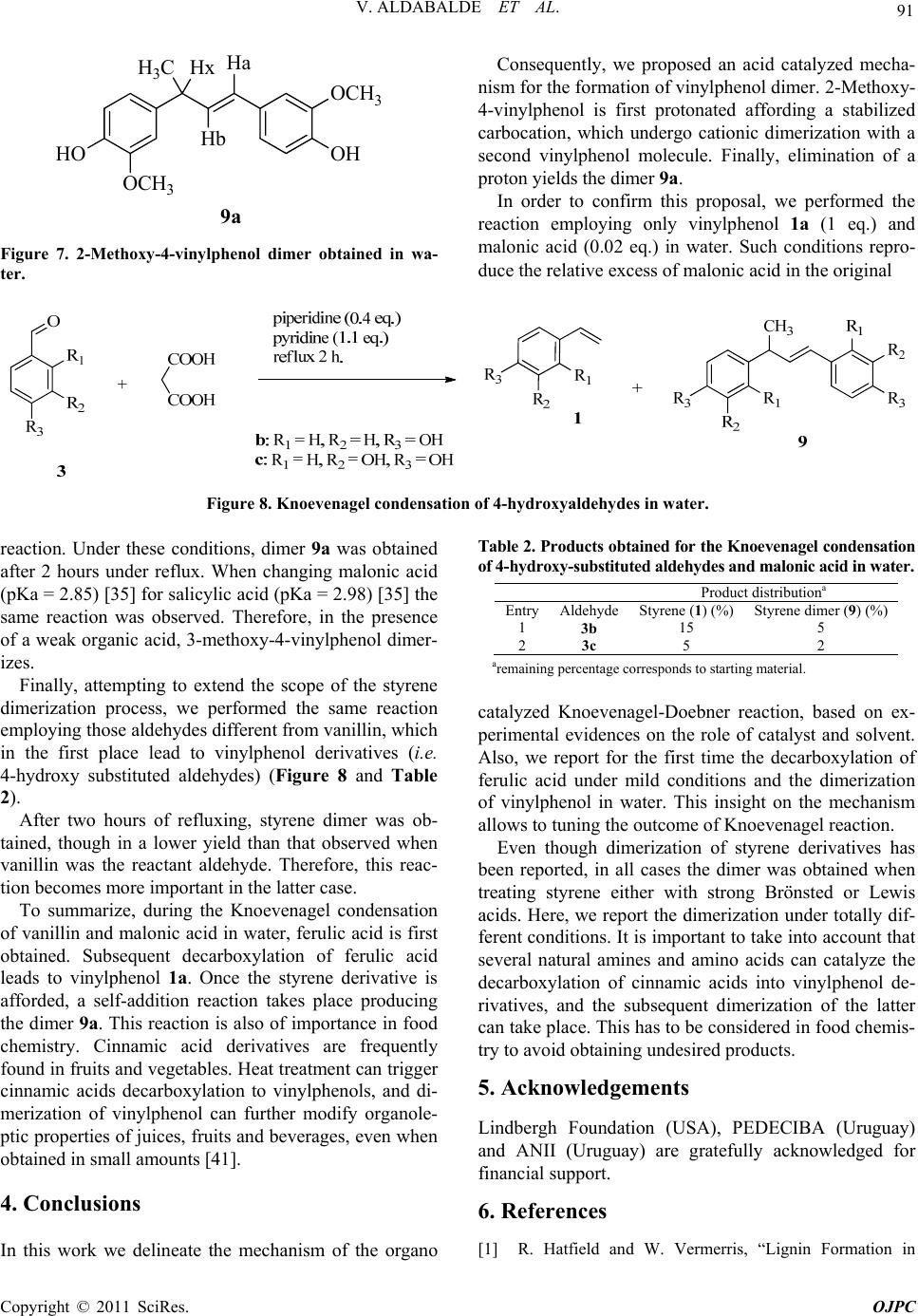 91 V. ALDABALDE ET AL. HO OCH3 OH OCH3 Hb Ha H3CHx 9a Figure 7. 2-Methoxy-4-vinylphenol dimer obtained in wa- ter. Consequently, we proposed an acid catalyzed mecha- nism for the formation of vinylphenol dimer. 2-Methoxy- 4-vinylphenol is first protonated affording a stabilized carbocation, which undergo cationic dimerization with a second vinylphenol molecule. Finally, elimination of a proton yields the dimer 9a. In order to confirm this proposal, we performed the reaction employing only vinylphenol 1a (1 eq.) and malonic acid (0.02 eq.) in water. Such conditions repro- duce the relative excess of malonic acid in the original Figure 8. Knoevenagel condensation of 4-hydroxyaldehydes in wate r. reaction. Under these conditions, dimer 9a was obtained after 2 hours under reflux. When changing malonic acid (pKa = 2.85) [35] for salicylic acid (pKa = 2.98) [35] the same reaction was observed. Therefore, in the presence of a weak organic acid, 3-methoxy-4-vinylphenol dimer- izes. Finally, attempting to extend the scope of the styrene dimerization process, we performed the same reaction employing those aldehydes different from vanillin, which in the first place lead to vinylphenol derivatives (i.e. 4-hydroxy substituted aldehydes) (Figure 8 and Table 2). After two hours of refluxing, styrene dimer was ob- tained, though in a lower yield than that observed when vanillin was the reactant aldehyde. Therefore, this reac- tion becomes more important in the latter case. To summarize, during the Knoevenagel condensation of vanillin and malonic acid in water, ferulic acid is first obtained. Subsequent decarboxylation of ferulic acid leads to vinylphenol 1a. Once the styrene derivative is afforded, a self-addition reaction takes place producing the dimer 9a. This reaction is also of importance in food chemistry. Cinnamic acid derivatives are frequently found in fruits and vegetables. Heat treatment can trigger cinnamic acids decarboxylation to vinylphenols, and di- merization of vinylphenol can further modify organole- ptic properties of juices, fruits and beverages, even when obtained in small amounts [41]. 4. Conclusions In this work we delineate the mechanism of the organo Table 2. Products o btained for the Knoeve nagel condensa tion of 4-hydroxy-substituted aldehydes and malonic acid in water. Product distributiona EntryAldehydeStyrene (1) (%) Styrene dimer (9) (%) 1 3b 15 5 2 3c 5 2 aremaining percentage corresponds to starting material. catalyzed Knoevenagel-Doebner reaction, based on ex- perimental evidences on the role of catalyst and solvent. Also, we report for the first time the decarboxylation of ferulic acid under mild conditions and the dimerization of vinylphenol in water. This insight on the mechanism allows to tuning the outcome of Knoevenagel reaction. Even though dimerization of styrene derivatives has been reported, in all cases the dimer was obtained when treating styrene either with strong Brönsted or Lewis acids. Here, we report the dimerization under totally dif- ferent conditions. It is important to take into account that several natural amines and amino acids can catalyze the decarboxylation of cinnamic acids into vinylphenol de- rivatives, and the subsequent dimerization of the latter can take place. This has to be considered in food chemis- try to avoid obtaining undesired products. 5. Acknowledgements Lindbergh Foundation (USA), PEDECIBA (Uruguay) and ANII (Uruguay) are gratefully acknowledged for financial support. 6. References [1] R. Hatfield and W. Vermerris, “Lignin Formation in Copyright © 2011 SciRes. OJPC  V. ALDABALDE ET AL. 92 Plants. The Dilemma of Linkage Specificity,” Plant Physiology, Vol. 126, No. 4, 2001, pp. 1351-1357. doi:10.1104/pp.126.4.1351 [2] A. -M. Boudet, “Lignins and Lignification: Selected Is- sues,” Plant Physiology and Biochemistry, Vol. 38, No. 1-2, 2000, pp. 82-96. doi:10.1016/S0981-9428(00)00166-2 [3] G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, “Sol- vent-Free Knoevenagel Condensations and Michael Addi- tions in the Solid State and in the Melt with Quantitative Yield,” Tetrahedron, Vol. 59, No. 21, 2003, pp. 3753- 3760. doi:10.1016/S0040-4020(03)00554-4 [4] B. List, A. Doehring, M. T. H. Fonseca, A. Job and R. Rios Torres, “A Practical, Efficient, and Atom Economic Alternative to the Wittig and Horner-Wadsworth-Emmons Reactions for the Synthesis of (E)-Unsaturated Esters from Aldehydes,” Tetrahedron, Vol. 62, No. 2-3, 2006, pp. 476-482. doi:10.1016/j.tet.2005.09.081 [5] M. Tanaka, O. Oota, H. Hiramatsu and K. Fujiwara, “The Knoevenagel Reactions of Aldehydes with Carboxy Com- pounds. I. Reactions of p-Nitrobenzaldehyde with Active Methine Compounds,” Bulletin of the Chemical Society of Japan, Vol. 61, No. 7, 1988, pp. 2473-2479. doi:10.1246/bcsj.61.2473 [6] K. A. Ahrendt, C. J. Borths and D. W. C. MacMillan, “New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction,” Journal of the American Chemical Society, Vol. 122, No. 17, 2000, pp. 4243-4244. doi:10.1021/ja000092s [7] P. I. Dalko and L. Moisan, “Enantioselective Organocata- lysis,” Angewandte Chemie International Edition, Vol. 40, 2001, pp. 3726-3748. doi:10.1002/1521-3773 [8] K. Juhl and K. A. Jorgensen, “The First Organocatalytic Enantioselective Inverse-Electron-Demand Hetero-Diels- Alder Reaction,” Angewandte Chemie International Edi- tion, Vol. 42, No. 13, 2003, pp. 1498-1501. doi:10.1002/anie.200250652 [9] D. W. C. MacMillan, “The Advent and Development of Organocatalysis,” Nature, Vol. 455, 2008, pp. 304-308. doi:10.1038/nature07367 [10] M. L. Derrudi, F. Geymonat, V. Aldabalde, D. Gamenara, G. Seoane and P. S. Méndez, “Síntesis Organocatalítica Eficiente de Precursors de Fenilglicidatos Funcionali- zados,” XVII-Simposio Nacional de Química Orgánica, Mendoza, 2009. [11] F. Geymonat, V. Aldabalde, M. L. Derrudi, D. Gamenara, G. Seoane and P. S. Méndez, “Efecto de las Condiciones en el Curso de la Reacción Descarboxilativa de Knoeve- nagel,” XVII-Simposio Nacional de Química Orgánica, Mendoza, 2009. [12] C. J. Simpson, M. J. Fitzhenry and N. P. J. Stamford, “Preparation of Vinylphenols from 2- and 4-Hydroxy- benzaldehydes,” Tetrahedron Letters, Vol. 46, No. 40, 2005, pp. 6893-6896. doi:10.1016/j.tetlet.2005.08.011 [13] A. K. Sinha, A. Sharma and B. P. Joshi, “One-Pot Two-Step Synthesis of 4-Vinylphenols from 4-Hydroxy Substituted Benzaldehydes under Microwave Irradiation: A New Perspective on the Classical Knoevenagel-Doeb- ner Reaction,” Tetrahedron, Vol. 63, No. 4, 2007, pp. 960- 965. doi:10.1016/j.tet.2006.11.023 [14] J. M. Ames and G. Macleod, “Volatile Components of Okra,” Phytochemistry, Vol. 29, No. 4, 1990, pp. 1201- 1207. [15] H. Y. Chung, “Volatile Flavor Components in Red Fer- mented Soybean (Glycin Max) Curds,” Journal of Agri- cultural and Food Chemistry, Vol. 48, No. 5, 2000, pp. 1803-1809. doi:10.1021/jf991272s [16] P. Pihlsgård, M. Larsson, A. Leufvén and H. Lingnert, “Volatile Compounds in the Production of Liquid Beet Sugar,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 10, 2000, pp. 4844-4850. doi:10.1021/jf000514h [17] D. Janes, D. Kantar, S. Kreft and H. Prosen, “Identifica- tion of Buckwheat (Fagopyrum Esculentum Moench) Aroma Compounds with GC-MS,” Food Chemistry, Vol. 112, No. 1, 2009, pp. 120-124. doi:10.1016/j.foodchem.2008.05.048 [18] R. Cong, R. Pelton, P. Russo and G. Doucet, “Factors Affecting the Size of Aqueous Poly (Vinylphenol-co-Po- tassium Styrenesulfonate)/Poly(Ethylene Oxide) Com- plexes,” Macromolecules, Vol. 36, No. 1, 2002, pp. 204- 209. doi:10.1021/ma020965y [19] S. N. Aslam, P. C. Steven son, S. J. Phy thian, N. C. Veitch and D. R. Hall, “Synthesis of Cicerfuran, an Antifungal Benzofuran, and Some Related Analogues,” Tetrahedron, Vol. 62, No. 17, 2006, pp. 4214-4226. doi:10.1016/j.tet.2006.02.015 [20] E. Bermúdez, O. N. Ventura and P. Saenz Méndez, “Mechanism of the Organocatalyzed Decarboxylative Knoevenagel-Doebner Reaction. A Theoretical Study,” The Journal of Physical Chemistry A, Vol. 114, No. 50, 2010, pp. 13086-13092. doi:10.1021/jp109703f [21] M. Takemoto and K. Achiwa, “Synthesis of Styrenes through the Decarboxylation of Trans-Cinnamic Acids by Plant Cell Cultures,” Tetrahedron Letters, Vol. 40, 1999, pp. 6595-6598. doi:10.1016/S0040-4039(99)01281-2 [22] K. Zeitler and C. A. Rose, “An Efficient Carbene-Ca- talyzed Access to 3,4-Dihydrocoumarins,” The Journal of Organic Chemistry, Vol. 74, No. 4, 2009, pp. 1759-1762. doi:10.1021/jo802285r [23] P. K. Upadhyay and P. Kumar, “A Novel Synthesis of Coumarins Employing Triphenyl([Alpha]-Carboxymethy- lene)Phosphorane Imidazolide as a C-2 Synthon,” Tetra- hedron Letters, Vol. 50, No. 2, 2009, pp. 236-238. doi:10.1016/j.tetlet.2008.10.133 [24] G. P. Rizzi and L. J. Boekley, “Observation of Ether- Linked Phenolic Products during Thermal Degradation of Ferulic Acid in the Presence of Alcohols,” Journal of Ag- ricultural and Food Chemistry, Vol. 40, No. 9, 1992, pp. 1666-1670. doi:10.1021/jf00021a037 [25] Q. Zhou and K. D. Turnbull, “Phosphodiester Alkylation with a Quinone Methide,” The Journal of Organic Chem- istry, Vol. 64, No. 8, 1999, pp. 2847-2851. doi:10.1021/jo9823745 Copyright © 2011 SciRes. OJPC  V. ALDABALDE ET AL. Copyright © 2011 SciRes. OJPC 93 [26] R. W. Van De Water and T. R. R. Pettus, “O-Quinones Methides: Intermediates Underdeveloped and Underuti- lized in Organic Synthesis,” Tetrahedron, Vol. 58, 2002, pp. 5367-5405. doi:10.1016/S0040-4020(02)00496-9 [27] G. Bouchoux, “Heats of Formation and Protonation Ther- mochemistry of Gaseous Benzaldehyde, Tropone and Quinone Methides,” Chemical Physics Letters, Vol. 495, No. 4-6, 2010, pp. 192-197. doi:10.1016/j.cplett.2010.07.008 [28] F. Woehrlin, H. Fry, K. Abraham and A. Preiss-Weigert, “Quantification of Flavoring Constituents in Cinnamon: High Variation of Coumarin in Cassia Bark from the German Retail Market and in Authentic Samples from Indonesia,” Journal of Agricultural and Food Chemistry, Vol. 58, 2010, pp. 10568-10575. doi:10.1021/jf102112p [29] B. List, “Proline-Catalyzed Asymmetric Reactions,” Tet- rahedron, Vol. 58, 2002, pp. 5573-5590. doi:10.1016/S0040-4020(02)00516-1 [30] S. M. Fleming, T. A. Robertson, G. J. Langley and T. D. H. Bugg, “Catalytic Mechanism of a C-C Hydrolase En- zyme: Evidence for a Gem-Diol Intermediate, Not an Acyl Enzyme,” Biochemistry, Vol. 39, No. 6, 2000, pp. 1522- 1531. doi:10.1021/bi9923095 [31] S. Coghe, K. Benoot, F. Delvaux, B. Vanderhaegen and F. R. Delvaux, “Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces Cere- visiae,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 3, 2004, pp. 602-608. doi:10.1021/jf0346556 [32] N. Vanbeneden, F. Gils, F. Delvaux and F. R. Delvaux, “Formation of 4-Vinyl and 4-Ethyl Derivatives from Hy- droxycinnamic Acids: Occurrence of Volatile Phenolic Flavour Compounds in Beer and Distribution of Pad1- Activity among Brewing Yeasts,” Food Chemistry, Vol. 107, No. 1, 2008, pp. 221-230. doi:10.1016/j.foodchem.2007.08.008 [33] L. Du and P. Yu, “Effect of Barley Variety and Growth Year on Ferulic and Para-Coumaric Acids, and Their Ra- tion in the Seed and Hull,” Cereal Research Communica- tions, Vol. 38, No. 4, 2010, pp. 521-532. doi:10.1556/CRC.38.2010.4.9 [34] L. Szabados and A. Savouré, “Proline: A Multifunctional Amino Acid,” Trends in Plant Science, Vol. 15, 2009, pp. 89-97. doi:10.1016/j.tplants.2009.11.009 [35] D. R. Lide, “CRC Handbook of Chemistry and Physics,” CRC Press, Boca Raton, 2003-2004. [36] B. B. Corson, J. Dorsky, J. E. Nickels, W. M. Kutz and H. I. Thayer, “Dimerization of Styrene in the Presence and Absence of Solvent,” The Journal of Organic Chemistry, Vol. 19, No. 1, 1954, pp. 17-26. doi:10.1021/jo01366a004 [37] M. J. Rosen, “Studies of the Dimerization of Styrene in Aqueous Sulfuric Acid,” The Journal of Organic Chemis- try, Vol. 18, No. 12, 1953, pp. 1701-1705. doi:10.1021/jo50018a012 [38] B. B. Corson, W. J. Heintzelman, H. Moe and C. R. Rous- seau, “Reactions of Styrene Dimers,” The Journal of Or- ganic Chemistry, Vol. 27, No. 5, 1962, pp. 1636-1640. doi:10.1021/jo01052a036 [39] T. Higashimura, M. Hiza and H. Hasegawa, “Catalytic Difference between Oxo Acids and Metal Halides in the Cationic Oligomerization of Styrene,” Macromolecules, Vol. 12, No. 2, 1979, pp. 217-222. doi:10.1021/ma60068a010 [40] J. Peng, J. Li, H. Qiu, J. Jia ng, K. Jiang, J. Mao and G. Lai, “Dimerization of Styrene to 1,3-Diphenyl-1-butene Cata- lyzed by Palladium-Lewis Acid in Ionic Liquid,” Journal of Molecular Catalysis A: Chemical, Vol. 255, No. 1-2, 2006, pp. 16-18. doi:10.1016/j.molcata.2006.03.058 [41] B. Fallico, M. C. Lanza, E. Maccarone, C. Nicolosi As- mundo and P. Rapisarda, “Role of Hydroxycinnamic Ac- ids and Vinylphenols in the Flavor Alteration of Blood Orange Juices,” Journal of Agricultural and Food Chem- istry, Vol. 44, No. 9, 1996, pp. 2654-2657. doi:10.1021/jf9503319
|