Journal of Materials Science and Chemical Engineering
Vol.06 No.07(2018), Article ID:87447,15 pages
10.4236/msce.2018.67018
Computation of Energy, Intensity and Thermodynamic Parameters for the Interaction of Ln(III) with Nucleic Acid: Analysis of Structural Conformations, Chemical Kinetics and Thermodynamic Behaviour through 4f-4f Transition Spectra as Probe
N. Bendangsenla1, T. Moainla1, Juliana Sanchu2, M. Indira Devi2*
1Department of Chemistry, Patkai Christian College, Chumoukedima, Nagaland, India
2Department of Chemistry, Nagaland University, Lumami, Zunheboto, Nagaland, India



Received: July 1, 2018; Accepted: July 28, 2018; Published: July 31, 2018
ABSTRACT
Nucleosides and Nucleotides are polydentate ligands, offering potential binding sites for metal ions. Energy interaction parameters: Slater Condon Fk (cm−1), Spin Orbit interaction (cm−1), Nephelauxetic Ratio (β), Bonding (b½) and Co-valency (δ) parameter for the interaction of Pr(III) with Nucleoside and Nucleotide are evaluated to study the mode of binding of the Nucleic Acid Components (Guanosine and Guanosine Triphosphate) with Pr(III). Further Intensity Parameters like Oscillation Strength and Judd Ofelt Parameter (T2, T4, T6) have been evaluated to investigate degree of inner or outer sphere co-ordination of Pr(III) with Nucliec Acid ligands. Comperative Absorption Spectra in different solvents substantiate the informations, gathered from the evaluated values of both energy interaction and intensity parameters. Further evaluation of Thermodynamic parameters (ΔG, ΔH and ΔS) through Kinetic studies enable to provide the detailed information about the reaction pathways and thermodynamic behavour of the complexation process of neucleosides and nucleotides with Pr(III) and Ca2+.
Keywords:
Nucleic Acid, 4f-4f Transition Spectra, Nephelauxetic Effect, Judd-Ofelt Parameters, Slator Condon, Reaction Rate, Thermodynamic

1. Introduction
Because of their remarkable and unmatched optical, spectroscopic, luminescent and magnetic properties, the lanthanides are under the limelight when it comes to high technology. The synthetic versatility, favorable optical and electronic properties are a wide spectrum for pharmacological, medicinal and biological activities of compounds. In view of the above applications and properties of lanthanide elements, it has become more important to understand the behaviors of lanthanide ion in biological system. Therefore the main idea behind this proposed work is to explore the potential of these fascinating lanthanides as spectral and structural probes in biochemical reactions which has immense importance in human metabolism. Solution spectral studies through kinetic and thermodynamic approach involving lanthanide complexes are very pertinent information about the mechanism, reaction pathways, and also about the chemical bonding and conformations.
The electronic transitions having high sensitivity of spectral intensities for ligand environment as a great phenomenon was first noticed by Moeller et al. [1] [2] for β-diketonate and EDTA complexes of Nd3+, Ho3+, and Er3+ much before the advent of Judd-Ofelt theory. Jorgensen and Judd have called such transitions HYPERSENSITIVE and these transitions obeyed selection rules |ΔS| = 0; |ΔL| ≤ 2; |ΔJ| ≤ 2 and these rules are the same as the selection rules for pure quadrupole transitions. But calculations have revealed that the intensities of some transitions even though they don’t obey selection rule show sensitivities even minor changes of the coordinating environment. Such transitions have been called pseudoquadrupole in character [3] [4]. The hypersensitive transition have widely been used as probe in extracting information regarding the binding characteristics of the co-ordinating ligands, relative binding capability of different binding sites, degree of outer and inner sphere coordination and identification of immediate coordination environment in the complex species [5] [6] [7] [8].
Much of the interest in lanthanide biochemistry flows from the ability of Ln(III) ion to replace Ca(II) in a specific and often isomorphous manner. It is worth comparing the properties of Ln(III) and Ca(II) in greater detail. Good reviews of the biologically important chemical properties of Ca(II) have been provided by Levine and Williams [9] and Einspahr and Bug [10]. Martin [11] has reviewed the structural chemistry of calcium and the lanthanides.
Nucleosides and nucleotides are the structural subunit of nucleic acids, the heredity controlling components of all living cells. Nucleosides and nucleotides contain two nearly planar rings, that of the base and that of ribofuranase.The anti conformer has the smaller H-6 (pyrimidine) or H-8 (purine) atom above the sugar ring, while the syn conformer has the larger O-2 (pyrimidine) or N-3 (purine) in that position. Both nucleosides and nucleotides have UV absorption profiles rather similar to those of their constituent’s bases and absorb strongly with λmax values close to 260 nm and molar extinction coefficient of around 104. This property finds uses in the detection and quantitative analysis not only of the free bases but also of nucleosides/nucleotides. They undergo marked changes when they are in close proximity to neighboring bases. Over the past 10 years there has been a resurgence of interest in the coordination chemistry of lanthanide complexes in solution. A renewed interest in this work may be connected with an enhanced appreciation of the rich functionality of lanthanide complexes [12] [13]. Much progress has been recently achieved in the coordination chemistry of lanthanides because of their medicinal and biochemical applications
Solution spectral studies through kinetic approach involving lanthanide complexes are very important because it can provide very pertinent information about the mechanism, reaction pathways, and also about the chemical bonding and conformations. Basing on the isomorphous characteristics of Ca2+ with Ln3+, we have chosen Praseodymium ion among the lanthanides, since these ions have optimum ionic radii for the effective isomorphic substitution of Ca2+ in biomolecules consequently mimicking the interaction between Ca2+ and the metabolites occurring in-vivo intracellularly. Ca2+ being diamagnetic are spectoscopically silent towards optical and magnetic techniques. Therefore the isomorphous substitution of Ca2+ by Pr3+ can provide a very useful supplement to understand the interaction of Guanosine and Guanosine Triphosphate (GTP) with Ca2+ since Ln3+ are paramagnetic and spectroscopically active.
Though the solution spectral analysis may not give very distinctive quantitative data but it provides useful data for structural determination, mechanistic studies and for creating optimum experimental condition required for product formation of some desired pre-determined configurations with advanced technology adopting much better resolution of solution spectral 4f-4f bands. Quantitative Absorption Spectroscopy can prove as an improved tool on mechanistic, diagnostic and condensation studies too [14].
In this Paper we will use comparative absorption and absorption difference spectrophotometry involving 4f-4f transition spectra as probe in understanding the binding characteristics of Guanosine and GTP with lanthanides (Pr3+) in the presence and absence of Ca2+; it will be further studied through kinetic and thermodynamic approach.
2. Methodology
The spectral analysis is made through Perkin Elmer Lambda-35 UV-V is Spectrophotometer at 298 K upgraded with computer attached.
The energy of 4f-4f transitions is composed of two main components, viz the electrostatic and spin orbit interaction between 4f electrons i.e.,
(1.1)
where FK and ASO are the angular part of electrostatic and spin orbit interaction respectively and their values can be calculated by applying tensor operator technique; FK and are radial integrals.
The energy Ej of the jth energy level is given by:
(1.2)
where Eoj is the zero order energy of the jth level.
Nephelauxetic effect, which measures the change in FK with respect to free ion and expressed by a nephelauxetic ratio “β”, and is given as:
and (1.3)
where F0k and Ffk (k = 2, 4, 6) refers to parameters in complex and free ion respectively.
The observed oscillator strength (Pobs) of the transition energies were expressed in terms of parameters defined by Judd and Ofelt known as the T2, T4 and T6 parameters which are given by the following equation.
(1.4)
These values are procurred using Carnall’s co-efficient for aquo system.
For praseodymium complexes, four equations exist for the observed values of the four bands. Since [(U2)]2 and [(U6)]2 has zero value for the 3P1 and 3P0 levels, the ordinary simultaneous equation gave the values of T2, T4 and T6 by simple calculation.
3. Methods for Chemical Kinetic Studies
All the spectra were recorded on a different temperatures i.e. 298 K, 303 K, 308 K, 313 K, 318 K and at pH-4 and pH-2 for Guanosine and GTP ligands using water circulating HAAKE DC 10 Thermotat.The activation energy for the complexationof Pr(III): (Guanosine and GTP) with Ca(II) in DMF is calculated from the plot of log k (k = rate constant) against 1/T using Arrhenius rate equation.
4. Results and Discussions
For praseodymium complex, four transitions (3P2, 3P1, 3P0, and 1D2) have been observed, originating from the symmetry forbidden 3H4 ground level, in the 400 - 600 nm spectral regions. The energy parameters of these transitions are shown in Table 1 and Table 2. The 4f-4f transitions 3H4 → 3P2, 3H4 → 3P1, 3H4 → 3P0, 3H4 → 1D2 of Pr(III) do not obey selection rule and so they are considered non-hypersensitive transition. Yet, they have been found to exhibit substantial sensitivity towards even minor co-ordination changes around Ln(III), due to the difference in the binding behavior and changes in the immediate co-ordination environment, and referred these transitions as ligand mediated pseudo-hypersensitive transitions [15]. Misra [16] [17] studied the high sensitivity of 3H4 → 3P2, 3H4 → 3P1, 3H4 → 3P0 and 3H4 → 1D2 transitions of Pr(III) and transition of Nd(III) chelates in their complexes with ligands having widely different binding characteristics. They have found that the nature of the co-ordinating sites, chelating power
Table 1. Computed values of energy interaction Slater Condon Fk (cm−1), Spin orbit interaction ξ4f (cm−1), Nephelauxetic ratio (β), bonding (b1/2) and covalency (δ) parameters of Pr(III), Pr(III): L, Pr(III): L: Ca(II), at 298 K (pH4) are given below (L = Guanosine).
Table 2. Computed value of energy interaction Slater Condon Fk (cm−1), Spin orbit interaction ξ4f (cm−1), Nephelauxetic ratio (β), bonding (b1/2) and covalency (δ) parameters of Pr(III), Pr(III): L, Pr(III): L: Ca(II), at 298 K (pH2) are given below (L = GTP).
of the ligand and nature as well as the geometry of the complex species induced unusual sensitivity to this pseudo-hypersensitve transition. Karraker [18] studied the hypersensitive transition correlating with the co-ordination number of lanthanide ions. As such the interaction of ligands (Guanosine and GTP) with Pr(III) in different aquated organic solvents like Dimethylforamide (DMF), Methanol (CH3OH), Acetonitrile (CH3CN), Dioxane (DX) and solvent mixture have been investigated, which gives results of the shape, energy and oscillator strength of pseudo-hypersensitive transition that correlates with co-ordination number.
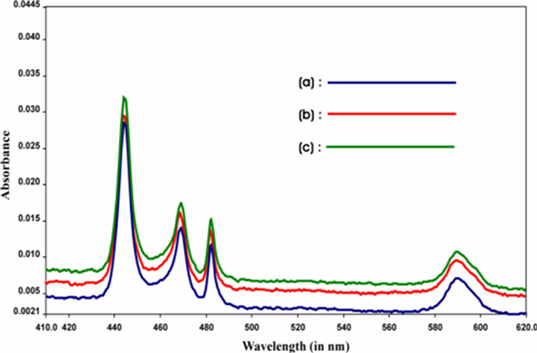
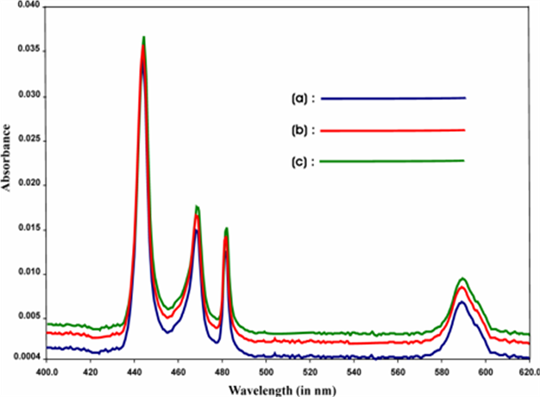
The comparative absorption spectra of Pr(III), Pr(III): Ligands, Pr(III): Ligands: Ca(II) (Ligands-Guanosine and GTP) are shown in Figure 1 and Figure 2, as sample among the various figures, which shows that the addition of ligands to Pr(III) ions enhances the intensities of the different 4f-4f transitions i.e. there is a red shift in all the energy bands. Also the intensities of the different 4f-4f transitions increases when Ca(II) ion is added to the solution. As a consequence,
Figure 1. Comparative absorption specta of (a) Pr(III), (b) Pr(III): Guanosine, Pr(III): Guanosine: Ca(II) in acetonitrile.
Figure 2. Comparative absorption specta of (a) Pr(III), (b) Pr(III): GTP (c) Pr(III): GTP: Ca(II) in acetonitrile.
we have observed noticeable increase in the magnitude of Judd-Ofelt intensity parameters (Tλ). These suggest the binding of Ligands to Pr(III) in solution state. The intensification of bands is interpreted in terms of increased interaction of 4f orbitals of Pr(III) with ligand orbitals. The intensification of bands especially 3H4 → 3P2 transition can be correlated with the lowering of the co-ordination number and shortening of metal-ligand distance.
As a result, the nephelauxetic effect increases when the co-ordination number decreases. Misra et al. [19] observed a general decrease in the values of Fk, Ek and ξ4f as compared to corresponding parameters of the free ion. Table 1 and Table 2 show the variations of the magnitude of energy interaction parameters like Slater-Condon (Fk), Lande spin orbit coupling parameter (ξ4f), Nephelauxatic ratio (β), bonding parameter (b1/2), Percentage covalency (δ) for Pr(III), Pr(III): L and Pr(III): L: Ca(II) in different aquated organic solvents. From these values it has been observed that, there is a steady decrease in the value of Fk, ξ4f which indicates the lowering of both coulombic (Fk) and spin-orbit interaction (ξ4f) parmeters thus results in the expansion of the central metal ion orbital on complexation. Further it has also been observed that the value of nephelauxetic ratio (β) in this system is also less than unity (0.9404 - 0.9575) and the values of bonding parameters (b1/2) are positive which indicates co-valent character in metal-ligand bond.
Table 3 and Table 4 give the absolute values of Oscillator strength and Judd-Ofelt intensity parameter Tλ (λ = 2, 4, 6) which have suggested three phenomenological parameters (T2, T4, T6) which are sensitive towards the changes in the immediate co-ordination environment. It has been observed that there is a significant change in the Oscillator strength of 4f-4f bands, when Pr(III) interacts with the ligands and also noticeable increase in the magnitude of Judd-Ofelt parameters suggesting the binding of ligands in solution. The intensification became more when Ca(II) ion is added to the binary mixture of Pr(III) and ligands showing the stimulated effect of Ca(II) towards the complexation. It has been observed that among the three Tλ parameters, T6 is the best defined while T2 is the least defined parameter for Pr(III) complex since by definition, Tλ ≥ 0 and their order is T6 > T4 > T2. In practice both the Oscillator strength of the transitions and the Tλ parameters have provided significant structural information about lanthanide co-ordination especially in solution state. The values of T2 for most of the complexes appear to be negative which is meaningless. This is because 3F2 → 3H4 transition has a significant U(2) matrix and it is not included in the data set of any of the complex as it is beyond the range of UV region. However, T4 and T6 are affected significantly. Both parameters are related to changes in symmetry properties of the complex species. These suggest that the symmetry of the complex species changed significantly and not only the immediate co-ordination of environment of Pr(III), these changes are considered to be a good evidence for the involvement of ligands in the inner sphere co-ordination of Pr(III).
The observation made from the Tables is further supported by the comparative absorption spectra shown in Figure 1 and Figure 2 (among the various spectra), where there is significant change in the intensity of the peak of the spectra when Pr(III) interacts with different ligands in solution, (Viz-Guanosine and GTP) resulting significant enhancement in the Oscillator strength of different 4f-4f transitions . As a result we have observed noticeable increase in the magnitude of Judd-Ofelt Tλ parameters. Such increase in the value of Oscillator strength and Tλ is more when Ca(II) is added to the solution of Pr(III):
Table 3. Observed and Computed values of Oscillator Strengths (P × 106) and Judd-Ofelt (Tλ × 1010 ) Parameters for Pr(
Table 4. Observed and Computed values of Oscillator Strengths (P × 106) and Judd-Ofelt (Tλ × 1010) Parameters for Pr(
Ligand. This is due to the involvement of Ca(II) to the other ligating site of the Ligands.
We have used comparative absorption and absorption difference spectroscopy, along with the variation of different spectral intensity parameters (P and Tλ) involving intra 4f?4f transitions as probe in following the simultaneous coordination of selected ligands with Pr(III) and Ca(II) and to investigate their rate of reaction, activation energy and consequently thermodynamic behavior, evaluating their thermodynamic parameters like ΔH0, ΔS0 and ΔG0.
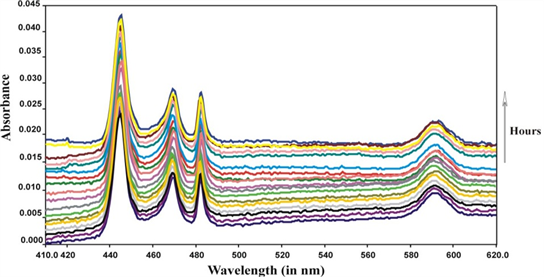
The time scan absorption spectra of the formation of the Pr(III): Guanosine complexes with Ca(II) respectively at 298 K, 308 K, 308 K, 310 K and 318 K are recorded . Several sets of kinetic experiments were conducted for the selected ligands but for brevity only ten sets of kinetic run showing changes in absorbance with time at different temperatures. From the selected Figure 3 it can be observed that there is an increase of absorbance and intensity with time. Both hypersensitive and pseudohypersensitive transitions of Pr(III) display significant changes during complexation, which can be seen from the spectral changes with time (Figure 3) The top most curve in all the cases represent the stage of apparent completion of the complexation and no further changes were noted over the next few days.
Only one Table 5 is selected from various tables which show the observed (Pobs) and calculated (Pcal) values of oscillator strengths and Judd-Ofelt intensity parameter, Tλ (λ = 2.4.6) for complexation of Pr(III): Nucloeside/Nucleotide with Ca(II) in DMF at different temperatures, viz; 298 K, 303 K, 308 K, 313 K and 318 K respectively. The rate of complexation for both Pr(III): (Nucloeside/Nucleotide): Ca(II) at different temperatures were evaluated by plotting the oscillator strength against time.
From Table 5, we can clearly see that the rates of complexation of Pr(III): (Nucloesides/Nucleotides) with Ca(II) linearly increases with the increase in time and temperature. Since the intensity of 4f-4f transitions show substantial
Figure 3. Comparative absorption spectra of Pr(III): Guanosine complexation with Ca(II) in DMF at 289 K (25˚C) and at different times (hour).
Table 5. Observed and calculated Oscillator strengths (P × 106) and Judd Ofelt parameter (Tλ, λ = 2, 4, 6 × 1010 cm−1) parameters for Pr(III): Guanosine: Ca(II) complex at 298 K (25˚C) at different time (hrs).
increase with Time, hence absorption spectral analysis of 4f-4f transitions can be used to explore the kinetics of the formation of the complexes. The observed values of rate (k) have been evaluated in terms of the complex formed during the progress of the reaction and the same has been evaluated from the plots of oscillator strength of 3H1 → 3P2 transitions of Pr(III) complex formation versus time (in hour) [Figure 4]. The values of activation energy (Ea) andthermodynamic parameters is evaluated from the Vant Hoff plot of lnk against 1/T [Figure 5] This technique can provide a means to determine indirectly the thermodynamic parameters of the complexation of Pr(III): ligands and Ca(II) ions in DMF medium.
From the above mentioned tables it was clearly seen that rate of complexation increases with increase in temperature and from which the rate of reaction and activation energy Ea of the complexation is evaluated as shwon in Table 6. From the values of the thermodynamic parameters in Table 7, we observe that the values of ΔH˚ and ΔS˚ are positive which indicates that the complexation reaction is endothermic and entropy increasing process. Further, since TΔS˚ > ΔH˚ the coordination reaction is entropy driven process. Negative values of standard
Figure 4. Plot of Pobs and time (Hr) for the 3H4 → 3P2 transition of Pr(III): Guanosine and Ca(II) at different temperatures.
Figure 5. Plot of lnk versus (1/T) × 10−3 for the complexation of Pr(III): Guanosine: Ca(II) in DMF medium.
Table 6. Rate at different temperatures; i.e. 298 K, 303 K, 308 K, 313 K and 318 K and activation energy Ea.
Table 7. Rate and thermodynamic parameters for the complexation of Pr(III): Guanosine: Ca(II) at different temperatures.
Gibbs energy (ΔG˚) indicates that the simultaneous coordination is a spontaneous processes and the process is favored in solution. We can further justify that the simultaneous complexation reaction between Pr(III) ions with (Nucloeside/Nucleotide) and metal ions, Ca(II) occurs but at a spontaneous pace, following the randomness of the system when it approaches higher temperatures (increasing ΔS˚ values). Further, we have also observed that the activation energies (Ea) for Guanosine was the lowest i.e., 0.008445 in Pr(III); the same trend was observed for GTP with 0.0047 in Pr(III), which supports our earlier report that the most sensitive ligand for nucleoside in binding with Pr(III) was Guanosine. The lower values of Ea gives further evidence that the reaction involved are fast reactions.
Acknowledgements
Authors wish to acknowledge the departments of Chemistry, Nagaland University and Manipur University, India for providing the laboratory facilities.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Bendangsenla, N., Moainla, T., Sanchu, J. and Devi, M.I. (2018) Computation of Energy, Intensity and Thermodynamic Parameters for the Interaction of Ln(III) with Nucleic Acid: Analysis of Structural Conformations, Chemical Kinetics and Thermodynamic Behaviour through 4f-4f Transition Spectra as Probe. Journal of Materials Science and Chemical Engineering, 6, 169-183. https://doi.org/10.4236/msce.2018.67018
References
- 1. Moeller, T. and Brantley, J.C. (1958) J. Am. Chem. Soc., 72, 5447. https://doi.org/10.1021/ja01168a022
- 2. Moeller, T. and Jackson, D.E. (1950) Anal. Chem., 22, 1393.
- 3. Gorller-Wairand, C. and Billemans, K. (1996) Spectral Intensities of f-f Transiton, In: Gschneidner, K.A. and Eyring, L., Eds., Handbook on Physics and Chemistry of Rare Earth, North Holland Amsterdam, Ch. 23, Vol. 155, 121.
- 4. Evers, A. and Chrysochoos (1973) Chem. Phys. Lelt., 18, 115. https://doi.org/10.1016/0009-2614(73)80353-7
- 5. Sinha, S.P. (1976) Struct. and Bonding., 25, 69. https://doi.org/10.1007/3-540-07508-9_3
- 6. Misra, S.N. (1985) J. Scient. Ind. Res., 44, 366.
- 7. Mason, S.F., Peacock, R.D. and Stewant, B. (1975) Molec. Phys., 30, 1829.
- 8. Mason, S.F. (1986) J. Indian Chem. Soc., 63, 73.
- 9. Levine, B.A. and William, R.J.P. (1982) The Chemistry of Calcium Ion and Its Biological Relevance, in the Role of Calcium in Biological System. In: Anghileri, L.J. and Tuffet-Anghileri, A.M., Eds., Vol. 1, CRC Press, Boca Raton, FL, 3-26.
- 10. Einspahr, H. and Bugg, C.E. Crystal Structure Studies of Calcium Complexes and Implications for Biological System. In: Sigel, H., Ed., Metal Ions in Biological System, Marcel Dekker, New York, 51-97.
- 11. Martin, R.B. (1983) Structural Chemistry of Calcium: Lanthanides as Probes. In: Spiro, T.G., Ed., Calcium Biology, Wiley, New York, 237-270.
- 12. Godin, B., Sakamoto, J.H., Serda, R.E., Grattoni, A., Boumarini, A. and Ferrari, M. (2010) Trends Pharmacol. Sci. [TiPS], 31, 199-205. https://doi.org/10.1016/j.tips.2010.01.003
- 13. Babailov, S.P. (2012) Inorg. Chem., 51, 1427-1433. https://doi.org/10.1021/ic201662q
- 14. Misra, S.N., Ramchandriah, G., Gagnani, M.A., Shukla, R.S. and Devi, M.I. (2006) Appl. Spectrosc. Rev., 38, 433-493. https://doi.org/10.1081/ASR-120026330
- 15. Devlin, M.T., Stephens, E.M. and Richardson, F.S. (1988) Inorg.Chem., 27, 1517. https://doi.org/10.1021/ic00282a003
- 16. Peacock, R.D. (1970) Chem. Phys. Litt, 7, 187. https://doi.org/10.1016/0009-2614(70)80282-2
- 17. Devi, M.I. and Misra, S.N. (1997) Spectrochim. Acta, 53, 1941. https://doi.org/10.1016/S1386-1425(97)00064-4
- 18. Karraker, D.G. (1969) J. Inorg. Nucl. Chem, 31, 2815-2832. https://doi.org/10.1016/0022-1902(69)80198-3
- 19. Abdi, S.H.R., Anjaiah, K., Joseph, G.K. and Misra, S.N. (1992) “B”. Indian J. Biochem. Biophys, 27, 285-290.